Exploring the Phytochemistry, Signaling Pathways, and Mechanisms of Action of Tanacetum parthenium (L.) Sch.Bip.: A Comprehensive Literature Review
Abstract
:1. Introduction
2. Literature Review Methodology
3. Phytochemistry
3.1. Sesquiterpene Lactones
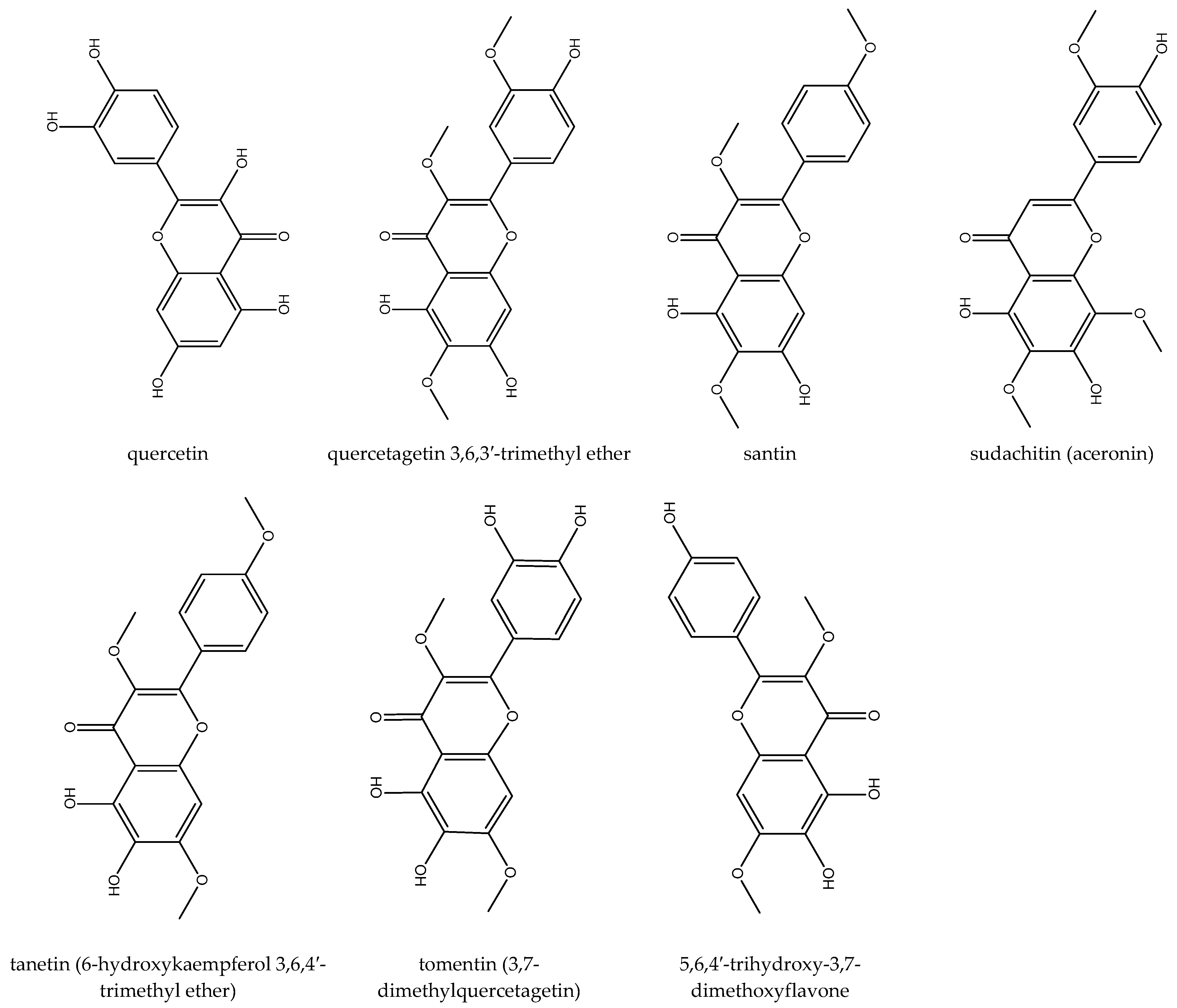
3.2. Flavonoids
3.3. Phytosterol and Triterpenoids
3.4. Essential Oil
3.5. Fatty Acids
3.6. Phenolic Acids
3.7. Other Phytochemicals
4. Feverfew’s Anti-Inflammatory and Antioxidant Properties
4.1. Inhibition of Pro-Inflammatory Enzyme Activity
4.2. Modulation of Pro-Inflammatory Mediators
4.3. Modulation of Adhesion Molecule Expression
4.4. Inhibition of NF- kB
| Mechanism of Action | Extract/Component | Study Type | Outcomes | Ref. | |
|---|---|---|---|---|---|
| Anti-inflammatory effect | Modulation of adhesion molecule expression | Parthenolide | in vitro/human RA synovial tissue | ↓Intercellular adhesion molecule-1 (ICAM-1) | [57] |
| Inhibition of pro- inflammatory enzyme activity | Parthenolide | in vivo In vitro/human epidermal keratinocytes | ↓5-lipoxygenase (IC50:11.8 ± 4.8 µg), phosphodiesterase-3 (IC50: 35.2 ± 12.3 µg/mL), and phosphodiesterase-4 (IC50: 20.8 ± 9.4 µg/mL) | [58] | |
| Modulation of pro- inflammatory mediators | Parthenolide | in vivo in vitro/human epidermal keratinocytes | ↓PGE2 production (IC50: 37.9 ± 4.16 µg/mL) and TNFa production (IC50: 31 ± 0.04 µg/mL) | [58] | |
| Parthenolide | in vitro/RAW264.7 cells | ↓IL-12 production | [59] | ||
| Aqueous extract | in silico | ↓ PGE2 and IL-1β, ↑ IL-10 | [60] | ||
| Parthenolide | in vitro/human RA synovial tissue | ↓TNF-α, and IFN-gamma | [57] | ||
| Feverfew’s extracts | in vitro/human monocytic THP-1 cells | ↓ LPS-mediated TNF-α and CCL2 (MCP-1) | [61] | ||
| Inhibition of NF- kB | Parthenolide | in vitro/peripheral blood T cells | Inhibition of NF- kB | [62] | |
| Antioxidant activity | Parthenolide | in vivo | ↑Total antioxidant capacity, glutathione (GSH) content, superoxide dismutase (SOD), and catalase (CAT) ↑The survival rate in mice pretreated with parthenolide compared to the control group when exposed to electron beam irradiation | [63] | |
| Methanol extract | in vitro | ↑Confirmed by evaluation by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay | [64] | ||
5. Feverfew’s Medicinal and Therapeutic Properties
5.1. Neurological Disorders
5.1.1. Migraine
5.1.2. Epilepsy
5.1.3. Neuroprotective Effects
5.1.4. Parkinson’s Disease
5.1.5. Neuropathic Pain
5.1.6. Anxiety and Depression
5.1.7. Hypnotic
5.2. Cancer
5.3. Respiratory System Disorders
5.3.1. Asthma
5.3.2. Acute Lung Injury
5.3.3. Acute Respiratory Distress Syndrome
5.4. Skin Disorders
5.5. Anti-Fibrotic Effects
5.6. Ulcerative Colitis
5.7. Anti-Osteoclastogenic Effects
5.8. Endometriosis
5.9. Obesity
5.10. Antiprotozoal Effect
6. Safety, Toxicity, and Adverse Effects of Feverfew
7. Guidelines for Healthcare Providers on the Use of Feverfew
- ✓ Pregnancy and Lactation: Avoid feverfew during pregnancy and breastfeeding due to potential risks.
- ✓ Pediatric Use: Not recommended for children under 2 years of age.
- ✓ Drug Interactions: Caution advised with anticoagulant medications and NSAIDs.
- ✓ Allergies: Be wary if allergic to plants in the Asteraceae family.
- ✓ Pre-surgery: Discontinue use before surgery to prevent bleeding complications.
- ✓ Monitoring Adverse Effects: Report any unusual symptoms promptly.
- ✓ Long-term Use: Lack of data on prolonged safety—regular monitoring recommended.
- ✓ Parthenolide-free Alternatives: Consider using parthenolide-free extracts for reduced allergic risk.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kenny, O.; Smyth, T.; Walsh, D.; Kelleher, C.; Hewage, C.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lechkova, B.; Benbassat, N.; Karcheva-Bahchevanska, D.; Ivanov, K.; Peychev, L.; Peychev, Z.; Dyankov, S.; Georgieva-Dimova, Y.; Kraev, K.; Ivanova, S. A Comparison between Bulgarian Tanacetum parthenium Essential Oil from Two Different Locations. Molecules 2024, 29, 1969. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F. Feverfew (Tanacetum parthenium (L.) Sch. Bip.). Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–225. [Google Scholar]
- Besharati-Seidani, A.; Jabbari, A.; Yamini, Y.; Saharkhiz, M. Rapid extraction and analysis of volatile organic compounds of Iranian feverfew (Tanacetum parthenium) using headspace solvent microextraction (HSME), and gas chromatography/mass spectrometry. Flavour Fragr. J. 2006, 21, 502–509. [Google Scholar] [CrossRef]
- Chavez, M.L.; Chavez, P.I. Feverfew. Hosp. Pharm. 1999, 34, 436–461. [Google Scholar] [CrossRef]
- Abhyankar, V.; Bland, P.; Fernandes, G. The Role of Systems Biologic Approach in Cell Signaling and Drug Development Responses-A Mini Review. Med. Sci. 2018, 6, 43. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- AntoinetteáGroenewegen, W. Sesquiterpene lactones from feverfew, Tanacetum parthenium: Iisolation, structural revision, activity against human blood platelet function and implications for migraine therapy. J. Chem. Soc. Perkin Trans. 1 1996, 1979–1986. [Google Scholar] [CrossRef]
- Abad, M.; Bermejo, P.; Villar, A. An approach to the genus Tanacetum, L. (Compositae): Phytochemical and pharmacological review. Phytother. Res. 1995, 9, 79–92. [Google Scholar] [CrossRef]
- Rateb, M.; El-Gendy, A.; El-Hawary, S.; El-Shamy, A. Phytochemical and biological studies on the different organs of Tanacetum parthenium L. cultivated in Egypt. J. Med. Plants 2008, 7, 8–22. [Google Scholar]
- Milbrodt, M.; Schröder, F.; König, W.A. 3, 4-β-Epoxy-8-deoxycumambrin B, A sesquiterpene lactone from Tanacetum parthenium. Phytochemistry 1997, 44, 471–474. [Google Scholar] [CrossRef]
- Begley, M.J.; Hewlett, M.J.; Knight, D.W. Revised structures for guaianolide α-methylenebutyro-lactones from feverfew. Phytochemistry 1989, 28, 940–943. [Google Scholar] [CrossRef]
- de Almeida, L.M.S.; Carvalho, L.S.A.; Gazolla, M.C.; Silva Pinto, P.L.; Silva, M.P.N.d.; de Moraes, J.; Da Silva Filho, A.A. Flavonoids and Sesquiterpene Lactones from Artemisia absinthium and Tanacetum parthenium against Schistosoma mansoni Worms. Evid.-Based Complement. Altern. Med. 2016, 2016, 9521349. [Google Scholar] [CrossRef] [PubMed]
- Rabito, M.F.; Britta, E.A.; Pelegrini, B.L.; Scariot, D.B.; Almeida, M.B.; Nixdorf, S.L.; Nakamura, C.V.; Ferreira, I.C. In vitro and in vivo antileishmania activity of sesquiterpene lactone-rich dichloromethane fraction obtained from Tanacetum parthenium (L.) Schultz-Bip. Exp. Parasitol. 2014, 143, 18–23. [Google Scholar] [CrossRef]
- Farzadfar, S.; Zarinkamar, F.; Hojati, M. Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. Biochem. 2017, 112, 207–217. [Google Scholar] [CrossRef]
- Yao, Y.; Song, L.; Zuo, Z.; Chen, Z.; Wang, Y.; Cai, H.; Gu, Y.; Lv, Z.; Guan, J.; Chen, R.; et al. Parthenolide attenuates hypoxia-induced pulmonary hypertension through inhibiting STAT3 signaling. Phytomedicine 2024, 134, 155976. [Google Scholar] [CrossRef]
- Pourianezhad, F.; Tahmasebi, S.; Nikfar, S.; Mirhoseini, M.; Abdusi, V. Review on feverfew, a valuable medicinal plant. J. HerbMed Pharmacol. 2016, 5, 45–49. [Google Scholar]
- Végh, K.; Riethmüller, E.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, Á.; Tóth, A.; Béni, S.; Könczöl, Á.; Balogh, G.T.; et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef]
- Rezaei, F.; Jamei, R.; Heidari, R. Evaluation of the Phytochemical and Antioxidant Potential of Aerial Parts of Iranian Tanacetum parthenium. Pharm. Sci. 2017, 23, 136. [Google Scholar] [CrossRef]
- Hanganu, D.; Benedec, D.; Vlase, L.; Popica, I.; Bele, C.; Raita, O.; Gheldiu, A.; Mihali, C.; Țarmure, V. Polyphenolic content and antioxidant activity of Chrysanthemum parthenium extract. Farmacia 2016, 64, 498–501. [Google Scholar]
- Végh, K.; Alberti, Á.; Riethmüller, E.; Tóth, A.; Béni, S.; Kéry, Á. Separation and analysis of bioactive flavonoids in Tanacetum parthenium supercritical fluid extracts. Planta Medica 2015, 81, PM_56. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Eagles, J. Variations in lipophilic and polar flavonoids in the genus Tanacetum. Phytochemistry 1999, 52, 1301–1306. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Hoult, J.R.S. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry 1999, 51, 417–423. [Google Scholar] [CrossRef]
- Williams, C.A.; Hoult, J.; Harborne, J.B.; Greenham, J.; Eagles, J. A biologically active lipophilic flavonol from Tanacetum parthenium. Phytochemistry 1995, 38, 267–270. [Google Scholar] [CrossRef]
- Long, C.; Sauleau, P.; David, B.; Lavaud, C.; Cassabois, V.; Ausseil, F.; Massiot, G. Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry 2003, 64, 567–569. [Google Scholar] [CrossRef]
- Wiłkomirski, B.; Dubielecka, B. Sterol content as a similarity marker of different organs of two varietas of Chrysanthemum parthenium. Phytochemistry 1996, 42, 1603–1604. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Bhakuni, R. Secondary metabolites of Chrysanthemum genus and their biological activities. Curr. Sci. 2005, 89, 1489–1501. [Google Scholar]
- Gu, J.Q.; Wang, Y.; Franzblau, S.G.; Montenegro, G.; Timmermann, B.N. Dereplication of pentacyclic triterpenoids in plants by GC-EI/MS. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2006, 17, 102–106. [Google Scholar] [CrossRef]
- Shahhoseini, R.; Azizi, M.; Asili, J.; Moshtaghi, N.; Samiei, L. Comprehensive Assessment of Phytochemical Potential of Tanacetum parthenium (L.): Phenolic Compounds, Antioxidant Activity, Essential Oil and Parthenolide. J. Essent. Oil Bear. Plants 2019, 22, 614–629. [Google Scholar] [CrossRef]
- Pavela, R.; Sajfrtová, M.; Sovová, H.; Bárnet, M.; Karban, J. The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip. extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crops Prod. 2010, 31, 449–454. [Google Scholar] [CrossRef]
- Végh, K.; Riethmüller, E.; Tóth, A.; Alberti, Á.; Béni, S.; Balla, J.; Kéry, Á. Convergence chromatographic determination of camphor in the essential oil of Tanacetum parthenium L. Biomed. Chromatogr. 2016, 30, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, F.; Chehregani, A.; Amiri, H. Chemical composition, antibacterial activity and cytotoxicity of essential oils of Tanacetum parthenium in different developmental stages. Pharm. Biol. 2011, 49, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Akpulat, H.A.; Tepe, B.; Sokmen, A.; Daferera, D.; Polissiou, M. Composition of the essential oils of Tanacetum argyrophyllum (C. Koch) Tvzel. var. argyrophyllum and Tanacetum parthenium (L.) Schultz Bip.(Asteraceae) from Turkey. Biochem. Syst. Ecol. 2005, 33, 511–516. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Kabudani, M.; Tabibzadeh, Z. Effect of drying methods on the essential oil content and composition of Tanacetum parthenium (L.) Schultz Bip cv. Zardband. J. Essent. Oil Bear. Plants 2007, 10, 26–30. [Google Scholar] [CrossRef]
- Mojab, F.; Tabatabai, S.A.; Naghdi-Badi, H.; Nickavar, N.; Ghadyani, F. Essential oil of the root of Tanacetum parthenium (L.) Schulz. Bip. (Asteraceae) from Iran. Iran. J. Pharm. Res. 2010, 291–293. [Google Scholar]
- Giuliani, C.; Bottoni, M.; Milani, F.; Spada, A.; Falsini, S.; Papini, A.; Santagostini, L.; Fico, G. An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry. Plants 2024, 13, 155. [Google Scholar] [CrossRef]
- Lechkova, B.; Karcheva-Bahchevanska, D.; Ivanov, K.; Todorova, V.; Benbassat, N.; Penkova, N.; Atanassova, P.; Peychev, L.; Hrischev, P.; Peychev, Z.; et al. A Study of the chemical composition, acute and subacute toxicity of Bulgarian Tanacetum parthenium essential oil. Molecules 2023, 28, 4906. [Google Scholar] [CrossRef]
- Wu, C.; Chen, F.; Wang, X.; Wu, Y.; Dong, M.; He, G.; Galyean, R.D.; He, L.; Huang, G. Identification of antioxidant phenolic compounds in feverfew (Tanacetum parthenium) by HPLC-ESI-MS/MS and NMR. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2007, 18, 401–410. [Google Scholar] [CrossRef]
- Kisiel, W.; Stojakowska, A. A sesquiterpene coumarin ether from transformed roots of Tanacetum parthenium. Phytochemistry 1997, 46, 515–516. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kisiel, W. Salicylate and methyl jasmonate differentially influence diacetylene accumulation pattern in transformed roots of feverfew. Plant Sci. 2002, 163, 1147–1152. [Google Scholar] [CrossRef]
- Stojakowska, A.; Burczyk, J.; Kisiel, W.; Zych, M.; Banas, A.; Duda, T. Effects of various elicitors on the accumulation and secretion of spiroketal enol ether diacetylenes in feverfew hairy root culture. Acta Soc. Bot. Pol. 2008, 77, 17–21. [Google Scholar] [CrossRef]
- Kouhestani, F.; Dayer, M.S.; Kamali, H. Reversed-phase Liquid Chromatographic Quantification of Pyrethrins in the Extract of Wild Tanacetum parthenium (Feverfew) from Northern Khorasan Province (Iran). J. Med. Plants By-Prod. 2018, 7, 99–104. [Google Scholar]
- Sy, L.-K.; Brown, G.D. Coniferaldehyde derivatives from tissue culture of Artemisia annua and Tanacetum parthenium. Phytochemistry 1999, 50, 781–785. [Google Scholar] [CrossRef]
- Murch, S.J.; Simmons, C.B. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Susan, J.M. Identification and Characterizatlon of Melatonin in Medicinal Plants: Feverfew Wang-Qin and St. John’s Wort; The University of Guelph: Guelph, ON, Canada, 2000. [Google Scholar]
- Marete, E.N.; Jacquier, J.C.; O’Riordan, D. Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food Chem. 2009, 117, 226–231. [Google Scholar] [CrossRef]
- Kahnt, A.S.; Angioni, C.; Göbel, T.; Hofmann, B.; Roos, J.; Steinbrink, S.D.; Rörsch, F.; Thomas, D.; Geisslinger, G.; Zacharowski, K.; et al. Inhibitors of Human 5-Lipoxygenase Potently Interfere With Prostaglandin Transport. Front. Pharmacol. 2022, 12, 782584. [Google Scholar] [CrossRef]
- Phillips, J.E. Inhaled Phosphodiesterase 4 (PDE4) Inhibitors for Inflammatory Respiratory Diseases. Front. Pharmacol. 2020, 11, 259. [Google Scholar] [CrossRef]
- Bergqvist, F.; Sundström, Y.; Shang, M.; Gunnarsson, I.; Lundberg, I.E.; Sundström, M.; Jakobsson, P.J.; Berg, L. Anti-Inflammatory Properties of Chemical Probes in Human Whole Blood: Focus on Prostaglandin E2 Production. Front. Pharmacol. 2020, 11, 613. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vaillant, A.A.J.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Wiesolek, H.L.; Bui, T.M.; Lee, J.J.; Dalal, P.; Finkielsztein, A.; Batra, A.; Thorp, E.B.; Sumagin, R. Intercellular Adhesion Molecule 1 Functions as an Efferocytosis Receptor in Inflammatory Macrophages. Am. J. Pathol. 2020, 190, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Piela-Smith, T.H.; Liu, X. Feverfew extracts and the sesquiterpene lactone parthenolide inhibit intercellular adhesion molecule-1 expression in human synovial fibroblasts. Cell. Immunol. 2001, 209, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Martin, K.; Liebel, F.; Lyte, P.; Shapiro, S.; Southall, M. Anti-inflammatory activity of parthenolide-depleted Feverfew (Tanacetum parthenium). Inflammopharmacology 2009, 17, 42–49. [Google Scholar] [CrossRef]
- Kang, B.Y.; Chung, S.W.; Kim, T.S. Inhibition of interleukin-12 production in lipopolysaccharide-activated mouse macrophages by parthenolide, a predominant sesquiterpene lactone in Tanacetum parthenium: Involvement of nuclear factor-κB. Immunol. Lett. 2001, 77, 159–163. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-inflammatory and neuromodulatory effects induced by Tanacetum parthenium water extract: Results from in silico, in vitro and ex vivo studies. Molecules 2020, 26, 22. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, C. Regulation of cellular metabolism and cytokines by the medicinal herb feverfew in the human monocytic THP-1 cells. Evid.-Based Complement. Altern. Med. 2009, 6, 91–98. [Google Scholar] [CrossRef]
- Li-Weber, M.; Giaisi, M.; Treiber, M.K.; Krammer, P.H. The anti-inflammatory sesquiterpene lactone parthenolide suppresses IL-4 gene expression in peripheral blood T cells. Eur. J. Immunol. 2002, 32, 3587–3597. [Google Scholar] [CrossRef]
- Pooja, S.; Shetty, P.; Kumari, N.S.; Shetty, K.J. Radioprotective and antioxidant potential of Tanacetum parthenium extract and synthetic parthenolide in Swiss albino mice exposed to electron beam irradiation. Int. J. Radiat. Res. 2021, 19, 145–154. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.-Y.; Guillen Quispe, Y.N.; Wang, Z.; Zuo, G.; Lim, S.S. Aldose Reductase, Protein Glycation Inhibitory and Antioxidant of Peruvian Medicinal Plants: The Case of Tanacetum parthenium L. and Its Constituents. Molecules 2019, 24, 2010. [Google Scholar] [CrossRef]
- Johnson, E.S.; Kadam, N.P.; Hylands, D.M.; Hylands, P.J. Efficacy of feverfew as prophylactic treatment of migraine. Br. Med. J. Clin. Res. Ed. 1985, 291, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.J.; Heptinstall, S.; Mitchell, J.R.A. Randomised Double-Blind Placebo-Controlled Trial of Feverfew in Migraine Prevention. Lancet 1988, 332, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Palevitch, D.; Earon, G.; Carasso, R. Feverfew (Tanacetum parthenium) as a prophylactic treatment for migraine: A double-blind placebo-controlled study. Phytother. Res. 1997, 11, 508–511. [Google Scholar] [CrossRef]
- Pfaffenrath, V.; Diener, H.C.; Fischer, M.; Friede, M.; Henneicke-Von Zepelin, H.H. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis—A double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia 2002, 22, 523–532. [Google Scholar] [CrossRef]
- Diener, H.C.; Pfaffenrath, V.; Schnitker, J.; Friede, M.; Henneicke-Von Zepelin, H.H. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention—A randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia 2005, 25, 1031–1041. [Google Scholar] [CrossRef]
- Moscano, F.; Guiducci, M.; Maltoni, L.; Striano, P.; Ledda, M.G.; Zoroddu, F.; Raucci, U.; Villa, M.P.; Parisi, P. An observational study of fixed-dose Tanacetum parthenium nutraceutical preparation for prophylaxis of pediatric headache. Ital. J. Pediatr. 2019, 45, 36. [Google Scholar] [CrossRef]
- Magni, P.; Ruscica, M.; Dozio, E.; Rizzi, E.; Beretta, G.; Facino, R.M. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-α and NF-κB nuclear translocation in BV-2 microglia. Phytother. Res. 2012, 26, 1405–1409. [Google Scholar] [CrossRef]
- Tassorelli, C.; Greco, R.; Morazzoni, P.; Riva, A.; Sandrini, G.; Nappi, G. Parthenolide is the component of Tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: Studies in an animal model of migraine. Cephalalgia 2005, 25, 612–621. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Bertels, Z.; Akerman, S. Targeted Nitric Oxide Synthase Inhibitors for Migraine. Neurotherapeutics 2018, 15, 391–401. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Lieb, K.; Engels, S.; Heinrich, M. Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J. Neuroimmunol. 2002, 132, 18–24. [Google Scholar] [CrossRef]
- Aviram, A.; Tsoukias, N.M.; Melnick, S.J.; Resek, A.P.; Ramachandran, C. Inhibition of nitric oxide synthesis in mouse macrophage cells by feverfew supercritical extract. Phytother. Res. 2012, 26, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.C.; Dodick, D.W. Other Preventive Anti-Migraine Treatments: ACE Inhibitors, ARBs, Calcium Channel Blockers, Serotonin Antagonists, and NMDA Receptor Antagonists. Curr. Treat. Options Neurol. 2019, 21, 17. [Google Scholar] [CrossRef]
- Mittra, S.; Datta, A.; Singh, S.; Singh, A. 5-Hydroxytryptamine-inhibiting property of Feverfew: Role of parthenolide content. Acta Pharmacol. Sin. 2000, 21, 1106–1114. [Google Scholar]
- Jäger, A.K.; Gauguin, B.; Adsersen, A.; Gudiksen, L. Screening of plants used in Danish folk medicine to treat epilepsy and convulsions. J. Ethnopharmacol. 2006, 105, 294–300. [Google Scholar] [CrossRef]
- Jäger, A.K.; Krydsfeldt, K.; Rasmussen, H.B. Bioassay-guided isolation of apigenin with GABA-benzodiazepine activity from Tanacetum parthenium. Phytother. Res. 2009, 23, 1642–1644. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, L.; Shi, L.L.; Zhao, Z.H.; Xu, H.; Liang, F.; Li, H.B.; Zhao, Y.; Xu, X.; Yang, K.; et al. Parthenolide attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β pathway in PC12 cells. Biomed. Pharmacother. 2017, 89, 1159–1165. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Hosseini, M.; Bargi, R.; Beheshti, F.; Rakhshandeh, H.; Mansouri, S.; Aghaei, A.; Sadeghnia, H.R.; Anaeigoudari, A. Effects of Hydro-ethanolic Extract of Tanacetum parthenium and its N-Butanol and Aqueous Fractions on Brain Oxidative Damage in Pentylenetetrazole-Induced Seizures in Mice. Pharm. Sci. 2020, 26, 252–260. [Google Scholar] [CrossRef]
- Ding, W.; Cai, C.; Zhu, X.; Wang, J.; Jiang, Q. Parthenolide ameliorates neurological deficits and neuroinflammation in mice with traumatic brain injury by suppressing STAT3/NF-κB and inflammasome activation. Int. Immunopharmacol. 2022, 108, 108913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, L.; Peng, Q.; Fan, X.; Song, W.; Yang, B.; Zhang, P.; Liu, G.; Liu, J. Parthenolide modulates cerebral ischemia-induced microglial polarization and alleviates neuroinflammatory injury via the RhoA/ROCK pathway. Phytomedicine 2022, 105, 154373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, S.; Zhu, D.; Tang, X.; Che, Y.; Feng, X. The parthenolide derivative ACT001 synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson’s disease in mice. Behav. Brain Res. 2020, 379, 112337. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.; Deiab, S.; Park, K.; Soliman, K.F. High throughput screening to identify natural human monoamine oxidase B inhibitors. Phytother. Res. 2013, 27, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Safakhah, H.A.; Tatar, M.; Ghanbari, A. Tanacetum parthenium relieves chronic constriction injury-induced neuropathic pain in male rats. Ann. Trop. Med. Public Health 2017, 10, 1500. [Google Scholar]
- Galeotti, N.; Maidecchi, A.; Mattoli, L.; Burico, M.; Ghelardini, C. St. John’s Wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia 2014, 92, 23–33. [Google Scholar] [CrossRef]
- Cárdenas, J.; Reyes-Pérez, V.; Hernández-Navarro, M.D.; Dorantes-Barrón, A.M.; Almazán, S.; Estrada-Reyes, R. Anxiolytic-and antidepressant-like effects of an aqueous extract of Tanacetum parthenium L. Schultz-Bip (Asteraceae) in mice. J. Ethnopharmacol. 2017, 200, 22–30. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Ghazavi, H.; Vahedi, M.M.; Tarrah, K.; Yavari, Z.; Hosseini, A.; Aghaee, A.; Rakhshandeh, H. Tanacetum parthenium enhances pentobarbital-induced sleeping behaviors. Avicenna J. Phytomedicine 2020, 10, 70–77. [Google Scholar]
- Liu, X.; Wang, X. Recent advances on the structural modification of parthenolide and its derivatives as anticancer agents. Chin. J. Nat. Med. 2022, 20, 814–829. [Google Scholar] [CrossRef]
- Parada-Turska, J.; Paduch, R.; Majdan, M.; Kandefer-Szerszeñ, M.; Rzeski, W. Antiproliferative activity of parthenolide against three human cancer cell lines and human umbilical vein endothelial cells. Pharmacol. Rep. 2007, 59, 233. [Google Scholar]
- Śmiech, M.; Leszczyński, P.; Kono, H.; Wardell, C.; Taniguchi, H. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; et al. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Liu, Y.C.; Kim, S.L.; Park, Y.R.; Lee, S.-T.; Kim, S.W. Parthenolide promotes apoptotic cell death and inhibits the migration and invasion of SW620 cells. Intest. Res. 2017, 15, 174. [Google Scholar] [CrossRef]
- Fan, Q.; Qiu, M.T.; Zhu, Z.; Zhou, J.H.; Chen, L.; Zhou, Y.; Gu, W.; Wang, L.H.; Li, Z.N.; Xu, Y.; et al. Twist induces epithelial-mesenchymal transition in cervical carcinogenesis by regulating the TGF-β/Smad3 signaling pathway. Oncol. Rep. 2015, 34, 1787–1794. [Google Scholar] [CrossRef]
- Sufian, H.B.; Santos, J.M.; Khan, Z.S.; Halim, S.A.; Khan, A.; Munir, M.T.; Zahid, M.K.; Al-Harrasi, A.; Gollahon, L.S.; Hussain, F.; et al. Parthenolide reverses the epithelial to mesenchymal transition process in breast cancer by targeting TGFbeta1: In vitro and in silico studies. Life Sci. 2022, 301, 120610. [Google Scholar] [CrossRef]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.-Y.E.; Lim, S.-T.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A.; et al. Parthenolide Covalently Targets and Inhibits Focal Adhesion Kinase in Breast Cancer Cells. Cell Chem. Biol. 2019, 26, 1027–1035.e22. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Dawood, M.; Ooko, E.; Efferth, T. Collateral sensitivity of parthenolide via NF-κB and HIF-α inhibition and epigenetic changes in drug-resistant cancer cell lines. Front. Pharmacol. 2019, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, Y.; Liu, D.; Cao, Y.; Li, Y. Parthenolide increases the sensitivity of gastric cancer cells to chemotherapy. J. Tradit. Chin. Med. 2020, 40, 908–916. [Google Scholar] [PubMed]
- Ramachandram, D.S.; Chitra, V.; Rini, R. Correlation of Anti-oxidant, Anti-angiogenic, and Cytotoxic activity of Tanacetum parthenium compared with Amlodipine using Chorioallantoic membrane Assay. Res. J. Pharm. Technol. 2020, 13, 1665–1671. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, Y.; Ma, J.; Ou, W.; Wang, H.; Wu, J.; Tang, J.; Zhang, B.; Liao, X.; Yang, D.; et al. Parthenolide inhibits angiogenesis in esophageal squamous cell carcinoma through suppression of VEGF. Onco Targets Ther. 2020, 13, 7447–7458. [Google Scholar] [CrossRef] [PubMed]
- Soni, U.K.; Jenny, L.; Hegde, R.S. IGF-1R targeting in cancer—does sub-cellular localization matter? J. Exp. Clin. Cancer Res. 2023, 42, 273. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, C.; Chen, F.; Feng, Z.; Jia, L.; Liu, P.; Yang, Z.X.; Hou, F.; Deng, Z.Y. M2-like tumour-associated macrophage-secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021, 24, 604. [Google Scholar] [CrossRef]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin Alleviates Oxidative Stress through Akt/Sirt1/FoxO3α Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, W.; Wen, G.; Yu, B.; Xu, F.; Gan, X.; Tang, J.; Zeng, Q.; Zhu, L.; Chen, C.; et al. Parthenolide inhibits human lung cancer cell growth by modulating the IGF-1R/PI3K/Akt signaling pathway. Oncol. Rep. 2020, 44, 1184–1193. [Google Scholar] [CrossRef]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Miglietta, A.; Bozzo, F.; Gabriel, L.; Bocca, C. Microtubule-interfering activity of parthenolide. Chem.-Biol. Interact. 2004, 149, 165–173. [Google Scholar] [CrossRef]
- Arıkan-Ayyıldız, Z.; Karaman, M.; Özbal, S.; Bağrıyanık, A.; Yilmaz, O.; Karaman, Ö.; Uzuner, N. Efficacy of parthenolide on lung histopathology in a murine model of asthma. Allergol. Et Immunopathol. 2017, 45, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, Y.; Li, F.; Fan, Y.; Li, Y.; Zhang, C.; Hou, H.; Shi, M.; Zhao, Z.; Chen, Z. ACT001 suppressing M1 polarization against inflammation via NF-κB and STAT1 signaling pathways alleviates acute lung injury in mice. Int. Immunopharmacol. 2022, 110, 108944. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, X.; Shen, N.; Di, Y.; Zhang, H.; Du, C.; Fang, T.; Guo, J. Administration of Parthenolide extract (ACT001) improved autophagy and attenuate inflammatory in Rats with ARDS. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100195. [Google Scholar] [CrossRef]
- Martin, K.; Sur, R.; Liebel, F.; Tierney, N.; Lyte, P.; Garay, M.; Oddos, T.; Anthonavage, M.; Shapiro, S.; Southall, M. Parthenolide-depleted Feverfew (Tanacetum parthenium) protects skin from UV irradiation and external aggression. Arch. Dermatol. Res. 2008, 300, 69–80. [Google Scholar] [CrossRef]
- Ohguchi, K.; Ito, M.; Yokoyama, K.; Iinuma, M.; Itoh, T.; Nozawa, Y.; Akao, Y. Effects of sesquiterpene lactones on melanogenesis in mouse B16 melanoma cells. Biol. Pharm. Bull. 2009, 32, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Q.; Chen, Y.; Peng, X.; Wang, Y.; Li, S.; Wu, J.; Luo, C.; Gong, W.; Yin, B.; et al. Parthenolide, an NF-κB inhibitor, alleviates peritoneal fibrosis by suppressing the TGF-β/Smad pathway. Int. Immunopharmacol. 2020, 78, 106064. [Google Scholar] [CrossRef]
- Jaffar, J.; Glaspole, I.; Symons, K.; Westall, G. Inhibition of NF-κB by ACT001 reduces fibroblast activity in idiopathic pulmonary fibrosis. Biomed. Pharmacother. 2021, 138, 111471. [Google Scholar] [CrossRef]
- Li, X.; Xiao, T.; Yang, J.; Qin, Y.; Gao, J.; Liu, H.; Zhou, H. Parthenolide attenuated bleomycin-induced pulmonary fibrosis via the NF-κB/Snail signaling pathway. Respir. Res. 2018, 19, 111. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Xiang, J.Y.; Liu, L.; Huang, X.L.; Gan, H.T. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice. Int. Immunopharmacol. 2012, 12, 169–174. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Xiao, Y.; Mao, X. Anti-inflammatory and antiosteoclastogenic activities of parthenolide on human periodontal ligament cells in vitro. Evid.-Based Complement. Altern. Med. 2014, 2014, 546097. [Google Scholar] [CrossRef]
- Kabil, S.L.; Rashed, H.E.; Mohamed, N.M.; Elwany, N.E. Parthenolide repressed endometriosis induced surgically in rats: Role of PTEN/PI3Kinase/AKT/GSK-3β/β-catenin signaling in inhibition of epithelial mesenchymal transition. Life Sci. 2023, 331, 122037. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kang, B.; Suh, H.J.; Choi, H.S. Parthenolide, a feverfew-derived phytochemical, ameliorates obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 pathway. Pharmacol. Res. 2019, 145, 104259. [Google Scholar] [CrossRef] [PubMed]
- Luize, P.S.; Tiuman, T.S.; Morello, L.G.; Maza, P.K.; Ueda-Nakamura, T.; Dias Filho, B.P.; Aparício Garcia Cortez, D.; Palazzo de Mello, J.; Vataru Nakamura, C. Effects of medicinal plant extracts on growth of Leishmania (L.) amazonensis and Trypanosoma cruzi. Rev. Bras. De Cienc. Farm./Braz. J. Pharm. Sci. 2005, 41, 85–94. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Ueda-Nakamura, T.; Garcia Cortez, D.A.; Dias Filho, B.P.; Morgado-Díaz, J.A.; De Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Izumi, E.; Morello, L.G.; Ueda-Nakamura, T.; Yamada-Ogatta, S.F.; Filho, B.P.D.; Cortez, D.A.G.; Ferreira, I.C.; Morgado-Díaz, J.A.; Nakamura, C.V. Trypanosoma cruzi: Antiprotozoal activity of parthenolide obtained from Tanacetum parthenium (L.) Schultz Bip. (Asteraceae, Compositae) against epimastigote and amastigote forms. Exp. Parasitol. 2008, 118, 324–330. [Google Scholar] [CrossRef]
- da Silva, B.P.; Cortez, D.A.; Violin, T.Y.; Filho, B.P.D.; Nakamura, C.V.; Ueda-Nakamura, T.; Ferreira, I.C. Antileishmanial activity of a guaianolide from Tanacetum parthenium (L.) Schultz Bip. Parasitol. Int. 2010, 59, 643–646. [Google Scholar] [CrossRef]
- Drugs.com. Feverfew Information from Drugs.com. 2024. Available online: https://www.drugs.com/npp/feverfew.html (accessed on 1 March 2024).
- Yao, M.; Ritchie, H.E.; Brown-Woodman, P.D. A Reproductive screening test of feverfew: Is a full reproductive study warranted? Reprod. Toxicol. 2006, 22, 688–693. [Google Scholar] [CrossRef]
- Nice, F.; Coghlan, R.J.; Birmingham, B.T. Herbals and breastfeeding. Birth Issues 2000, 9, 77–84. [Google Scholar]
- Ataollahi, M.; Akrami, E.; Kalani, M.; Zarei, M.; Chijan, M.R.; Sedigh-Rahimabadi, M.; Alipanah, H. Evaluation of anticoagulant and inflammatory effects of Tanacetum parthenium (L.) in a randomized controlled clinical trial. J. Herb. Med. 2022, 36, 100613. [Google Scholar] [CrossRef]
- Leite, P.M.; Martins, M.A.P.; Castilho, R.O. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed. Pharmacother. 2016, 83, 14–21. [Google Scholar] [CrossRef]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health-Syst. Pharm. 2000, 57, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y. The Chemopreventive Property of Parthenolide, A Sesquiterpene Lactone. Ph.D. Thesis, National University of Singapore, Singapore, 2006. [Google Scholar]
- Sharma, V.K.; Verma, P.; Maharaja, K. Parthenium dermatitis. Photochem. Photobiol. Sci. 2012, 12, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Wasan, A. Allergic contact dermatitis to parthenolide. J. Allergy Clin. Immunol. Pract. 2016, 4, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Angus-Leppan, H.; Benson, K. Migraine prevention: Initial treatment options. BMJ 2023, 382, e069494. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Sharma, V.; Gupta, M.; Chauhan, P.S.; Mehta, K.S.; Garg, S. Parthenium dermatitis: Is parthenolide an effective choice for patch testing? Contact Dermat. 2014, 70, 340–343. [Google Scholar] [CrossRef]
- Paulsen, E.; El-Houri, R.B.; Andersen, K.E.; Christensen, L.P. Parthenolide in Danish biodynamic and organic milk: A new source of exposure to an allergenic sesquiterpene lactone. Contact Dermat. 2018, 79, 208–212. [Google Scholar] [CrossRef]
- Paulsen, E.; Christensen, L.P.; Andersen, K.E. Compositae dermatitis from airborne parthenolide. Br. J. Dermatol. 2007, 156, 510–515. [Google Scholar] [CrossRef]
- Rodriguez, K.J.; Wong, H.-K.; Oddos, T.; Southall, M.; Frei, B.; Kaur, S. A purified feverfew extract protects from oxidative damage by inducing DNA repair in skin cells via a PI3-kinase-dependent Nrf2/ARE pathway. J. Dermatol. Sci. 2013, 72, 304–310. [Google Scholar] [CrossRef]
- Ernst, E.; Pittler, M. The efficacy and safety of feverfew (Tanacetum parthenium L.): An update of a systematic review. Public Health Nutr. 2000, 3, 509–514. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashkooe, A.; Jalali, A.; Zarshenas, M.M.; Hamedi, A. Exploring the Phytochemistry, Signaling Pathways, and Mechanisms of Action of Tanacetum parthenium (L.) Sch.Bip.: A Comprehensive Literature Review. Biomedicines 2024, 12, 2297. https://doi.org/10.3390/biomedicines12102297
Kashkooe A, Jalali A, Zarshenas MM, Hamedi A. Exploring the Phytochemistry, Signaling Pathways, and Mechanisms of Action of Tanacetum parthenium (L.) Sch.Bip.: A Comprehensive Literature Review. Biomedicines. 2024; 12(10):2297. https://doi.org/10.3390/biomedicines12102297
Chicago/Turabian StyleKashkooe, Ali, Atefeh Jalali, Mohammad M. Zarshenas, and Azadeh Hamedi. 2024. "Exploring the Phytochemistry, Signaling Pathways, and Mechanisms of Action of Tanacetum parthenium (L.) Sch.Bip.: A Comprehensive Literature Review" Biomedicines 12, no. 10: 2297. https://doi.org/10.3390/biomedicines12102297






