Zebrafish as a Model for Multiple Sclerosis
Abstract
:1. Introduction
1.1. Introduction to Zebrafish in Medicine
1.2. What Are Zebrafish?
1.3. Advantages of Zebrafish Models
1.4. Current Areas of Study and the Future of Zebrafish in Research
1.5. What Is Multiple Sclerosis?
1.6. Epidemiology and Etiology of Multiple Sclerosis
2. In Vitro Models
2.1. Microglia
2.2. Oligodendrocytes
2.3. Astrocytes
2.4. Neurons
2.5. Brain Slices and Aggregate Systems
| Cell Type | Cell Line | Derivation | Culture Conditions | Efficacy Readout | References |
|---|---|---|---|---|---|
| Microglia | HMO6 | Human. Generated by transfection of embryonic human microglia with a retroviral vector containing cDNA encoding for v-myc oncogene. | Maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% horse serum, 5 mg/mL d-glucose, 25 mg/mL gentamicin, and 2.5 mg/mL amphotericin B (feeding medium). | Immunocytochemistry Fura-2 Ca21-Fluorescence Quantitative Real-Time PCR Analysis Gene Expression of Cytokines and Chemokines ELISA Analysis | [56] |
| Oligodendrocytes | MO3.13 | Human. Fusion of the rhabdomyosarcoma cell line with primary oligodendrocytes [57]. | Maintained in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 1× penicillin–streptomycin solution. | Immunocytochemistry: Primary antibodies used were NCAM2 and CNPase Cell-based ELISA: Biotinylated anti-human IgG and IgM antibodies were used | [58] |
| HOG | Human. Clone derived from oligodendroglioma [59]. | ||||
| Astrocytes | C6 (rat) | Rat. Derived from a rat glial tumour induced by N-nitrosomethylurea. | Maintained in DMEM containing 10% FCS and antibiotics (100 i.u./mL penicillin and 100 μg/mL streptomycin). | mRNA analysis Luciferase activity assay measurements Glutathione assay Immunoblot analysis Immunocytochemical staining Transcription factor DNA-binding activity assay | [60] |
| A172 (human) | Human glioblastoma | Cells were cultivated in α-MEM with glutamine, 10 or 5% fetal calf serum (FCS), 0.1 mg/mL streptomycin, and 100 units penicillin G at 37 °C and 5% CO2. Cells were subcultured with trypsin/EDTA every 3–4 days. | Morphology and immunocytochemistry Flow cytometry Quantitative Real-Time PCR Analysis | [61] | |
| U-87MG (human) | Human glioblastoma | Nicotine was diluted in PBS and added at various concentrations (1, 5, 10, 50, 100, 500, and 1000 μg/well) after a four-hour cell attachment. Cells were incubated for 48 h at 37 °C and then removed from the medium. | MTT assay used for cell viability measurement Evaluation of MMP-2 activity by zymoanalysis | [62] | |
| Neurons | SH-SY5Y | Human. A thrice-cloned sub-line of bone marrow biopsy-derived line SK-N-SH [63]. | Maintained in DMEM supplemented with 2 mM L-glutamine 100 units/mL penicillin/streptomycin 1% nonessential amino acids (11140-035, Invitrogen, Paisley, Scotland, U.K.), and 10% (v/v) heat-inactivated FBS. | MTT assay used for cell viability measurement Determination of Reactive Oxygen Species Immunocytochemistry: Primary antibody used was anti-human Apo D Quantitative Real-Time PCR Analysis | [64] |
| Brain Slices and Aggregate Systems | N/A | Human | Sixteen coronally cut, 10 mm thick full-hemispheric brain slices of 10 patients with chronic MS were selected at autopsy and were formalin-fixed for several weeks. | Magnetic resonance imaging (MRI) Neuropathology and immunohistochemistry Regional analysis of cortical gray matter Global analysis of cortical gray matter | [65] |
3. In Vivo Models
3.1. Experimental Autoimmune Encephalomyelitis (EAE) Model
3.2. Chemically Induced Model
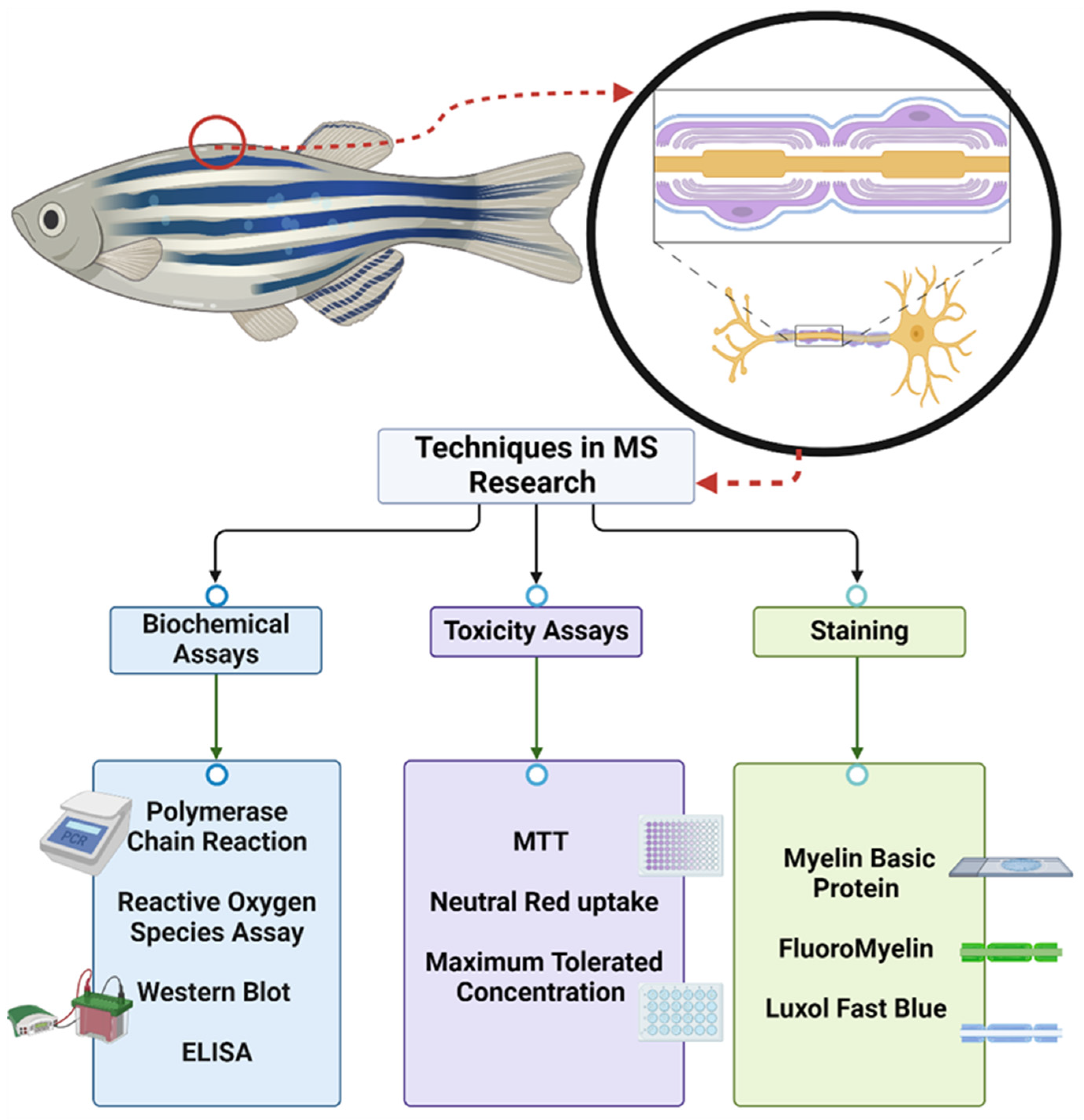
3.3. Zebrafish Models of MS
4. Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arunachalam, M.; Raja, M.; Vijayakumar, C.; Malaiammal, P.; Mayden, R.L. Natural history of zebrafish (Danio rerio) in India. Zebrafish 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Patterson, L.B.; Rao, A.A.; Parichy, D.M. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 2007, 4, 21–40. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models. Princ. Anim. Res. Grad. Undergrad. Stud. 2017, 117–175. [Google Scholar] [CrossRef]
- Why Mouse Matters. Available online: https://www.genome.gov/10001345/importance-of-mouse-genome (accessed on 16 February 2024).
- 000664-B6 Strain Details. Available online: https://www.jax.org/strain/000664 (accessed on 19 February 2024).
- ZIRC Prices. Available online: https://zebrafish.org/documents/fees.php (accessed on 27 February 2024).
- Cheng, R.-K.; Jesuthasan, S.; Penney, T.B. Time for Zebrafish. Front. Integr. Neurosci. 2011, 5, 40. [Google Scholar] [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic Characterization of Larval Zebrafish (Danio rerio) with Partial Knockdown of the cacna1a Gene. Mol. Neurobiol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Svara, F.; Förster, D.; Kubo, F.; Januszewski, M.; dal Maschio, M.; Schubert, P.J.; Kornfeld, J.; Wanner, A.A.; Laurell, E.; Denk, W.; et al. Automated synapse-level reconstruction of neural circuits in the larval zebrafish brain. Nat. Methods 2022, 19, 1357–1366. [Google Scholar] [CrossRef]
- Multiple Sclerosis|National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/multiple-sclerosis (accessed on 26 February 2024).
- Multiple Sclerosis: What You Need to Know. Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/diseases/17248-multiple-sclerosis (accessed on 10 June 2024).
- White Matter Disease: What It Is, Symptoms & Treatment. Available online: https://my.clevelandclinic.org/health/diseases/23018-white-matter-disease (accessed on 10 June 2024).
- Pukoli, D.; Vécsei, L. Smouldering Lesion in MS: Microglia, Lymphocytes and Pathobiochemical Mechanisms. Int. J. Mol. Sci. 2023, 24, 12631. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Myelin: MedlinePlus Medical Encyclopedia. Available online: https://medlineplus.gov/ency/article/002261.htm (accessed on 10 June 2024).
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How patients with multiple sclerosis acquire disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef]
- Lamb, Y.N. Ocrelizumab: A Review in Multiple Sclerosis. Drugs 2022, 82, 323–334. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- López-Gómez, J.; Sacristán-Enciso, B.; Caro-Miró, M.A.; Querol Pascual, M.R. Clinically isolated syndrome: Diagnosis and risk of developing clinically definite multiple sclerosis. Neurologia 2021, 38, 663–670. [Google Scholar] [CrossRef]
- Drug Treatment of Clinically Isolated Syndrome|CNS Drugs. Available online: https://link.springer.com/article/10.1007/s40263-019-00647-x (accessed on 10 June 2024).
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2; Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.A.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef]
- The Immune Response in Multiple Sclerosis|Annual Reviews. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-pathol-052920-040318 (accessed on 10 June 2024).
- Epstein–Barr Virus and Multiple Sclerosis|Nature Reviews Microbiology. Available online: https://www.nature.com/articles/s41579-022-00770-5 (accessed on 10 June 2024).
- Multiple Sclerosis—Symptoms and Causes—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/symptoms-causes/syc-20350269 (accessed on 10 June 2024).
- Burrows, D.J.; McGown, A.; Jain, S.A.; De Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal models of multiple sclerosis: From rodents to zebrafish. Mult. Scler. J. 2019, 25, 306–324. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- van der Star, B.J.; Vogel, D.Y.S.; Kipp, M.; Puentes, F.; Baker, D.; Amor, S. In vitro and in vivo models of multiple sclerosis. CNS Neurol. Disord. Drug Targets 2012, 11, 570–588. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Dragunow, M. Adult human brain cell culture for neuroscience research. Int. J. Biochem. Cell Biol. 2010, 42, 844–856. [Google Scholar] [CrossRef]
- De Vries, G.H.; Boullerne, A.I. Glial cell lines: An overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Zuiderwijk-Sick, E.A.; van der Putten, C.; Bsibsi, M.; Deuzing, I.P.; de Boer, W.; Persoon-Deen, C.; Kondova, I.; Boven, L.A.; van Noort, J.M.; ’t Hart, B.A.; et al. Differentiation of primary adult microglia alters their response to TLR8-mediated activation but not their capacity as APC. Glia 2007, 55, 1589–1600. [Google Scholar] [CrossRef]
- de Groot, C.J.; Hulshof, S.; Hoozemans, J.J.; Veerhuis, R. Establishment of microglial cell cultures derived from postmortem human adult brain tissue: Immunophenotypical and functional characterization. Microsc. Res. Tech. 2001, 54, 34–39. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Tichauer, J.; Eugenín-von Bernhardi, L. Proliferating culture of aged microglia for the study of neurodegenerative diseases. J. Neurosci. Methods 2011, 202, 65–69. [Google Scholar] [CrossRef]
- Fancy, S.P.J.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.M.; Rowitch, D.H. Myelin regeneration: A recapitulation of development? Annu. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- De Groot, C.J.; Langeveld, C.H.; Jongenelen, C.A.; Montagne, L.; Van Der Valk, P.; Dijkstra, C.D. Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: Immunophenotypical and functional characterization. J. Neurosci. Res. 1997, 49, 342–354. [Google Scholar] [CrossRef]
- Sharif, A.; Prevot, V. Isolation and culture of human astrocytes. Methods Mol. Biol. Clifton NJ 2012, 814, 137–151. [Google Scholar] [CrossRef]
- Losciuto, S.; Dorban, G.; Gabel, S.; Gustin, A.; Hoenen, C.; Grandbarbe, L.; Heuschling, P.; Heurtaux, T. An efficient method to limit microglia-dependent effects in astroglial cultures. J. Neurosci. Methods 2012, 207, 59–71. [Google Scholar] [CrossRef]
- Bertrand, S.J.; Aksenova, M.V.; Aksenov, M.Y.; Mactutus, C.F.; Booze, R.M. Endogenous amyloidogenesis in long-term rat hippocampal cell cultures. BMC Neurosci. 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Copray, S.; Balasubramaniyan, V.; Levenga, J.; de Bruijn, J.; Liem, R.; Boddeke, E. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells 2006, 24, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.; Dang, J.; Misiak, M.; Tameh Abolfazl, A.; Beyer, C.; Kipp, M. Combined 17beta-oestradiol and progesterone treatment prevents neuronal cell injury in cortical but not midbrain neurones or neuroblastoma cells. J. Neuroendocrinol. 2009, 21, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, A.; Shimizu, N. Differentiation of mouse embryonic stem cells into neurons using conditioned medium of dorsal root ganglia. J. Biosci. Bioeng. 2005, 100, 94–99. [Google Scholar] [CrossRef]
- Salli, U.; Reddy, A.P.; Salli, N.; Lu, N.Z.; Kuo, H.-C.; Pau, F.K.-Y.; Wolf, D.P.; Bethea, C.L. Serotonin neurons derived from rhesus monkey embryonic stem cells: Similarities to CNS serotonin neurons. Exp. Neurol. 2004, 188, 351–364. [Google Scholar] [CrossRef]
- Brewer, G.J.; Espinosa, J.; McIlhaney, M.P.; Pencek, T.P.; Kesslak, J.P.; Cotman, C.; Viel, J.; McManus, D.C. Culture and regeneration of human neurons after brain surgery. J. Neurosci. Methods 2001, 107, 15–23. [Google Scholar] [CrossRef]
- Cano-Abad, M.F.; Herrera-Peco, I.; Sola, R.G.; Pastor, J.; García-Navarrete, E.; Moro, R.C.; Pizzo, P.; Ruiz-Nuño, A. New insights on culture and calcium signalling in neurons and astrocytes from epileptic patients. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2011, 29, 121–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Drzewiecki, G.J.; Hom, J.T.; May, P.C.; Hyslop, P.A. Human cortical neuronal (HCN) cell lines: A model for amyloid beta neurotoxicity. Neurosci. Lett. 1994, 177, 162–164. [Google Scholar] [CrossRef]
- Pleasure, S.J.; Page, C.; Lee, V.M. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 1802–1815. [Google Scholar] [CrossRef]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. JAD 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Forsby, A.; Bal-Price, A.K.; Camins, A.; Coecke, S.; Fabre, N.; Gustafsson, H.; Honegger, P.; Kinsner-Ovaskainen, A.; Pallas, M.; Rimbau, V.; et al. Neuronal in vitro models for the estimation of acute systemic toxicity. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2009, 23, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.W.; Byun, J.H.; Vahidi, B.; Rhee, S.W.; Jeon, N.L. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann. Biomed. Eng. 2012, 40, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Smorodchenko, A.; Brocke, S.; Infante-Duarte, C.; Schulze Topphoff, U.; Vogt, J.; Prozorovski, T.; Meier, S.; Osmanova, V.; Pohl, E.; et al. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 2005, 46, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.; Roth, K.; Zurbuchen, U.; Deisz, R.; Bechmann, I.; Lehmann, T.-N.; Meier, S.; Nitsch, R.; Zipp, F. Tumor-necrosis-factor-related apoptosis-inducing-ligand (TRAIL)-mediated death of neurons in living human brain tissue is inhibited by flupirtine-maleate. J. Neuroimmunol. 2005, 167, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Nakagawa, E.; Hatori, K.; Choi, H.B.; McLarnon, J.G.; Lee, M.A.; Kim, S.U. Generation and characterization of immortalized human microglial cell lines: Expression of cytokines and chemokines. Neurobiol. Dis. 2001, 8, 1057–1068. [Google Scholar] [CrossRef]
- McLaurin, J.; Trudel, G.C.; Shaw, I.T.; Antel, J.P.; Cashman, N.R. A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J. Neurobiol. 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Nazir, F.H.; Wiberg, A.; Müller, M.; Mangsbo, S.; Burman, J. Antibodies from serum and CSF of multiple sclerosis patients bind to oligodendroglial and neuronal cell-lines. Brain Commun. 2023, 5, fcad164. [Google Scholar] [CrossRef]
- Post, G.R.; Dawson, G. Characterization of a cell line derived from a human oligodendroglioma. Mol. Chem. Neuropathol. 1992, 16, 303–317. [Google Scholar] [CrossRef]
- Lin, S.X.; Lisi, L.; Russo, C.D.; Polak, P.E.; Sharp, A.; Weinberg, G.; Kalinin, S.; Feinstein, D.L. The Anti-Inflammatory Effects of Dimethyl Fumarate in Astrocytes Involve Glutathione and Haem Oxygenase-1. ASN Neuro 2011, 3, AN20100033. [Google Scholar] [CrossRef]
- Kiseleva, L.N.; Kartashev, A.V.; Vartanyan, N.L.; Pinevich, A.A.; Samoilovich, M.P. A172 and T98G cell lines characteristics. Cell Tissue Biol. 2016, 10, 341–348. [Google Scholar] [CrossRef]
- Naddafi, F.; Reza Haidari, M.; Azizi, G.; Sedaghat, R.; Mirshafiey, A. Novel Therapeutic Approach by Nicotine in Experimental Model of Multiple Sclerosis. Innov. Clin. Neurosci. 2013, 10, 20–25. [Google Scholar] [PubMed]
- Authenticated SH-SY5Y Cell Line Sigma Aldrich. Available online: http://www.sigmaaldrich.com/ (accessed on 17 June 2024).
- Martínez-Pinilla, E.; Rubio-Sardón, N.; Peláez, R.; García-Álvarez, E.; del Valle, E.; Tolivia, J.; Larráyoz, I.M.; Navarro, A. Neuroprotective Effect of Apolipoprotein D in Cuprizone-Induced Cell Line Models: A Potential Therapeutic Approach for Multiple Sclerosis and Demyelinating Diseases. Int. J. Mol. Sci. 2021, 22, 1260. [Google Scholar] [CrossRef] [PubMed]
- Seewann, A.; Vrenken, H.; Kooi, E.-J.; van der Valk, P.; Knol, D.L.; Polman, C.H.; Pouwels, P.J.; Barkhof, F.; Geurts, J.J. Imaging the tip of the iceberg: Visualization of cortical lesions in multiple sclerosis. Mult. Scler. J. 2011, 17, 1202–1210. [Google Scholar] [CrossRef]
- Gold, R.; Linington, C.; Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain J. Neurol. 2006, 129, 1953–1971. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Fujinami, R.S. Experimental Autoimmune Encephalomyelitis as a Testing Paradigm for Adjuvants and Vaccines. Vaccine 2011, 29, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Gran, B.; O’Brien, K.; Fitzgerald, D.; Rostami, A.A.M. Experimental autoimmune encephalomyelitis (EAE). In Handbook of Neurochemistry and Molecular Neurobiology; Galoyan, A., Besedovsky, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 356–377. [Google Scholar]
- Yednock, T.A.; Cannon, C.; Fritz, L.C.; Sanchez-Madrid, F.; Steinman, L.; Karin, N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992, 356, 63–66. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Meshorer, A.; Hirshfeld, T.; Arnon, R.; Sela, M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur. J. Immunol. 1971, 1, 242–248. [Google Scholar] [CrossRef]
- Sriram, S.; Steiner, I. Experimental allergic encephalomyelitis: A misleading model of multiple sclerosis. Ann. Neurol. 2005, 58, 939–945. [Google Scholar] [CrossRef]
- Kleinschmidt-DeMasters, B.K.; Tyler, K.L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005, 353, 369–374. [Google Scholar] [CrossRef]
- Praet, J.; Guglielmetti, C.; Berneman, Z.; Van der Linden, A.; Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci. Biobehav. Rev. 2014, 47, 485–505. [Google Scholar] [CrossRef]
- Pasquini, L.A.; Calatayud, C.A.; Bertone Uña, A.L.; Millet, V.; Pasquini, J.M.; Soto, E.F. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem. Res. 2007, 32, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Torre-Fuentes, L.; Moreno-Jiménez, L.; Pytel, V.; Matías-Guiu, J.A.; Gómez-Pinedo, U.; Matías-Guiu, J. Experimental models of demyelination and remyelination. Neurologia 2020, 35, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.-A.; Blakemore, W.F. Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol. 2006, 175, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cuprizone-Containing Pellets Are Less Potent to Induce Consistent Demyelination in the Corpus Callosum of C57BL/6 Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28238065/ (accessed on 9 June 2024).
- Strain Differences in Cuprizone Induced Demyelination|Cell & Bioscience|Full Text. Available online: https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-017-0181-3 (accessed on 9 June 2024).
- Leopold, P.; Schmitz, C.; Kipp, M. Animal Weight Is an Important Variable for Reliable Cuprizone-Induced Demyelination. J. Mol. Neurosci. MN 2019, 68, 522–528. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Eames, R.A.; Smith, K.J.; Mcdonald, W.I. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J. Neurol. Sci. 1977, 33, 31–43. [Google Scholar] [CrossRef]
- Dousset, V.; Brochet, B.; Vital, A.; Gross, C.; Benazzouz, A.; Boullerne, A.; Bidabe, A.M.; Gin, A.M.; Caille, J.M. Lysolecithin-induced demyelination in primates: Preliminary in vivo study with MR and magnetization transfer. Am. J. Neuroradiol. 1995, 16, 225–231. [Google Scholar]
- Foote, A.K.; Blakemore, W.F. Inflammation stimulates remyelination in areas of chronic demyelination. Brain 2005, 128, 528–539. [Google Scholar] [CrossRef]
- Girard, C.; Bemelmans, A.-P.; Dufour, N.; Mallet, J.; Bachelin, C.; Nait-Oumesmar, B.; Evercooren, A.B.-V.; Lachapelle, F. Grafts of Brain-Derived Neurotrophic Factor and Neurotrophin 3-Transduced Primate Schwann Cells Lead to Functional Recovery of the Demyelinated Mouse Spinal Cord. J. Neurosci. 2005, 25, 7924–7933. [Google Scholar] [CrossRef]
- White Matter Plasticity and Enhanced Remyelination in the Maternal CNS|Journal of Neuroscience. Available online: https://www.jneurosci.org/content/27/8/1812?utm_source=TrendMD&utm_medium=cpc&utm_campaign=JNeurosci_TrendMD_0 (accessed on 9 June 2024).
- Blakemore, W.F.; Franklin, R.J.M. Remyelination in experimental models of toxin-induced demyelination. Curr. Top. Microbiol. Immunol. 2008, 318, 193–212. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.-Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Blakemore, W.F. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J. Neurocytol. 1995, 24, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Glia|Neurobiology Journal|Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/glia.1085?casa_token=oggwspny6KQAAAAA%3AzHNXgo7DDGRQ7Xh1UC6kJ5g24CbAqEEg7n0lbpiWHru17Vk5xC-hUSTMmrXDLVPQJMZvMvnELemSilk (accessed on 9 June 2024).
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.E. In Vitro and In Vivo Pharmacological Models to Assess Demyelination and Remyelination. Neuropsychopharmacology 2009, 34, 55–73. [Google Scholar] [CrossRef]
- Baxi, E.G.; DeBruin, J.; Tosi, D.M.; Grishkan, I.V.; Smith, M.D.; Kirby, L.A.; Strasburger, H.J.; Fairchild, A.N.; Calabresi, P.A.; Gocke, A.R. Transfer of Myelin-Reactive Th17 Cells Impairs Endogenous Remyelination in the Central Nervous System of Cuprizone-Fed Mice. J. Neurosci. 2015, 35, 8626–8639. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, M.M.; Chen, V.S.; Suzuki, K.; Ting, J.P.-Y.; Matsushima, G.K. MHC class II exacerbates demyelination in vivo independently of T cells. J. Neuroimmunol. 2008, 203, 23–32. [Google Scholar] [CrossRef]
- Soulika, A.M.; Lee, E.; McCauley, E.; Miers, L.; Bannerman, P.; Pleasure, D. Initiation and Progression of Axonopathy in Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2009, 29, 14965–14979. [Google Scholar] [CrossRef]
- Rüther, B.J.; Scheld, M.; Dreymueller, D.; Clarner, T.; Kress, E.; Brandenburg, L.-O.; Swartenbroekx, T.; Hoornaert, C.; Ponsaerts, P.; Fallier-Becker, P.; et al. Combination of cuprizone and experimental autoimmune encephalomyelitis to study inflammatory brain lesion formation and progression. Glia 2017, 65, 1900–1913. [Google Scholar] [CrossRef]
- Preston, M.A.; Macklin, W.B. Zebrafish as a Model to Investigate CNS Myelination. Glia 2015, 63, 177–193. [Google Scholar] [CrossRef]
- Ackerman, S.D.; Monk, K.R. The Scales and Tales of Myelination: Using zebrafish and mouse to study myelinating glia. Brain Res. 2016, 1641, 79–91. [Google Scholar] [CrossRef]
- Gong, Z.; Ju, B.; Wan, H. Green fluorescent protein (GFP) transgenic fish and their applications. Genetica 2001, 111, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Dubińska-Magiera, M.; Daczewska, M.; Lewicka, A.; Migocka-Patrzałek, M.; Niedbalska-Tarnowska, J.; Jagla, K. Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. Int. J. Mol. Sci. 2016, 17, 1941. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.B.; Takada, N.; Latimer, A.J.; Shin, J.; Carney, T.J.; Kelsh, R.N.; Appel, B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 2006, 9, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Udvadia, A.J.; Linney, E. Windows into development: Historic, current, and future perspectives on transgenic zebrafish. Dev. Biol. 2003, 256, 1–17. [Google Scholar] [CrossRef]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, P.; Zheng, M.; Zhang, W.; Yang, F.; Hong, L.; Yu, X.; Xu, H. Cuprizone-induced dopaminergic hyperactivity and locomotor deficit in zebrafish larvae. Brain Res. 2022, 1780, 147802. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Guo, S.-Y.; Xia, B.; Li, C.-Q.; Wang, L.; Wang, Y.-H. Development of zebrafish demyelination model for evaluation of remyelination compounds and RORγt inhibitors. J. Pharmacol. Toxicol. Methods 2019, 98, 106585. [Google Scholar] [CrossRef]
- Promotion of Remyelination by Sulfasalazine in a Transgenic Zebrafish Model of Demyelination—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26549504/ (accessed on 9 June 2024).
- Münzel, E.J.; Becker, C.G.; Becker, T.; Williams, A. Zebrafish regenerate full thickness optic nerve myelin after demyelination, but this fails with increasing age. Acta Neuropathol. Commun. 2014, 2, 77. [Google Scholar] [CrossRef]
- Buckley, C.E.; Marguerie, A.; Alderton, W.K.; Franklin, R.J.M. Temporal dynamics of myelination in the zebrafish spinal cord. Glia 2010, 58, 802–812. [Google Scholar] [CrossRef]
- Kulkarni, P.; Yellanki, S.; Medishetti, R.; Sriram, D.; Saxena, U.; Yogeeswari, P. Novel Zebrafish EAE model: A quick in vivo screen for multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 11, 32–39. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. Rev. Mag. Anim. Agric. 2019, 9, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.E.; Goldsmith, P.; Franklin, R.J.M. Zebrafish myelination: A transparent model for remyelination? Dis. Model. Mech. 2008, 1, 221–228. [Google Scholar] [CrossRef]
| Strain | Fish Mode | Fish Age | Behavioral Assessment (Yes/No) | Efficacy Readout | Findings | References |
|---|---|---|---|---|---|---|
| Wild-type zebrafish (Danio rerio) | EAE | 4–6-month-old fish | No | Optimization of immunization dose Histopathological evaluation used for validation of the model | The EAE model was developed by disease induction with myelin oligodendrocyte glycoprotein (0.6 mg/mL of MOG), model validated using fingolimod | [108] |
| Wild type | Chemically Induced: Cuprizone | Larvae Cuprizone exposure: 6–8 h post-fertilization (hpf) Behavioral Analysis: 120 hpf | Yes, post-demyelination | Automated video-tracking system used for behavioral analysis Neurotransmitter measurement RNA-seq and bioinformatic analysis Quantitative Real-Time PCR Analysis Whole mount in situ hybridization | Cuprizone reduced overall locomotor activity and diminished responses to acoustic and light stimuli; effects were associated with the upregulation of several dopamine receptor genes | [103,106] |
| Wild-type (WIK) tg (olig2:DsRed) tg (claudink:GFP) tg (claudink: GFP/olig2:DsRed) tg (FoxD3:GFP) | Chemically Induced: Lysophosphatidylcholine | 4–7 months young adult 15–18 months aged adult | No | Axonal tracing Tissue processing and immunohistochemistry Electron microscopy | Applying LPC onto gelatin foam induced demyelination, which peaked at day 3 and recovered by day 28 Zebrafish regenerate optic nerve myelin post-demyelination; this ability diminishes with age, suggesting age-related changes in remyelination processes | |
| Wild-type AB line Green-fluorescent-protein transgenic zebrafish Neutrophil green-fluorescent-protein transgenic zebrafish | Chemically Induced: Ethidium Bromide | Larvae 2–6 dpf | Yes | Determination of no observed adverse effect level (NOAEL) Zebrafish demyelination model validation: Motility assay and FluoroMyelin staining and whole mount anti-MBP immunostaining for demyelination model validation Compound effect assessments: Dose-response assay of remyelination, whole mount anti-acetylated tubulin immunostaining, promotion of peripheral motor neuron, reduction in neutrophil infiltration, and reduction in macrophage recruitment Video-track motion detector used for motility assay | 75 μM EB for 72 h effectively induced demyelination, decreased motility Thyroxine (T4) promoted remyelination and improved motility | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maktabi, B.; Collins, A.; Safee, R.; Bouyer, J.; Wisner, A.S.; Williams, F.E.; Schiefer, I.T. Zebrafish as a Model for Multiple Sclerosis. Biomedicines 2024, 12, 2354. https://doi.org/10.3390/biomedicines12102354
Maktabi B, Collins A, Safee R, Bouyer J, Wisner AS, Williams FE, Schiefer IT. Zebrafish as a Model for Multiple Sclerosis. Biomedicines. 2024; 12(10):2354. https://doi.org/10.3390/biomedicines12102354
Chicago/Turabian StyleMaktabi, Briana, Abigail Collins, Raihaanah Safee, Jada Bouyer, Alexander S. Wisner, Frederick E. Williams, and Isaac T. Schiefer. 2024. "Zebrafish as a Model for Multiple Sclerosis" Biomedicines 12, no. 10: 2354. https://doi.org/10.3390/biomedicines12102354
APA StyleMaktabi, B., Collins, A., Safee, R., Bouyer, J., Wisner, A. S., Williams, F. E., & Schiefer, I. T. (2024). Zebrafish as a Model for Multiple Sclerosis. Biomedicines, 12(10), 2354. https://doi.org/10.3390/biomedicines12102354






