The Mechanism and Latest Research Progress of Blood–Brain Barrier Breakthrough
Abstract
:1. Introduction
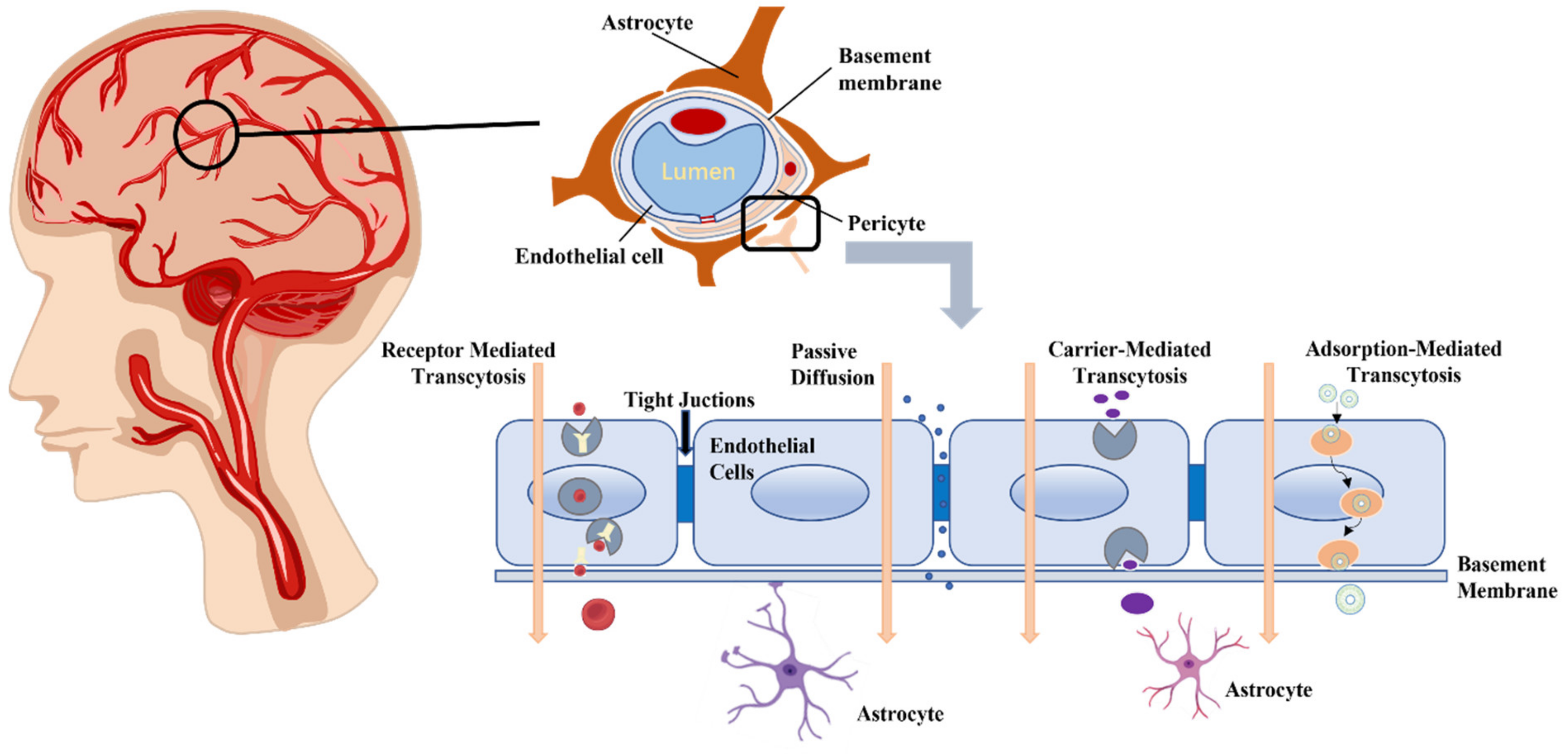
2. Physiological Basis of the BBB
2.1. The Structure of BBB
2.1.1. Endothelial Cells
2.1.2. Basement Membrane
2.1.3. Pericytes
2.1.4. Astrocytes
2.2. The Discovery of BBB
2.3. Function of the BBB
3. Methods of BBB Breach
3.1. Passive Diffusion
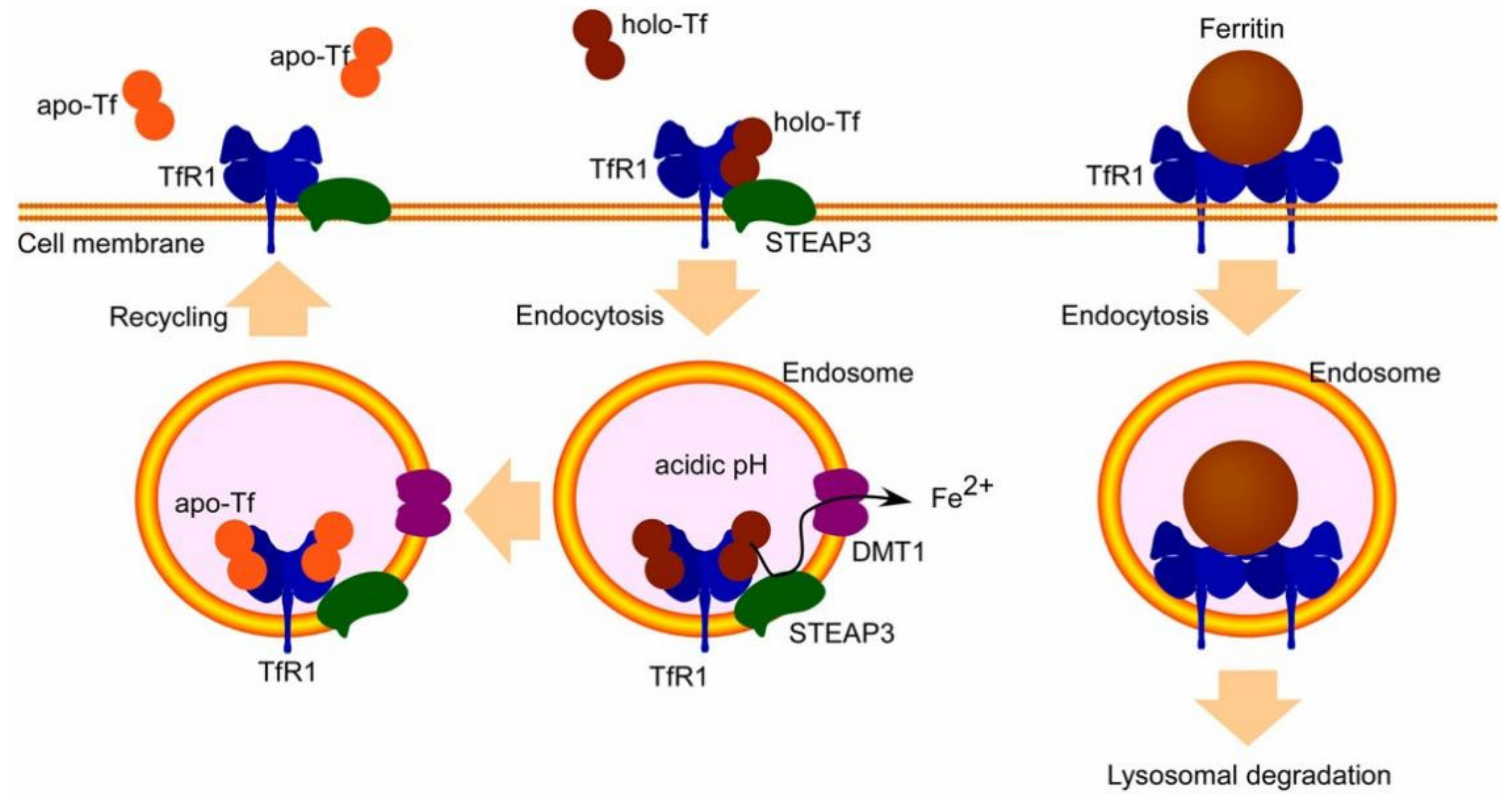
3.2. Receptor Mediated BBB Crossing
3.2.1. Transferrin Receptor
3.2.2. Insulin Receptor
3.2.3. LDL Receptor
3.2.4. Lactoferrin Receptor
3.3. Carrier-Mediated Transport
3.3.1. Au Nanoparticles (Au NPs)
3.3.2. Silver Nanoparticles (Ag NPs)
3.3.3. Zinc Oxide Nanoparticles (ZnO NPs)
3.3.4. Magnetic Nanoparticles
3.3.5. Carbon Nanomaterials (CNs)
3.3.6. Shuttle Peptide
3.4. Adsorption-Mediated Transport
3.4.1. Liposomes
3.4.2. Polylactic Acid-Glycolic Acid
3.4.3. Quantum Dots
3.4.4. Mesoporous Silica Nanoparticles
3.4.5. Exosomes
3.4.6. Other NPs
3.5. Physical Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uchikawa, H.; Uekawa, K.; Hasegawa, Y. Perivascular macrophages in cerebrovascular diseases. Exp. Neurol. 2024, 374, 114680. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Blood–Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Transcellular routes of blood–brain barrier disruption. Exp. Biol. Med. 2022, 247, 788–796. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999, 5, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef]
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The blood-brain barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Schäfer, J.; Lyck, R.; Makrides, V.; Brunner, S.; Schaeren-Wiemers, N.; Deutsch, U.; Engelhardt, B. Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011, 122, 601–614. [Google Scholar] [CrossRef]
- Sohet, F.; Lin, C.; Munji, R.N.; Lee, S.Y.; Ruderisch, N.; Soung, A.; Arnold, T.D.; Derugin, N.; Vexler, Z.S.; Yen, F.T. LSR/angulin-1 is a tricellular tight junction protein involved in blood–brain barrier formation. J. Cell Biol. 2015, 208, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, H.; Yilmaz, O.; Karaman, M.; Firinci, F.; Turkeli, A.; Kanik, E.T.; Inan, S. Vascular endothelial growth factor antagonism restores epithelial barrier dysfunction via affecting zonula occludens proteins. Exp. Ther. Med. 2015, 10, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.J.; Asselin, M.-C.; Hinz, R.; Parkes, L.M.; Allan, S.; Schiessl, I.; Boutin, H.; Dickie, B.R. In vivo methods for imaging blood–brain barrier function and dysfunction. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1051–1083. [Google Scholar] [CrossRef] [PubMed]
- LeBowitz, J.H. A breach in the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 14485–14486. [Google Scholar] [CrossRef]
- Daneman, R. The blood–brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Quanguo, H.; Jun, L.; Jing, L.; Xiaopeng, L.; Wen, L.; Zhi, L.; Ziyu, D.; Du, T. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke—ScienceDirect. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef]
- Osada, T.; Gu, Y.-H.; Kanazawa, M.; Tsubota, Y.; Hawkins, B.T.; Spatz, M.; Milner, R.; Del Zoppo, G.J. Interendothelial claudin-5 expression depends on cerebral endothelial cell–matrix adhesion by β1-integrins. J. Cereb. Blood Flow Metab. 2011, 31, 1972–1985. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and specificity of astrocyte–neuron communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.-S.; Martel, S. Remote control of the permeability of the blood–brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef]
- Booth, B.B.; Dunstone, N.J.; Halloran, P.R.; Andrews, T.; Bellouin, N. Aerosols implicated as a prime driver of twentieth-century North Atlantic climate variability. Nature 2012, 484, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the blood–brain barrier: Successes and challenges in developing nanoparticle-mediated drug delivery systems for the treatment of brain tumours. Int. J. Nanomed. 2020, 15, 2999–3022. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Helgeson, M.E.; Mitragotri, S. Delivery of nanoparticles and macromolecules across the blood–brain barrier. Adv. Ther. 2020, 3, 1900073. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X. Drug delivery to the brain across the blood–brain barrier using nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef] [PubMed]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood–brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, 1801362. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Lan, M.; Yan, L.; Chen, X. Getting drugs to the brain: Advances and prospects of organic nanoparticle delivery systems for assisting drugs to cross the blood–brain barrier. J. Mater. Chem. B 2022, 10, 9314–9333. [Google Scholar] [CrossRef]
- Vlieghe, P.; Khrestchatisky, M. Medicinal Chemistry Based Approaches and Nanotechnology-Based Systems to Improve CNS Drug Targeting and Delivery. Med. Res. Rev. 2013, 33, 457–516. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Kim, J.; Bai, Y.; Pabla, N. Role of Passive diffusion, Transporters, and membrane trafficking-mediated processes in cellular drug transport. Clin. Pharmacol. Ther. 2017, 101, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Chu, C.; Walczak, P.; Searson, P.C. Modeling hyperosmotic blood–brain barrier opening within human tissue-engineered in vitro brain microvessels. J. Cereb. Blood Flow Metab. 2020, 40, 1517–1532. [Google Scholar] [CrossRef]
- Choi, C.; Kim, H.M.; Shon, J.; Park, J.; Kim, H.-T.; Oh, S.-H.; Kim, N.K.; Kim, O.J. Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability. Biochem. Biophys. Res. Commun. 2018, 497, 769–775. [Google Scholar] [CrossRef]
- Shen, X.; Cui, Z.; Wei, Y.; Huo, Y.; Yu, D.; Zhang, X.; Mao, S. Exploring the potential to enhance drug distribution in the brain subregion via intranasal delivery of nanoemulsion in combination with borneol as a guider. Asian J. Pharm. Sci. 2023, 18, 100778. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.-Y.; Liu, Y.; Zhang, L.; Guo, R.-B.; Li, X.-T.; Ma, L.-Y.; Kong, L. Borneol-modified docetaxel plus tetrandrine micelles for treatment of drug-resistant brain glioma. Drug Dev. Ind. Pharm. 2024, 50, 135–149. [Google Scholar] [CrossRef]
- Lin, Y. A model of cell motility leading to biphasic dependence of transport speed on adhesive strength. J. Mech. Phys. Solids 2010, 58, 502–514. [Google Scholar] [CrossRef]
- Fang, F.; Zou, D.; Wang, W.; Yin, Y.; Yin, T.; Hao, S.; Wang, B.; Wang, G.; Wang, Y. Non-invasive approaches for drug delivery to the brain based on the receptor mediated transport. Mater. Sci. Eng. C 2017, 76, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Lázaro, M.Á.; Brugada-Vila, P.; Porcar, I.; Morera, I.; Guerra-Rebollo, M.; Garrido, C.; Rubio, N.; Blanco, J.; Cascante, A. Application of an assay Cascade methodology for a deep preclinical characterization of polymeric nanoparticles as a treatment for gliomas. Drug Deliv. 2018, 25, 472–483. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. PEGylation: An approach for drug delivery. A review. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 403–447. [Google Scholar] [CrossRef]
- Jain, A.; Chasoo, G.; Singh, S.K.; Saxena, A.K.; Jain, S.K. Transferrin-appended PEGylated nanoparticles for temozolomide delivery to brain: In vitro characterisation. J. Microencapsul. 2011, 28, 21–28. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, L.; Yin, Q.; Feng, L.; Li, Y. Transferrin-modified c [RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol. Pharm. 2012, 9, 1590–1598. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sun, T.; Rama, E.; Motta, A.; Zhang, Y.; Power, C.; Moeckel, D.; Fletcher, S.M.; Moosavifar, M.; Barmin, R. Transferrin Receptor-Targeted Nonspherical Microbubbles for Blood–Brain Barrier Sonopermeation. Adv. Mater. 2023, 35, 2308150. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Visser, C.C.; de Boer, A.G. Targeted delivery across the blood–brain barrier. Expert Opin. Drug Deliv. 2005, 2, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Eisenberg, J.; Yang, J. Human blood—Brain barrier insulin receptor. J. Neurochem. 1985, 44, 1771–1778. [Google Scholar] [CrossRef]
- Bickel, U.; Yoshikawa, T.; Pardridge, W.M. Delivery of peptides and proteins through the blood–brain barrier. Adv. Drug Deliv. Rev. 2001, 46, 247–279. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef]
- Méresse, S.; Delbart, C.; Fruchart, J.C.; Cecchelli, R. Low-density lipoprotein receptor on endothelium of brain capillaries. J. Neurochem. 1989, 53, 340–345. [Google Scholar] [CrossRef]
- Malavolti, M.; Fromm, H.; Ceryak, S.; Shehan, K.L. Cerebral low-density lipoprotein (LDL) uptake is stimulated by acute bile drainage. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1991, 1081, 106–108. [Google Scholar] [CrossRef]
- Traber, M.; Kayden, H. Vitamin E is delivered to cells via the high affinity receptor for low-density lipoprotein. Am. J. Clin. Nutr. 1984, 40, 747–751. [Google Scholar] [CrossRef]
- Candela, P.; Gosselet, F.; Miller, F.; Buee-Scherrer, V.; Torpier, G.; Cecchelli, R.; Fenart, L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: Transcytosis through brain capillary endothelial cells in vitro. Endothelium 2008, 15, 254–264. [Google Scholar] [CrossRef]
- Dehouck, B.; Fenart, L.; Dehouck, M.-P.; Pierce, A.; Torpier, G.; Cecchelli, R. A new function for the LDL receptor: Transcytosis of LDL across the blood–brain barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lönnerdal, B. Characterization of mammalian receptors for lactoferrin. Biochem. Cell Biol. 2002, 80, 75–80. [Google Scholar] [CrossRef]
- Huang, R.-q.; Ke, W.-l.; Qu, Y.-h.; Zhu, J.-h.; Pei, Y.-y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Tamai, I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Kuzmanov, I.; Hucke, S.; Gross, C.C.; Posevitz, V.; Dreykluft, A.; Schulte-Mecklenbeck, A.; Janoschka, C.; Lindner, M.; Herold, M. B7-H1 shapes T-cell–mediated brain endothelial cell dysfunction and regional encephalitogenicity in spontaneous CNS autoimmunity. Proc. Natl. Acad. Sci. USA 2016, 113, E6182–E6191. [Google Scholar] [CrossRef]
- Burrack, K.S.; Huggins, M.A.; Taras, E.; Dougherty, P.; Henzler, C.M.; Yang, R.; Alter, S.; Jeng, E.K.; Wong, H.C.; Felices, M. Interleukin-15 complex treatment protects mice from cerebral malaria by inducing interleukin-10-producing natural killer cells. Immunity 2018, 48, 760–772.e764. [Google Scholar] [CrossRef]
- Jain, S.; Mishra, V.; Singh, P.; Dubey, P.; Saraf, D.; Vyas, S. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 2003, 261, 43–55. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Weintraub, K. Biomedicine: The new gold standard. Nature 2013, 495, S14–S16. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Ma, T.; Liu, Q.; Wu, S.; Hua, K.; Zhang, C.; Chen, M.; Cui, Y. Enhanced Stability of Gold Magnetic Nanoparticles with Poly(4-styrenesulfonic acid-co-maleic acid): Tailored Optical Properties for Protein Detection. Nanoscale Res. Lett. 2017, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010, 62, 346–361. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Li, J.; Wu, D.; Liu, Z.; Guan, C.; Guan, Y.; Lu, X. Effect of gold-conjugated resveratrol nanoparticles on glioma cells and its underlying mechanism. Bio-Med. Mater. Eng. 2024, 35, 279–292. [Google Scholar] [CrossRef]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. S4), 547–551. [Google Scholar]
- Salazar-García, S.; Delgado-Buenrostro, N.L.; Rodríguez-Escamilla, J.C.; Davalos-Rivas, G.; Chirino, Y.I.; Castillo Martín del Campo, C.G.; Martínez-Castañón, G.A.; Vargas-Morales, J.M.; Gonzalez, C. Zinc protects the rat brain from damage induced by 24 h exposure to silver nanoparticles. J. Nanoparticle Res. 2019, 21, 172. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Strużyński, W.; Strużyńska, L. Prolonged exposure to silver nanoparticles results in oxidative stress in cerebral myelin. Neurotox. Res. 2019, 35, 495–504. [Google Scholar] [CrossRef]
- Lin, H.-C.; Ho, M.-Y.; Tsen, C.-M.; Huang, C.-C.; Wu, C.-C.; Huang, Y.-J.; Hsiao, I.-L.; Chuang, C.-Y. From the cover: Comparative proteomics reveals silver nanoparticles alter fatty acid metabolism and amyloid beta clearance for neuronal apoptosis in a triple cell coculture model of the blood–brain barrier. Toxicol. Sci. 2017, 158, 151–163. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Song, W.; Wu, C.; Yin, H.; Liu, X.; Sa, P.; Hu, J. Preparation of PbS nanoparticles by phase-transfer method and application to Pb2+-selective electrode based on PVC membrane. Anal. Lett. 2008, 41, 2844–2859. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Zhang, T.; Ren, G.; Yang, Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012, 19, 14. [Google Scholar] [CrossRef]
- Liu, X.; Fu, C.; Wang, M.; Wang, J.; Zou, H.; Le, Y.; Chen, J. High-gravity technology intensified Knoevenagel condensation-Michael addition polymerization of poly (ethylene glycol)-poly (n-butyl cyanoacrylate) for blood-brain barrier delivery. Chin. J. Chem. Eng. 2022, 46, 94–103. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef]
- Ying, J.Y.; Zheng, Y.; Li, Y. Forming Glutathione-Capped and Metal-Doped Zinc Selenide/Zinc Sulfide Core-Shell Quantum Dots in Aqueous Solution. WO2009099397A1, 1 August 2009. [Google Scholar]
- Park, J.; Porter, M.D.; Granger, M.C. Colloidally Assembled Zinc Ferrite Magnetic Beads: Superparamagnetic Labels with High Magnetic Moments for MR Sensors. ACS Appl. Mater. Interfaces 2017, 9, 19569–19577. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Gubin, S.; Taranov, I.; Khomutov, G.; Gulyaev, Y.V. Magnetic nanoparticles in medicine: Progress, problems, and advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
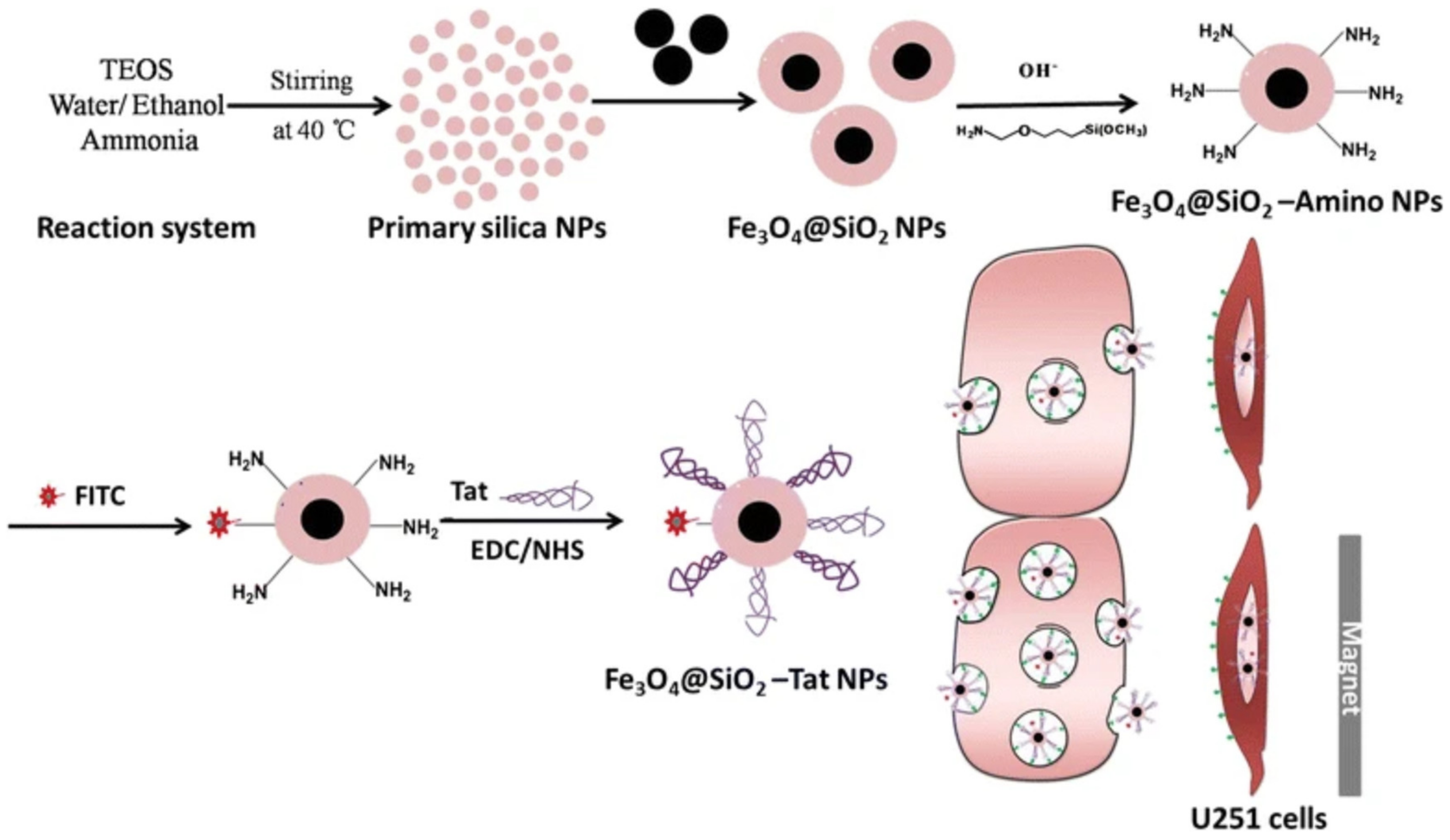
- Zhao, X.; Shang, T.; Zhang, X.; Ye, T.; Wang, D.; Rei, L. Passage of Magnetic tat-conjugated Fe 3 O 4@ SiO 2 nanoparticles across in vitro blood-brain barrier. Nanoscale Res. Lett. 2016, 11, 451. [Google Scholar] [CrossRef]
- Nosrati, H.; Tarantash, M.; Bochani, S.; Charmi, J.; Bagheri, Z.; Fridoni, M.; Abdollahifar, M.-A.; Davaran, S.; Danafar, H.; Kheiri Manjili, H. Glutathione (GSH) peptide conjugated magnetic nanoparticles as blood–brain barrier shuttle for MRI-monitored brain delivery of paclitaxel. ACS Biomater. Sci. Eng. 2019, 5, 1677–1685. [Google Scholar] [CrossRef]
- Ye, E.; Park, E.; Kim, E.; Lee, J.E.; Yang, S.H.; Park, S.-M. Transcranial application of magnetic pulses for improving brain drug delivery efficiency via intranasal injection of magnetic nanoparticles. Biomed. Eng. Lett. 2023, 13, 417–427. [Google Scholar] [CrossRef]
- Beola, L.; Iturrioz-Rodríguez, N.; Pucci, C.; Bertorelli, R.; Ciofani, G. Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme. ACS Nano 2023, 17, 18441–18455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sigdel, G.; Mintz, K.J.; Seven, E.S.; Zhou, Y.; Wang, C.; Leblanc, R.M. Carbon dots: A future Blood–Brain Barrier penetrating nanomedicine and drug nanocarrier. Int. J. Nanomed. 2021, 16, 5003–5016. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mohammadinejad, R.; Kailasa, S.K.; Ahmadi, Z.; Afshar, E.G.; Pardakhty, A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv. Colloid Interface Sci. 2020, 278, 102123. [Google Scholar] [CrossRef]
- Henna, T.; Raphey, V.; Sankar, R.; Shirin, V.A.; Gangadharappa, H.; Pramod, K. Carbon nanostructures: The drug and the delivery system for brain disorders. Int. J. Pharm. 2020, 587, 119701. [Google Scholar] [CrossRef]
- Chiang, W.H.; Li, Y.S.; Liao, J.L. Modified Graphene, Method for Producing a Modified Graphene and Applications Thereof. US9708190B2, 18 July 2017. [Google Scholar]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood–brain barrier. Cell. Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, N.; He, L.; Shi, C.; Zhang, D.; Liu, Y.; Luo, L.; Chen, T. Designing dual-functionalized carbon nanotubes with high blood–brain-barrier permeability for precise orthotopic glioma therapy. Dalton Trans. 2019, 48, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, U. Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Kristensen, M.; Birch, D.; Mørck Nielsen, H. Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef]
- Kristensen, M.; Brodin, B. Routes for drug translocation across the blood-brain barrier: Exploiting peptides as delivery vectors. J. Pharm. Sci. 2017, 106, 2326–2334. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Knipp, G.T.; Ekins, S.; Swaan, P.W. Structural biology and function of solute transporters: Implications for identifying and designing substrates. Drug Metab. Rev. 2002, 34, 709–750. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J. A brain-to-blood carrier-mediated transport system for small, N-tyrosinated peptides. Pharmacol. Biochem. Behav. 1984, 21, 943–946. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar] [PubMed]
- Banks, W.A. Peptides and the blood–brain barrier. Peptides 2015, 72, 16–19. [Google Scholar] [CrossRef]
- Shadmani, N.; Makvandi, P.; Parsa, M.; Azadi, A.; Nedaei, K.; Mozafari, N.; Poursina, N.; Mattoli, V.; Tay, F.R.; Maleki, A.; et al. Enhancing Methotrexate Delivery in the Brain by Mesoporous Silica Nanoparticles Functionalized with Cell-Penetrating Peptide using in Vivo and ex Vivo Monitoring. Mol. Pharm. 2023, 20, 1531–1548. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.F. Physiology of Blood–Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Ross, A.M.; Mc Nulty, D.; O’Dwyer, C.; Grabrucker, A.M.; Cronin, P.; Mulvihill, J.J.E. Standardization of research methods employed in assessing the interaction between metallic-based nanoparticles and the blood-brain barrier: Present and future perspectives. J. Control. Release 2019, 296, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chen, H.; Zhao, H.; Yang, W.; Song, Y.; Li, X.; Wang, Y.; Du, D.; Liao, H.; Pan, W.; et al. New insight into brain disease therapy: Nanomedicines-crossing blood–brain barrier and extracellular space for drug delivery. Expert Opin. Drug Deliv. 2022, 19, 1618–1635. [Google Scholar] [CrossRef] [PubMed]
- Scherrmann, J.-M. Drug delivery to brain via the blood–brain barrier. Vasc. Pharmacol. 2002, 38, 349–354. [Google Scholar] [CrossRef]
- Vorbrodt, A. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J. Neurocytol. 1989, 18, 359–368. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier delivery of protein and non-viral gene therapeutics with molecular Trojan horses. J. Control. Release 2007, 122, 345–348. [Google Scholar] [CrossRef]
- Han, E.L.; Padilla, M.S.; Palanki, R.; Kim, D.; Mrksich, K.; Li, J.J.; Tang, S.; Yoon, I.-C.; Mitchell, M.J. Predictive High-Throughput Platform for Dual Screening of mRNA Lipid Nanoparticle Blood–Brain Barrier Transfection and Crossing. Nano Lett. 2024, 24, 1477–1486. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Liu, Y.; Yu, Y.-W.; Gong, B.-F.; Ruan, J.; Fan, H.-Y. Application of targeted liposomes-based salvianolic acid A for the treatment of ischemic stroke. Neurotherapeutics 2024, 21, e00342. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Tabatabaei Mirakabad, F.S.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug Deliv. Sci. Technol. 2020, 55, 101405. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, C.; Ning, J.; Ding, X.; Wang, H.; Zhou, Y. A polysaccharide-based hydrogel and PLGA microspheres for sustained P24 peptide delivery: An in vitro and in vivo study based on osteogenic capability. Chem. Res. Chin. Univ. 2019, 35, 908–915. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in-PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2020, 106, 110294. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef]
- Kaya, S.; Callan, B.; Hawthorne, S. Non-Invasive, Targeted Nanoparticle-Mediated Drug Delivery across a Novel Human BBB Model. Pharmaceutics 2023, 15, 1382. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G. PLGA-PEG-ANG-2 nanoparticles for blood–brain barrier crossing: Proof-of-concept study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef]
- Mansur, H.S. Quantum dots and nanocomposites. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 113–129. [Google Scholar] [CrossRef]
- Ekimov, A.I. Quantum size effect in three-dimensional microscopic semiconductor crystals. JETP Lett. 1981, 34, 345. [Google Scholar] [CrossRef]
- Díaz-González, M.; de la Escosura-Muñiz, A.; Fernandez-Argüelles, M.T.; Alonso, F.J.G.; Costa-Fernandez, J.M. Quantum dot bioconjugates for diagnostic applications. In Surface-Modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 133–176. [Google Scholar]
- Li, H.; Zhang, Y.; Ding, J.; Wu, T.; Cai, S.; Zhang, W.; Cai, R.; Chen, C.; Yang, R. Synthesis of carbon quantum dots for application of alleviating amyloid-β mediated neurotoxicity. Colloids Surf. B Biointerfaces 2022, 212, 112373. [Google Scholar] [CrossRef]
- Ho, Y.-P.; Leong, K.W. Quantum dot-based theranostics. Nanoscale 2010, 2, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Gerasimovich, E.; Baryshnikova, M.; Sokolova, Z.; Samokhvalov, P.; Guhrenz, C.; Gaponik, N.; Karaulov, A.; Nabiev, I. Dependence of quantum dot toxicity in vitro on their size, chemical composition, and surface charge. Nanomaterials 2022, 12, 2734. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, T.; Tang, M. Toxicity of quantum dots on target organs and immune system. J. Appl. Toxicol. 2022, 42, 17–40. [Google Scholar] [CrossRef]
- Li, S.; Skromne, I.; Peng, Z.; Dallman, J.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. “Dark” carbon dots specifically “light-up” calcified zebrafish bones. J. Mater. Chem. B 2016, 4, 7398–7405. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Leblanc, R.M. Method to determine protein concentration in the protein–nanoparticle conjugates aqueous solution using circular dichroism spectroscopy. Anal. Chem. 2015, 87, 6455–6459. [Google Scholar] [CrossRef]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Cilingir, E.K.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M. Metformin derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef]
- Zheng, C.; An, X.; Gong, J. Novel pH sensitive N-doped carbon dots with both long fluorescence lifetime and high quantum yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Cao, Y.; Yang, H.; Li, M.; Wu, F.; Zhang, Y.; Chen, G.; Wang, Q. Multiscale NIR-II Imaging-Guided Brain-Targeted Drug Delivery Using Engineered Cell Membrane Nanoformulation for Alzheimer’s Disease Therapy. ACS Nano 2023, 17, 5033–5046. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In vitro study of receptor-mediated silica nanoparticles delivery across blood–brain barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef] [PubMed]
- Pellen-Mussi, P.; Tricot-Doleux, S.; Neaime, C.; Nerambourg, N.; Cabello-Hurtado, F.; Cordier, S.; Grasset, F.; Jeanne, S. Evaluation of functional SiO2 nanoparticles toxicity by a 3D culture model. J. Nanosci. Nanotechnol. 2018, 18, 3148–3157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cao, F.; Zhang, J.; Tan, Y.; Yao, S. Temozolomide and chloroquine co-loaded mesoporous silica nanoparticles are effective against glioma. Heliyon 2023, 9, e18490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, S.; Jiang, X.; Wu, Q.; Shen, W.; Zou, Z.; Wei, W.; Wu, C.; Gao, Y. Functional paclitaxel-manganese-doped mesoporous silica nanoparticles for orthotopic brain glioma targeted therapy. Mater. Des. 2024, 238, 112715. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Gaurav, I.; Sweta, K.; Chakraborty, P.; Thakur, S. 17—Modified biopolymer-based systems for drug delivery to the brain. In Tailor-Made and Functionalized Biopolymer Systems; Bera, H., Layek, B., Singh, J., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 571–611. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New players in cell–cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Joshi, H.; Kumar, G.; Tuli, H.S.; Mittal, S. Inhibition of cancer cell metastasis by nanotherapeutics: Current achievements and future trends. In Nanotherapeutics in Cancer; Jenny Stanford Publishing: Singapore, 2022; pp. 161–209. [Google Scholar]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Thakur, A.; Guan, X.-J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.-F.; Lau, K.-F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target. Ther. 2023, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, Y.; Zhao, C.; Liu, H.; Shao, Y.; Zhu, C.; An, L.; Chen, X.; Chen, Z. Engineered exosomes with enhanced stability and delivery efficiency for glioblastoma therapy. J. Control. Release 2024, 368, 170–183. [Google Scholar] [CrossRef]
- Ashokan, A.; Sarkar, S.; Kamran, M.Z.; Surnar, B.; Kalathil, A.A.; Spencer, A.; Dhar, S. Simultaneous targeting of peripheral and brain tumors with a therapeutic nanoparticle to disrupt metabolic adaptability at both sites. Proc. Natl. Acad. Sci. USA 2024, 121, e2318119121. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, K.; Mukhambetiyar, K.; Lee, N.K.; Sabaté del Río, J.; Joo, J.; Park, C.G.; Kwon, T.; Park, T.-E. Organ-on-a-Chip Approach for Accelerating Blood–Brain Barrier Nanoshuttle Discovery. ACS Nano 2024, 18, 14388–14402. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, V.A.; Gould, A.; Kim, K.-S.; Habashy, K.J.; Dmello, C.; Vázquez-Cervantes, G.I.; Palacín-Aliana, I.; McManus, G.; Amidei, C.; Gomez, C.; et al. Ultrasound-mediated delivery of doxorubicin to the brain results in immune modulation and improved responses to PD-1 blockade in gliomas. Nat. Commun. 2024, 15, 4698. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.I.; Carpenter, J.S.; Mehta, R.I.; Haut, M.W.; Ranjan, M.; Najib, U.; Lockman, P.; Wang, P.; D’haese, P.-F.; Rezai, A.R. Blood-brain barrier opening with MRI-guided focused ultrasound elicits meningeal venous permeability in humans with early Alzheimer disease. Radiology 2021, 298, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sutharsan, R.; Lee, J.L.; Cruz, E.; Asnicar, B.; Palliyaguru, T.; Wasielewska, J.M.; Gaudin, A.; Song, J.; Leinenga, G. Claudin-5 binder enhances focused ultrasound-mediated opening in an in vitro blood-brain barrier model. Theranostics 2022, 12, 1952. [Google Scholar] [CrossRef]
- Huang, R.; Lin, C.; Jiang, G.; Zhang, M.; Gao, W.; Aihemaiti, K.; Liu, Q.; Shi, J.; Shi, W.; Huang, R. BBB-penetrating magnetoelectric nanoparticles with sustainable Gel formulation for enhanced chemotherapy and reduced postoperative glioma recurrence. Chem. Eng. J. 2024, 496, 154208. [Google Scholar] [CrossRef]
- Shin, D.W.; Fan, J.; Luu, E.; Khalid, W.; Xia, Y.; Khadka, N.; Bikson, M.; Fu, B.M. In Vivo Modulation of the Blood-Brain Barrier Permeability by Transcranial Direct Current Stimulation (tDCS). Ann. Biomed. Eng. 2020, 48, 1256–1270. [Google Scholar] [CrossRef]
- Murphy, K.; Aycock, K.; Marsh, S.; Hay, A.; Chang, C.; Bracha, S.; Gourdie, R.; Davalos, R.; Rossmeisl, J.H.; Dervisis, N. Abstract 4917: High-frequency irreversible electroporation ablation of glioma alters extracellular vesicles and disrupts the blood-brain barrier endothelium. Cancer Res. 2023, 83 (Suppl. S7), 4917. [Google Scholar] [CrossRef]

| Method | Content | Reference |
|---|---|---|
| low-intensity pulsed ultrasound (LIPU) mediated transport | Intravenous microvesicles (MB) and LIPU were utilized to break down the BBB and raise the levels of PD-1-blocking antibody (aPD-1) and liposomal adriamycin. | [157] |
| Intravascular MBs in conjunction with MRI-guided targeted ultrasound activated the BBB. The findings demonstrated that MRI-guided combination therapy produced accurate and short-term BBB, turning on in the targeted hippocampus and internal entorhinal cortex, which fully recovered to normalcy in less than 24 h. | [158] | |
| The combination of FUS-MB with claudin-5 adhesive maximizes the transport of medication to the brain. The permeability of the BBB increases when the two types are combined. | [159] | |
| magnetic-field-enhanced permeation | A magnetoelectric nanoparticle for glioma-targeted therapy that has a pegylated IP peptide surface coating (CBPI), a piezoelectric shell made of BaTiO, and a magnetic core made of CoFeO. Through magnetoelectric effects, the nanoparticle down-regulates the BBB’s TJ protein, working in concert with IP peptides to increase BBB permeability and target gliomas. | [160] |
| electromagnetic field | In a rat model, after 20 min of tDCS treatment at 0.1–1.0 mA, the increase in membrane permeability lasted for about 20 min, rather than immediately returning to normal. | [161] |
| electroporation | The proteomic cargo of tumor-derived extracellular vesicles, TDEVs, is markedly altered by high-frequency irreversible electroperforation (H-FIRE) ablation of gliomas. This increases the permeability of the BBB endothelium and increases the internalization of TDEVs by brain endothelial cells. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Qi, L.; Zhang, Z.; Duan, H.; Wang, Y.; Zhang, K.; Li, J. The Mechanism and Latest Research Progress of Blood–Brain Barrier Breakthrough. Biomedicines 2024, 12, 2302. https://doi.org/10.3390/biomedicines12102302
Wang F, Qi L, Zhang Z, Duan H, Wang Y, Zhang K, Li J. The Mechanism and Latest Research Progress of Blood–Brain Barrier Breakthrough. Biomedicines. 2024; 12(10):2302. https://doi.org/10.3390/biomedicines12102302
Chicago/Turabian StyleWang, Fei, Liujie Qi, Zhongna Zhang, Huimin Duan, Yanchao Wang, Kun Zhang, and Jingan Li. 2024. "The Mechanism and Latest Research Progress of Blood–Brain Barrier Breakthrough" Biomedicines 12, no. 10: 2302. https://doi.org/10.3390/biomedicines12102302






