Characterization and Physiological Differences of Two Primary Cultures of Human Normal and Hypertrophic Scar Dermal Fibroblasts: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Dermal Fibroblasts
2.2. Karyotype Analysis
2.3. Flow Cytometry
2.3.1. Phenotype Analysis
2.3.2. Growth Curve
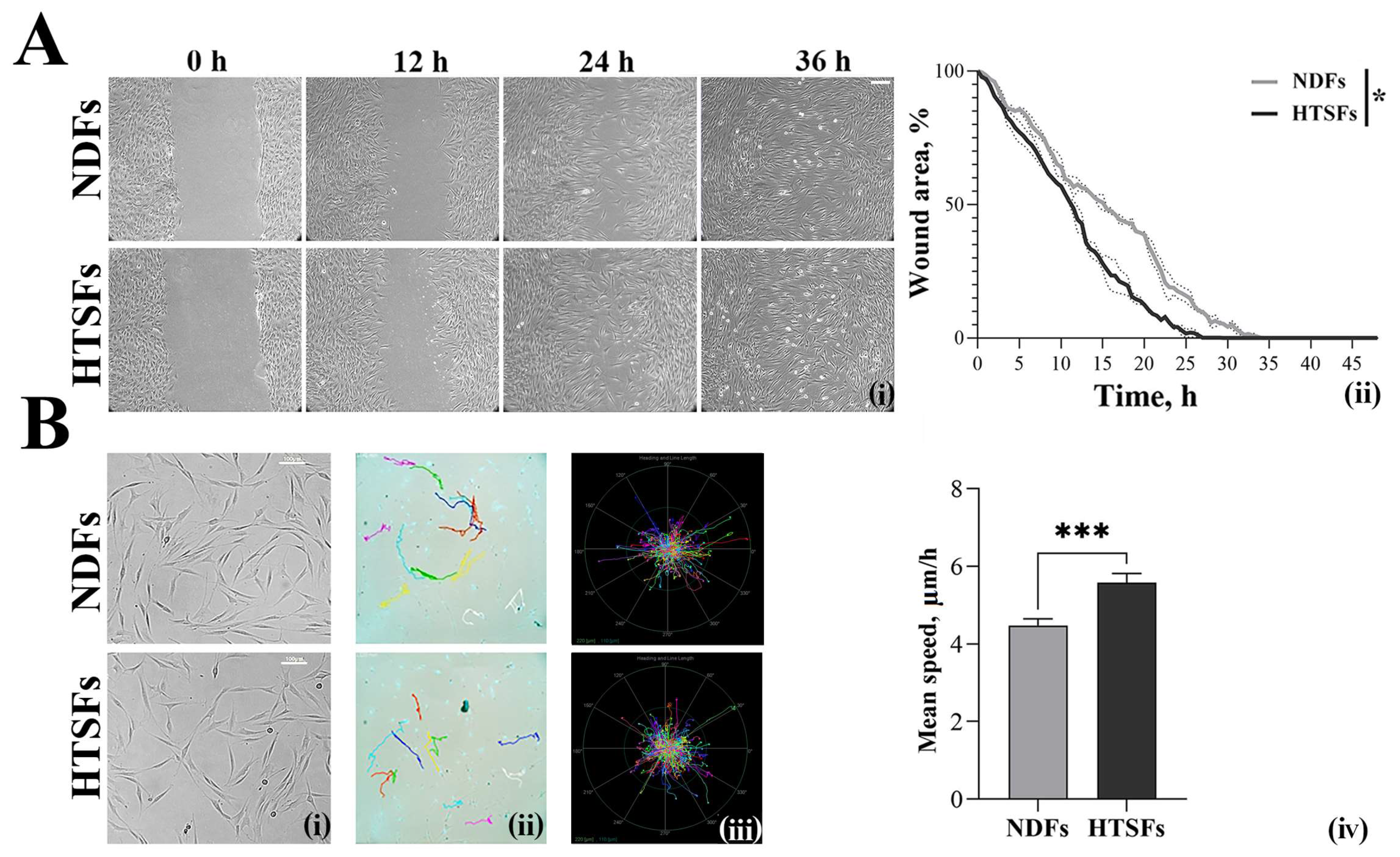
2.4. Assessment of Cell Motility
2.4.1. Wound Healing Assay
2.4.2. Single Cell Motility Assay
2.5. Isolation and Separation of the Cell Secretome
2.6. Liquid Chromatography and Mass Spectrometry
2.7. Statistical Analysis
3. Results
3.1. Cell Morphology and Karyotype Analysis
3.2. Phenotype Analysis and Curve of Cell Proliferation
3.3. Analysis of Cell Motility
3.4. Characterization of NDFs and HTSFs Proteome by Mass Spectrometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Limitations of The Study
Conflicts of Interest
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Verhaegen, P.D.H.M.; Van Zuijlen, P.P.M.; Pennings, N.M.; Van Marle, J.; Niessen, F.B.; Van Der Horst, C.M.A.M.; Middelkoop, E. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Suarez, E.; Syed, F.; Alonso-Rasgado, T.; Bayat, A. Identification of biomarkers involved in differential profiling of hypertrophic and keloid scars versus normal skin. Arch. Dermatol. Res. 2015, 307, 115–133. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, Y.J. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef]
- Elsaie, M.L. Update on management of keloid and hypertrophic scars: A systemic review. J. Cosmet. Dermatol. 2021, 20, 2729–2738. [Google Scholar] [CrossRef]
- El Kinani, M.; Duteille, F. Scar Epidemiology and Consequences. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 45–49. [Google Scholar]
- Griffin, M.F.; Borrelli, M.R.; Garcia, J.T.; Januszyk, M.; King, M.; Lerbs, T.; Cui, L.; Moore, A.L.; Shen, A.H.; Mascharak, S.; et al. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci. Transl. Med. 2021, 13, eabb3312. [Google Scholar] [CrossRef]
- Honardoust, D.; Kwan, P.; Momtazi, M.; Ding, J.; Tredget, E.E. Novel methods for the investigation of human hypertrophic scarring and other dermal fibrosis. Methods Mol. Biol. 2013, 1037, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ebrahim, S.; Sandercock, P.; Bracken, M.B.; Roberts, I. Where is the evidence that animal research benefits humans? BMJ (Clin. Res. Ed.) 2004, 328, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Takamiya, M.; Biwasaka, H.; Saigusa, K.; Nakayashiki, N.; Aoki, Y. Wound age estimation by simultaneous detection of 9 cytokines in human dermal wounds with a multiplex bead-based immunoassay: An estimative method using outsourced examinations. Leg. Med. 2009, 11, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Fujita, S.; Saigusa, K.; Aoki, Y. Simultaneous detections of 27 cytokines during cerebral wound healing by multiplexed bead-based immunoassay for wound age estimation. J. Neurotrauma 2007, 24, 1833–1844. [Google Scholar] [CrossRef]
- Hu, R.; Xu, W.; Ling, W.; Wang, Q.; Wu, Y.; Han, D. Characterization of extracellular matrix proteins during wound healing in the lamina propria of vocal fold in a canine model: A long-term and consecutive study. Acta Histochem. 2014, 116, 730–735. [Google Scholar] [CrossRef]
- Amadeu, T.P.; Braune, A.S.; Porto, L.C.; Desmoulière, A.; Costa, A.M. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2004, 12, 169–174. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Clément, V.; Roy, V.; Paré, B.; Goulet, C.R.; Deschênes, L.T.; Berthod, F.; Bolduc, S.; Gros-Louis, F. Tridimensional cell culture of dermal fibroblasts promotes exosome-mediated secretion of extracellular matrix proteins. Sci. Rep. 2022, 12, 19786. [Google Scholar] [CrossRef]
- Tu, L.; Lin, Z.; Huang, Q.; Liu, D. USP15 Enhances the Proliferation, Migration, and Collagen Deposition of Hypertrophic Scar-Derived Fibroblasts by Deubiquitinating TGF-βR1 In Vitro. Plast. Reconstr. Surg. 2021, 148, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, B.; Lin, S.; Chang, P.; Tao, K. Apigenin inhibits growth and migration of fibroblasts by suppressing FAK signaling. Aging 2019, 11, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gao, X.; Shi, K.; Zhao, J.; Zhang, X.; Zhou, X.; Liu, X.; Yu, J. Interferon-α2b-Induced RARRES3 Upregulation Inhibits Hypertrophic Scar Fibroblasts’ Proliferation and Migration Through Wnt/β-Catenin Pathway Suppression. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2023, 43, 23–34. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Fu, R.; Xue, Y.; Zhao, D.; Han, D. Platycodin D inhibits the proliferation and migration of hypertrophic scar-derived fibroblasts and promotes apoptosis through a caspase-dependent pathway. Arch. Dermatol. Res. 2023, 315, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Philippeos, C.; Telerman, S.B.; Oulès, B.; Pisco, A.O.; Shaw, T.J.; Elgueta, R.; Lombardi, G.; Driskell, R.R.; Soldin, M.; Lynch, M.D.; et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J. Investig. Dermatol. 2018, 138, 811–825. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 2015, 94, 483–512. [Google Scholar] [CrossRef]
- Griffin, M.F.; desJardins-Park, H.E.; Mascharak, S.; Borrelli, M.R.; Longaker, M.T. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis. Models Mech. 2020, 13, dmm044164. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Xue, M.; Zhao, R.; March, L.; Jackson, C. Dermal Fibroblast Heterogeneity and Its Contribution to the Skin Repair and Regeneration. Adv. Wound Care 2022, 11, 87–107. [Google Scholar] [CrossRef]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Shao, J.; Sun, J.; Xu, J. Exosomes released by melanocytes modulate fibroblasts to promote keloid formation: A pilot study. J. Zhejiang Univ. Sci. B 2022, 23, 699–704. [Google Scholar] [CrossRef]
- Vu, R.; Jin, S.; Sun, P.; Haensel, D.; Nguyen, Q.H.; Dragan, M.; Kessenbrock, K.; Nie, Q.; Dai, X. Wound healing in aged skin exhibits systems-level alterations in cellular composition and cell-cell communication. Cell Rep. 2022, 40, 111155. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Long, X.; Zhao, Q.; Zheng, Y.; Song, M.; Ma, S.; Jing, Y.; Wang, S.; He, Y.; Esteban, C.R.; et al. A Single-Cell Transcriptomic Atlas of Human Skin Aging. Dev. Cell 2021, 56, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Horan, M.A.; Ferguson, M.W. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J. Investig. Dermatol. 1997, 108, 430–437. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Horan, M.A.; Ferguson, M.W. The effects of ageing on wound healing: Immunolocalisation of growth factors and their receptors in a murine incisional model. J. Anat. 1997, 190 Pt 3, 351–365. [Google Scholar] [CrossRef]
- Belužić, R.; Šimunić, E.; Podgorski, I.I.; Pinterić, M.; Hadžija, M.P.; Balog, T.; Sobočanec, S. Gene Expression Profiling Reveals Fundamental Sex-Specific Differences in SIRT3-Mediated Redox and Metabolic Signaling in Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2024, 25, 3868. [Google Scholar] [CrossRef]
- Moset Zupan, A.; Nietupski, C.; Schutte, S.C. Cyclic Adenosine Monophosphate Eliminates Sex Differences in Estradiol-Induced Elastin Production from Engineered Dermal Substitutes. Int. J. Mol. Sci. 2021, 22, 6358. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Dodsworth, J.; van Boxtel, E.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef]
- Ma, L.; Gan, C.; Huang, Y.; Wang, Y.; Luo, G.; Wu, J. Comparative proteomic analysis of extracellular matrix proteins secreted by hypertrophic scar with normal skin fibroblasts. Burn. Trauma 2014, 2, 76–83. [Google Scholar] [CrossRef]
- Tsai, K.H.-Y.; Shi, H.; Parungao, R.J.; Naficy, S.; Ding, X.; Ding, X.; Hew, J.J.; Wang, X.; Chrzanowski, W.; Lavery, G.G.; et al. Skin 11β-hydroxysteroid dehydrogenase type 1 enzyme expression regulates burn wound healing and can be targeted to modify scar characteristics. Burn. Trauma 2023, 11, tkac052. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, P.; Bettler, E.; Vadon-Le Goff, S.; Moali, C. Procollagen C-proteinase enhancer-1 (PCPE-1), a potential biomarker and therapeutic target for fibrosis. Matrix Biol. Plus 2021, 11, 100062. [Google Scholar] [CrossRef] [PubMed]
- Niada, S.; Giannasi, C.; Magagnotti, C.; Andolfo, A.; Brini, A.T. Proteomic analysis of extracellular vesicles and conditioned medium from human adipose-derived stem/stromal cells and dermal fibroblasts. J. Proteom. 2021, 232, 104069. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Vetter, T.R. Fundamentals of Research Data and Variables: The Devil Is in the Details. Anesth. Analg. 2017, 125, 1375–1380. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Gadea, G.; Sanz-Moreno, V.; Self, A.; Godi, A.; Marshall, C.J. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr. Biol. CB 2008, 18, 1456–1465. [Google Scholar] [CrossRef]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging 2016, 8, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.; Baur, M.; Guerrero, J.; Pötzel, T.; Stoyanov, J. Autofluorescence is a Reliable in vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells. Sci. Rep. 2019, 9, 2074. [Google Scholar] [CrossRef] [PubMed]
- Terman, A. Garbage catastrophe theory of aging: Imperfect removal of oxidative damage? Redox Rep. Commun. Free Radic. Res. 2001, 6, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ströele, V.; Crivano, R.; Send Email To Zimbrao, I.; Souza, J.; Campos, F.; David, J.M.; Braga, R. Rational Erdös number and maximum flow as measurement models for scientific social network analysis. J. Braz. Comput. Soc. 2018, 24, 6. [Google Scholar] [CrossRef]
- Dhanani, K.C.H.; Samson, W.J.; Edkins, A.L. Fibronectin is a stress responsive gene regulated by HSF1 in response to geldanamycin. Sci. Rep. 2017, 7, 17617. [Google Scholar] [CrossRef]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Li, C.X.; Gao, X.Q.; Qiu, W.Y.; Chen, Q.; Li, X.M.; Zhou, X.; Tian, X.; Tang, Z.P.; Zhao, T.; et al. Identification and functional characterization of a novel transglutaminase 1 gene mutation associated with autosomal recessive congenital ichthyosis. Int. J. Dermatol. 2016, 55, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, N.; Lee, S.H.; Liu, S.; Li, G.Y.; Smith, M.J.; Reichardt, L.F.; Ikura, M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 2010, 141, 117–128. [Google Scholar] [CrossRef]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2023, 10, 1894–1907. [Google Scholar] [CrossRef]
- Shimaoka, M.; Kawamoto, E.; Gaowa, A.; Okamoto, T.; Park, E.J. Connexins and Integrins in Exosomes. Cancers 2019, 11, 106. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J. Cell Biol. 2020, 219, e202006178. [Google Scholar] [CrossRef] [PubMed]
- Lofaro, F.D.; Costa, S.; Simone, M.L.; Quaglino, D.; Boraldi, F. Fibroblasts’ secretome from calcified and non-calcified dermis in Pseudoxanthoma elasticum differently contributes to elastin calcification. Commun. Biol. 2024, 7, 577. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Chang, Y.H.; Lin, Y.C.; Chen, B.; Guevara, B.E.K.; Hsu, C.K. Inflammation in Wound Healing and Pathological Scarring. Adv. Wound Care 2023, 12, 288–300. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Kim, D.H.; Joo, S.Y.; Cho, Y.S.; Kim, J.B.; Seo, C.H. Exosomes derived from human hypertrophic scar fibroblasts induces smad and TAK1 signaling in normal dermal fibroblasts. Arch. Biochem. Biophys. 2022, 722, 109215. [Google Scholar] [CrossRef]
- Stacey, M.; Chang, G.W.; Davies, J.Q.; Kwakkenbos, M.J.; Sanderson, R.D.; Hamann, J.; Gordon, S.; Lin, H.H. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 2003, 102, 2916–2924. [Google Scholar] [CrossRef]
- Mulligan, M.S.; Lentsch, A.B.; Huber-Lang, M.; Guo, R.F.; Sarma, V.; Wright, C.D.; Ulich, T.R.; Ward, P.A. Anti-inflammatory effects of mutant forms of secretory leukocyte protease inhibitor. Am. J. Pathol. 2000, 156, 1033–1039. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Ding, F.; Zhang, Y.; Luo, A.; Wang, M.; Liu, Z. A novel p53 target gene, S100A9, induces p53-dependent cellular apoptosis and mediates the p53 apoptosis pathway. Biochem. J. 2009, 422, 363–372. [Google Scholar] [CrossRef]
- Nakatani, Y.; Yamazaki, M.; Chazin, W.J.; Yui, S. Regulation of S100A8/A9 (calprotectin) binding to tumor cells by zinc ion and its implication for apoptosis-inducing activity. Mediat. Inflamm. 2005, 2005, 280–292. [Google Scholar] [CrossRef]
- Bertin, J.; Wang, L.; Guo, Y.; Jacobson, M.D.; Poyet, J.L.; Srinivasula, S.M.; Merriam, S.; DiStefano, P.S.; Alnemri, E.S. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-kappa B. J. Biol. Chem. 2001, 276, 11877–11882. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Umehara, Y.; Yue, H.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Ikutama, R.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide Human β-Defensin-3 Accelerates Wound Healing by Promoting Angiogenesis, Cell Migration, and Proliferation Through the FGFR/JAK2/STAT3 Signaling Pathway. Front. Immunol. 2021, 12, 712781. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Gu, Y.; Xiong, Y.; Zheng, G.; He, Z. Microarray-assisted pathway analysis identifies MT1X & NFκB as mediators of TCRP1-associated resistance to cisplatin in oral squamous cell carcinoma. PLoS ONE 2012, 7, e51413. [Google Scholar] [CrossRef]
- Tan, J.; He, W.; Luo, G.; Wu, J. Involvement of impaired desmosome-related proteins in hypertrophic scar intraepidermal blister formation. Burns 2015, 41, 1517–1523. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Moukette, B.; Sepúlveda, M.N.; Hayasaka, T.; Aonuma, T.; Haskell, A.K.; Mah, J.; Liangpunsakul, S.; Tang, Y.; Conway, S.J.; et al. SPRR1A is a key downstream effector of MiR-150 during both maladaptive cardiac remodeling in mice and human cardiac fibroblast activation. Cell Death Dis. 2023, 14, 446. [Google Scholar] [CrossRef]
- Kjell, J.; Götz, M. Filling the Gaps—A Call for Comprehensive Analysis of Extracellular Matrix of the Glial Scar in Region- and Injury-Specific Contexts. Front. Cell. Neurosci. 2020, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Yiu, T.W.; Holman, S.R.; Kaidonis, X.; Graham, R.M.; Iismaa, S.E. Transglutaminase 2 Facilitates Murine Wound Healing in a Strain-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 11475. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Ali-Bahar, M.; Bauer, B.; Tredget, E.E.; Ghahary, A. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2004, 12, 175–182. [Google Scholar] [CrossRef]
- Baranyi, U.; Winter, B.; Gugerell, A.; Hegedus, B.; Brostjan, C.; Laufer, G.; Messner, B. Primary Human Fibroblasts in Culture Switch to a Myofibroblast-Like Phenotype Independently of TGF Beta. Cells 2019, 8, 721. [Google Scholar] [CrossRef]
- Walmsley, G.G.; Rinkevich, Y.; Hu, M.S.; Montoro, D.T.; Lo, D.D.; McArdle, A.; Maan, Z.N.; Morrison, S.D.; Duscher, D.; Whittam, A.J.; et al. Live fibroblast harvest reveals surface marker shift in vitro. Tissue Eng. Part C Methods 2015, 21, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]



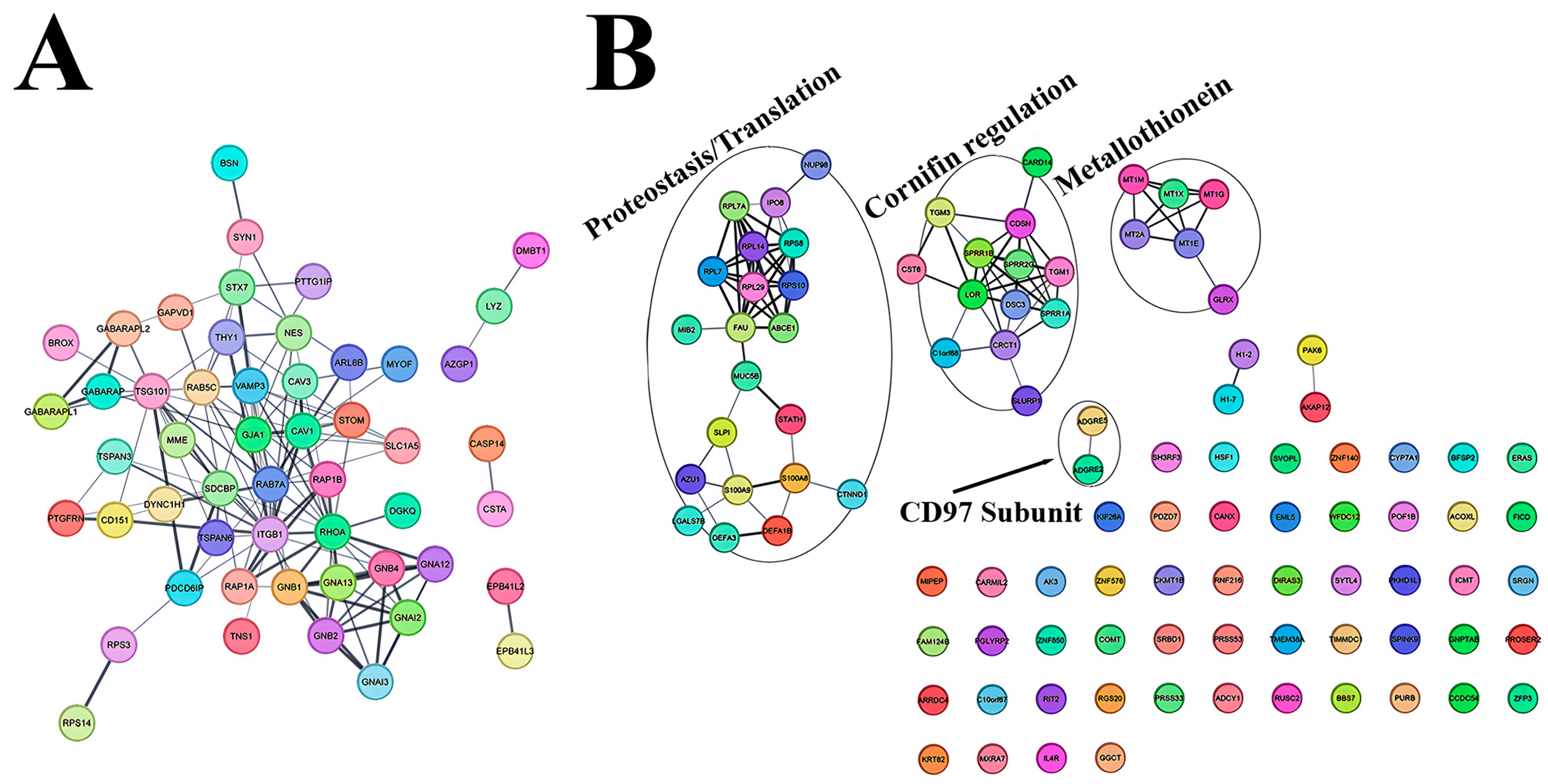

| Soluble Fraction | Vesicular Fraction | |
|---|---|---|
| NDFs | Cell differentiation, Chaperome, ECM, Hemostasis, Histone group, mRNA binding, Vesicular transport, Proteasomal proteins, Keratinization, Lysosomal proteins | Chaperome, Cytoskeleton, ECM, Ribosomal proteins, Histone group, Small GTPases, Chaperome, Vesicular proteins group, Vesicular transport, tRNAgroup |
| HTSFs | Cell differentiation, Chaperome, ECM, Hemostasis, Histone group, mRNA binding, Vesicular transport, Proteasomal proteins, Cytoskeleton, Carbon metabolism | Cytoskeleton, Chaperome, ECM, Ribosomal proteins, Histone group, Small GTPases, Cell differentiation, Keratinization, Actin contraction, Annexin group, Calmodulin group |
| vHTSFs | vNDFs | sHTSFs | sNDFs |
|---|---|---|---|
| STARD9 | NBPF10 | NBPF10 | NBPF10 |
| PRSS53 | NBPF19-2 | NBPF19-2 | NBPF19-2 |
| TRAPPC2L | SLC43A3 | ||
| ARRDC4 | FAM234A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudintceva, N.M.; Kolesnichenko, Y.V.; Shatrova, A.N.; Aksenov, N.D.; Yartseva, N.M.; Shevtsov, M.A.; Fedorov, V.S.; Khotin, M.G.; Ziganshin, R.H.; Mikhailova, N.A. Characterization and Physiological Differences of Two Primary Cultures of Human Normal and Hypertrophic Scar Dermal Fibroblasts: A Pilot Study. Biomedicines 2024, 12, 2295. https://doi.org/10.3390/biomedicines12102295
Yudintceva NM, Kolesnichenko YV, Shatrova AN, Aksenov ND, Yartseva NM, Shevtsov MA, Fedorov VS, Khotin MG, Ziganshin RH, Mikhailova NA. Characterization and Physiological Differences of Two Primary Cultures of Human Normal and Hypertrophic Scar Dermal Fibroblasts: A Pilot Study. Biomedicines. 2024; 12(10):2295. https://doi.org/10.3390/biomedicines12102295
Chicago/Turabian StyleYudintceva, Natalia M., Yulia V. Kolesnichenko, Alla N. Shatrova, Nikolay D. Aksenov, Natalia M. Yartseva, Maxim A. Shevtsov, Viacheslav S. Fedorov, Mikhail G. Khotin, Rustam H. Ziganshin, and Natalia A. Mikhailova. 2024. "Characterization and Physiological Differences of Two Primary Cultures of Human Normal and Hypertrophic Scar Dermal Fibroblasts: A Pilot Study" Biomedicines 12, no. 10: 2295. https://doi.org/10.3390/biomedicines12102295
APA StyleYudintceva, N. M., Kolesnichenko, Y. V., Shatrova, A. N., Aksenov, N. D., Yartseva, N. M., Shevtsov, M. A., Fedorov, V. S., Khotin, M. G., Ziganshin, R. H., & Mikhailova, N. A. (2024). Characterization and Physiological Differences of Two Primary Cultures of Human Normal and Hypertrophic Scar Dermal Fibroblasts: A Pilot Study. Biomedicines, 12(10), 2295. https://doi.org/10.3390/biomedicines12102295







