Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope?
Abstract
:1. Introduction
2. Pathophysiological Mechanisms Underlying the Increased Cardiovascular Risk in OSA
2.1. Blood Pressure Disturbance
2.2. Endothelial Dysfunction
2.3. Inflammation and Metabolic Dysregulation
3. Clinical Evidence on the Association Between OSA and Cardiovascular Disease
3.1. OSA and Subclinical Atherosclerosis
3.2. Incident Cardiovascular Disease in OSA
4. GLP-1 and GIP/GLP-1 Receptor Agonists and OSA
5. SGLT2 Inhibitors and OSA
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- Lee, C.-H.; Sethi, R.; Li, R.; Ho, H.-H.; Hein, T.; Jim, M.-H.; Loo, G.; Koo, C.-Y.; Gao, X.-F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Baillieul, S.; Shah, N.M.; Dharmasena, R.; Schiza, S.; Testelmans, D.; Pataka, A. CPAP for secondary cardiovascular prevention in obstructive sleep apnoea patients: Not only one moon, but many stars. Breathe 2022, 18, 220148. [Google Scholar] [CrossRef]
- Tsuyumu, M.; Tsurumoto, T.; Iimura, J.; Nakajima, T.; Kojima, H. Ten-year adherence to continuous positive airway pressure treatment in patients with moderate-to-severe obstructive sleep apnea. Sleep Breath. 2020, 24, 1565–1571. [Google Scholar] [CrossRef]
- Bikov, A.; Bentley, A.; Csoma, B.; Smith, N.; Morris, B.; Bokhari, S. Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project. J. Clin. Med. 2024, 13, 2891. [Google Scholar] [CrossRef]
- Qiao, M.; Xie, Y.; Wolff, A.; Kwon, J. Long term adherence to continuous positive Airway pressure in mild obstructive sleep apnea. BMC Pulm. Med. 2023, 23, 320. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Dong, J.; Yang, W.; Liu, Z. Effects of continuous positive airway pressure on cardiac events and metabolic components in patients with moderate to severe obstructive sleep apnea and coronary artery disease: A meta-analysis. J. Clin. Sleep Med. 2023, 19, 2015–2025. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Francis, C.E.; Quinnell, T. Mandibular Advancement Devices for OSA: An Alternative to CPAP? Pulm. Ther. 2021, 7, 25–36. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Rizzo, M.; Voulgaris, A.; Xanthoudaki, M.; Kouratzi, M.; Ragia, G.; Manolopoulos, V.; Zissimopoulos, A.; et al. Metabolic Syndrome and Vitamin D Levels in Patients with Obstructive Sleep Apnea Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 190–196. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Kapen, S.; Lee-Chiong, T.; Alessi, C.; Boehlecke, B.; Brown, T.; Coleman, J.; Friedman, L.; Kapur, V.; Owens, J.; et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 2006, 29, 1031–1035. [Google Scholar]
- Hudgel, D.W.; Patel, S.R.; Ahasic, A.M.; Bartlett, S.J.; Bessesen, D.H.; Coaker, M.A.; Fiander, P.M.; Grunstein, R.R.; Gurubhagavatula, I.; Kapur, V.K.; et al. The Role of Weight Management in the Treatment of Adult Obstructive Sleep Apnea. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e70–e87. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Voulgaris, A.; Papanas, N.; Nena, E.; Xanthoudaki, M.; Pataka, A.; Schiza, S.; Rizzo, M.; Froudarakis, M.E.; Steiropoulos, P. Metabolic Syndrome in Patients with Coexistent Obstructive Sleep Apnea Syndrome and Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Metab. Syndr. Relat. Disord. 2020, 18, 296–301. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Heinzer, R.; Eckert, D. Treatment for obstructive sleep apnoea and cardiovascular diseases: Are we aiming at the wrong target? Lancet Respir. Med. 2020, 8, 323–325. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Janez, A.; Muzurovic, E.; Bogdanski, P.; Czupryniak, L.; Fabryova, L.; Fras, Z.; Guja, C.; Haluzik, M.; Kempler, P.; Lalic, N.; et al. Modern Management of Cardiometabolic Continuum: From Overweight/Obesity to Prediabetes/Type 2 Diabetes Mellitus. Recommendations from the Eastern and Southern Europe Diabetes and Obesity Expert Group. Diabetes Ther. 2024, 15, 1865–1892. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, T.; Malandris, K.; Avgerinos, I.; Stamati, A.; Kakotrichi, P.; Liakos, A.; Vasilakou, D.; Kakaletsis, N.; Tsapas, A.; Bekiari, E. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: A systematic review and network meta-analysis of randomised controlled trials. Diabetologia 2024, 67, 1206–1222. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Pamporis, K.; Stachteas, P.; Bougioukas, K.I.; Klisic, A.; Fragakis, N.; Rizzo, M. Safety and efficacy of the new, oral, small-molecule, GLP-1 receptor agonists orforglipron and danuglipron for the treatment of type 2 diabetes and obesity: Systematic review and meta-analysis of randomized controlled trials. Metabolism 2023, 149, 155710. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Klisic, A.; Rizzo, M. Effect of tirzepatide on albuminuria levels and renal function in patients with type 2 diabetes mellitus: A systematic review and multilevel meta-analysis. Diabetes Obes. Metab. 2024, 26, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Bernal-López, M.R.; Gómez-Huelgas, R. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors combination therapy versus monotherapy and major adverse cardiovascular events: Do the benefits add up? Eur. J. Intern. Med. 2024. [Google Scholar] [CrossRef]
- Patoulias, D.; Karakasis, P.; El-Tanani, M.; Rizzo, M. Can tirzepatide untie the Gordian knot of eating disorders among individuals with type 2 diabetes and obesity? J. Diabetes Its Complicat. 2024, 38, 108812. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef]
- de Mesquita, Y.L.L.; Calvi, I.P.; Marques, I.R.; Cruz, S.A.; Padrao, E.M.H.; Carvalho, P.E.d.P.; da Silva, C.H.A.; Cardoso, R.; Moura, F.A.; Rafalskiy, V.V. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: A meta-analysis of randomized controlled trials. Int. J. Obes. 2023, 47, 883–892. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Kassimis, G.; Koufakis, T.; Klisic, A.; Doumas, M.; Fragakis, N.; Rizzo, M. Therapeutic Potential of Sodium-glucose Co-transporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists for Patients with Acute Coronary Syndrome: A Review of Clinical Evidence. Curr. Pharm. Des. 2024, 30, 2109–2119. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Tzeis, S.; Fragakis, N. Glucagon-Like Peptide-1 Receptor Agonists and Atrial Fibrillation Recurrence After Ablation: A Fire without the Smoke? Clin. Electrophysiol. 2024, 10, 1940–1941. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Stachteas, P.; Patoulias, D.; Bougioukas, K.I.; Fragakis, N. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: An overview of 36 systematic reviews. Heart Fail. Rev. 2023, 28, 1033–1051. [Google Scholar] [CrossRef]
- Karakasis, P.; Popovic, D.S.; Patoulias, D.; Koufakis, T.; Papanas, N.; Fragakis, N.; Rizzo, M. The Effect of Sodium-Glucose Cotransporter Inhibitors on Renal Function as Adjunctive to Insulin in Adults with Type 1 Diabetes: An Updated Multilevel Meta-analysis of Randomized Controlled Trials. Diabetes Ther. 2024, 15, 521–532. [Google Scholar] [CrossRef]
- Stachteas, P.; Karakasis, P.; Patoulias, D.; Clemenza, F.; Fragakis, N.; Rizzo, M. The effect of sodium-glucose co-transporter-2 inhibitors on markers of subclinical atherosclerosis. Ann. Med. 2023, 55, 2304667. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Karakasis, P.; Karagiannidis, E.; Patoulias, D.; Athanasiadou, P.; Nasoufidou, A.; Papadopoulos, C.; Kassimis, G.; Fragakis, N. Efficacy of sodium-glucose cotransporter 2 inhibitors in preventing atrial fibrillation recurrence after catheter ablation. Hell. J. Cardiol. 2024. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Giannakoulas, G.; Rosenkranz, S.; Fragakis, N. Effect of sodium-glucose cotransporter-2 inhibitors on pulmonary arterial wedge pressure. Eur. J. Intern. Med. 2024, 124, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.-A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Giannakoulas, G.; Fragakis, N. Are We Ready for Expanding the Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Acute Myocardial Infarction? J. Cardiovasc. Pharmacol. 2024, 84, 26–28. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Kouskouras, K.; Karamitsos, T.; Patoulias, D.; Rizzo, M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Acute Coronary Syndrome: A Modern Cinderella? Clin. Ther. 2024. [Google Scholar] [CrossRef]
- Mylonas, N.; Nikolaou, P.E.; Karakasis, P.; Stachteas, P.; Fragakis, N.; Andreadou, I. Endothelial Protection by Sodium-Glucose Cotransporter 2 Inhibitors: A Literature Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 7274. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Nasoufidou, A.; Karagiannidis, E.; Patoulias, D.; Karakasis, P.; Alexiou, S.; Samaras, A.; Zormpas, G.; Stavropoulos, G.; Tsalikakis, D.; et al. The Role of Sodium Glucose Co-Transporter 2 Inhibitors in Atrial Fibrillation: A Comprehensive Review. J. Clin. Med. 2024, 13, 5408. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Ruža, I.; Marra, A.M.; Gómez-Huelgas, R. Comparative safety and efficacy analysis of GLP-1 receptor agonists and SGLT-2 inhibitors among frail individuals with type 2 diabetes in the era of continuous population ageing. Eur. J. Intern. Med. 2024. [Google Scholar] [CrossRef]
- Siddiquee, A.T.; Kim, S.; Thomas, R.J.; Lee, M.-H.; Ku Lee, S.; Shin, C. Obstructive sleep apnoea and long-term risk of incident diabetes in the middle-aged and older general population. ERJ Open Res. 2023, 9. [Google Scholar] [CrossRef]
- Wondie, A.; Taderegew, M.M.; Girma, B.; Getawey, A.; Tsega, D.; Terefe, T.F.; Mitiku, S.; Berhanu, H. Obstructive sleep apnea risk and its associated factors among type 2 diabetes mellitus patients at wolkite university specialized hospital, Wolkite, Southern Ethiopia, 2021. A comparative cross-sectional study. Diabetol. Metab. Syndr. 2022, 14, 157. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef]
- Blanchard, M.; Gervès-Pinquié, C.; Feuilloy, M.; Le Vaillant, M.; Trzepizur, W.; Meslier, N.; Goupil, F.; Pigeanne, T.; Balusson, F.; Oger, E.; et al. Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur. Respir. J. 2020, 57, 2004022. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Taranto-Montemurro, L.; Vena, D.; Sofer, T.; Kim, S.-W.; Stone, K.L.; White, D.P.; Wellman, A.; Redline, S. The Sleep Apnea-Specific Hypoxic Burden Predicts Incident Heart Failure. Chest 2020, 158, 739–750. [Google Scholar] [CrossRef]
- Kim, J.S.; Azarbarzin, A.; Wang, R.; Djonlagic, I.E.; Punjabi, N.M.; Zee, P.C.; Koo, B.B.; Soliman, E.Z.; Younes, M.; Redline, S. Association of novel measures of sleep disturbances with blood pressure: The Multi-Ethnic Study of Atherosclerosis. Thorax 2020, 75, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Umesi, C.; Gaston, S.A.; Azarbarzin, A.; Lunyera, J.; McGrath, J.A.; Ii, W.B.J.; Diamantidis, C.J.; Boulware, E.; Lutsey, P.L.; et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: Findings from the Multi-Ethnic Study of Atherosclerosis. Thorax 2021, 76, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.-M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea-Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Stradling, J.R. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 2010, 7, 677–685. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Wolf, J.; Lopez-Jimenez, F.; Somers, V.K. Obstructive sleep apnea and hypertension. Curr. Cardiol. Rep. 2005, 7, 435–440. [Google Scholar] [CrossRef]
- Panza, G.S.; Puri, S.; Lin, H.-S.; Badr, M.S.; Mateika, J.H. Daily Exposure to Mild Intermittent Hypoxia Reduces Blood Pressure in Male Patients with Obstructive Sleep Apnea and Hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 949–958. [Google Scholar] [CrossRef]
- Fung, M.M.; Peters, K.; Redline, S.; Ziegler, M.G.; Ancoli-Israel, S.; Barrett-Connor, E.; Stone, K.L.; For the Osteoporotic Fractures in Men Research Group. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertens 2011, 58, 596–603. [Google Scholar] [CrossRef]
- Javaheri, S.; Zhao, Y.Y.; Punjabi, N.M.; Quan, S.F.; Gottlieb, D.J.; Redline, S. Slow-Wave Sleep Is Associated with Incident Hypertension: The Sleep Heart Health Study. Sleep 2017, 41, A250–A251. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Hagen, E.W.; Finn, L.A.; Hla, K.M.; Carter, J.R.; Peppard, P.E. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: A longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 2015, 70, 1062–1069. [Google Scholar] [CrossRef]
- Labarca, G.; Schmidt, A.; Dreyse, J.; Jorquera, J.; Enos, D.; Torres, G.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101446. [Google Scholar] [CrossRef] [PubMed]
- Grover-Páez, F.; Zavalza-Gómez, A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.M.; Melehan, K.L.; Liu, P.Y.; Grunstein, R.R.; Phillips, C.L. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med. Rev. 2015, 20, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Tkacova, R.; Rizzo, M.; Berneis, K. Therapy with noninvasive ventilation in patients with obstructive sleep apnoea: Effects on atherogenic lipoprotein phenotype. Med. Hypotheses 2009, 73, 441–444. [Google Scholar] [CrossRef]
- Sopkova, Z.; Berneis, K.; Rizzo, M.; Spinas, G.A.; Dorkova, Z.; Tisko, R.; Tkacova, R. Size and subclasses of low-density lipoproteins in patients with obstructive sleep apnea. Angiology 2012, 63, 617–621. [Google Scholar] [CrossRef]
- Vekic, J.; Joppa, P.; Habalova, V.; Tisko, R.; Zeljkovic, A.; Pobeha, P.; Gojkovic, T.; Spasojevic-Kalimanovska, V.; Strbova, Z.; Kuklisova, Z.; et al. Relationship Between the Apolipoprotein E Genotype and LDL Particle Size in Patients with Obstructive Sleep Apnea. Angiology 2016, 67, 937–944. [Google Scholar] [CrossRef]
- Nieto, F.J.; Herrington, D.M.; Redline, S.; Benjamin, E.J.; Robbins, J.A. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am. J. Respir. Crit. Care Med. 2004, 169, 354–360. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Tse, H.-F.; Lam, B.; Tsang, K.W.T.; Lam, W.-K. Endothelial function in obstructive sleep apnea and response to treatment. Am. J. Respir. Crit. Care Med. 2004, 169, 348–353. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Lam, B.; Chan, L.Y.; Zheng, L.; Tsang, K.W.T.; Fung, P.C.W.; Lam, W.-K. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 2000, 162, 2166–2171. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef]
- Huang, Z.-W.; Ouyang, W.; Zhang, L.-J.; Li, H.; Ye, Y.-M.; Lin, X.-J.; Xu, Q.-Z.; Lin, L.; Chen, L.-D. Association of continuous positive airway pressure with F2-isoprostanes in adults with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2019, 23, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fernandez, A.; Garcia-Rio, F.; Arias, M.A.; Hernanz, A.; de la Pena, M.; Pierola, J.; Barcelo, A.; Lopez-Collazo, E.; Agusti, A. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: A randomised trial. Thorax 2009, 64, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Emin, M.; Thoma, T.; Pastellas, K.; Castagna, F.; Shah, R.; Jimenez, A.; Patel, N.; Wei, Y.; Jelic, S. Complement promotes endothelial von Willebrand factor and angiopoietin-2 release in obstructive sleep apnea. Sleep 2021, 44, zsaa286. [Google Scholar] [CrossRef]
- Calvin, A.D.; Albuquerque, F.N.; Lopez-Jimenez, F.; Somers, V.K. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab. Syndr. Relat. Disord. 2009, 7, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, J.; Liu, Y. Roles and Mechanisms of Obstructive Sleep Apnea-Hypopnea Syndrome and Chronic Intermittent Hypoxia in Atherosclerosis: Evidence and Prospective. Oxidative Med. Cell. Longev. 2016, 2016, 8215082. [Google Scholar] [CrossRef]
- Baessler, A.; Nadeem, R.; Harvey, M.; Madbouly, E.; Younus, A.; Sajid, H.; Naseem, J.; Asif, A.; Bawaadam, H. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—A meta-analysis. J. Inflamm. 2013, 10, 13. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Jenny, N.S.; Wang, R.; Shea, S.J.; Kaufman, J.D.; Allison, M.; Punjabi, N.M.; Tracy, R.; Libby, P.; Redline, S. Abstract 13147: Obstructive Sleep Apnea Associates with Elevated Leukocytes and Markers of Inflammation in the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016, 134, A13147. [Google Scholar] [CrossRef]
- Ryan, S. Adipose tissue inflammation by intermittent hypoxia: Mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J. Physiol. 2017, 595, 2423–2430. [Google Scholar] [CrossRef]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.-L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef]
- Baud, M.O.; Magistretti, P.J.; Petit, J.-M. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J. Sleep Res. 2013, 22, 3–12. [Google Scholar] [CrossRef]
- Shechter, A.; Grandner, M.A.; St-Onge, M.-P. The Role of Sleep in the Control of Food Intake. Am. J. Lifestyle Med. 2014, 8, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Duprez, D.A.; Jacobs, D.R.; Nagayoshi, M.; McClelland, R.L.; Shahar, E.; Budoff, M.; Redline, S.; Shea, S.; Carr, J.J.; et al. Obstructive sleep apnea and progression of coronary artery calcium: The multi-ethnic study of atherosclerosis study. J. Am. Heart Assoc. 2014, 3, e001241. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.S.; Kern, M.J.; Patel, P.M. Influence of obstructive sleep apnea and treatment with continuous positive airway pressure on fractional flow reserve measurements for coronary lesion assessment. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2010, 75, 207–213. [Google Scholar] [CrossRef]
- Querejeta Roca, G.; Shah, A.M. Sleep Disordered Breathing: Hypertension and Cardiac Structure and Function. Curr. Hypertens. Rep. 2015, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef]
- Yeboah, J.; Redline, S.; Johnson, C.; Tracy, R.; Ouyang, P.; Blumenthal, R.S.; Burke, G.L.; Herrington, D.M. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011, 219, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Reyes-Nuñez, N.; Caballero-Martinez, I.; Catalan-Serra, P.; Almeida-Gonzalez, C.V. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am. J. Respir. Crit. Care Med. 2014, 189, 1544–1550. [Google Scholar] [CrossRef]
- Nakashima, H.; Kurobe, M.; Minami, K.; Furudono, S.; Uchida, Y.; Amenomori, K.; Nunohiro, T.; Takeshita, S.; Maemura, K. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 75–84. [Google Scholar] [CrossRef]
- Mazaki, T.; Kasai, T.; Yokoi, H.; Kuramitsu, S.; Yamaji, K.; Morinaga, T.; Masuda, H.; Shirai, S.; Ando, K. Impact of Sleep-Disordered Breathing on Long-Term Outcomes in Patients with Acute Coronary Syndrome Who Have Undergone Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2016, 5, e003270. [Google Scholar] [CrossRef]
- Wu, X.; Lv, S.; Yu, X.; Yao, L.; Mokhlesi, B.; Wei, Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest 2015, 147, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Buchner, S.; Satzl, A.; Debl, K.; Hetzenecker, A.; Luchner, A.; Husser, O.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Zeman, F.; et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur. Heart J. 2014, 35, 192–199. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lin, Y.; Luo, M.J.; Considine, G.; Cox, A.L.; Bowsman, L.M.; Robins, D.A.; Haupt, A.; Duffin, K.L.; Ruotolo, G. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes. Metab. 2022, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Jastreboff, A.M.; Aronne, L.J.; Aronne, L.J.; Ahmad, N.N.; Ahmad, N.N.; Wharton, S.; Wharton, S.; Connery, L.; Connery, L.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Malhotra, A.; Bednarik, J.; Chakladar, S.; Dunn, J.P.; Weaver, T.; Grunstein, R.; Fietze, I.; Redline, S.; Azarbarzin, A.; Sands, S.A.; et al. Tirzepatide for the treatment of obstructive sleep apnea: Rationale, design, and sample baseline characteristics of the SURMOUNT -OSA phase 3 trial. Contemp. Clin. Trials 2024, 141, 107516. [Google Scholar] [CrossRef]
- O’donnell, C.; Crilly, S.; O’mahony, A.; O’riordan, B.; Traynor, M.; Gitau, R.; McDonald, K.; Ledwidge, M.; O’shea, D.; Murphy, D.J.; et al. Continuous Positive Airway Pressure but Not GLP1-mediated Weight Loss Improves Early Cardiovascular Disease in Obstructive Sleep Apnea: A Randomized Proof-of-Concept Study. Ann. Am. Thorac. Soc. 2024, 21, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, W.; Cheng, J.; Li, W.; Cheng, F. Efficacy and safety of liraglutide in patients with type 2 diabetes mellitus and severe obstructive sleep apnea. Sleep Breath. 2023, 27, 1687–1694. [Google Scholar] [CrossRef]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Castro, J.C.; Alcarria, E.; Cruz-Bravo, M.; Garcia-Llorente, M.J.; Albornos, C.; Moreno, C.; Cepeda, M.; Almodóvar, F. An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes. BMC Endocr. Disord. 2015, 15, 78. [Google Scholar] [CrossRef]
- Idris, I.; Abdulla, H.; Tilbrook, S.; Dean, R.; Ali, N. Exenatide improves excessive daytime sleepiness and wakefulness in obese patients with type 2 diabetes without obstructive sleep apnoea. J. Sleep Res. 2013, 22, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sprung, V.S.; Kemp, G.J.; Wilding, J.P.; Adams, V.; Murphy, K.; Burgess, M.; Emegbo, S.; Thomas, M.; Needham, A.J.; Weimken, A.; et al. Randomised, cOntrolled Multicentre trial of 26 weeks subcutaneous liraglutide (a glucagon-like peptide-1 receptor Agonist), with or without contiNuous positive airway pressure (CPAP), in patients with type 2 diabetes mellitus (T2DM) and obstructive sleep. BMJ Open 2020, 10, e038856. [Google Scholar] [CrossRef]
- Cistulli, P.; Dexter, B.; Woodford, C.; Alpert, N.; Mcconnell, W.; Sterling, K.; Pépin, J.-L.; Malhotra, A.; Cole, K. Adherence to Glucagon-like Peptide-1 Receptor Agonists (GLP-1s) in Obstructive Sleep Apnea Patients With and Without Type 2 Diabetes. Am. J. Respir. Crit. Care Med. 2024, 209, A6593. [Google Scholar] [CrossRef]
- Weiss, T.; Yang, L.; Carr, R.D.; Pal, S.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res. Care 2022, 10, e002517. [Google Scholar] [CrossRef] [PubMed]
- Wojeck, B.S.; Inzucchi, S.E.; Neeland, I.J.; Mancuso, J.P.; Frederich, R.; Masiukiewicz, U.; Cater, N.B.; McGuire, D.K.; Cannon, C.P.; Yaggi, H.K. Ertugliflozin and incident obstructive sleep apnea: An analysis from the VERTIS CV trial. Sleep Breath. 2023, 27, 669–672. [Google Scholar] [CrossRef]
- Kusunoki, M.; Hisano, F.; Wakazono, N.; Tsutsumi, K.; Oshida, Y.; Miyata, T. Effect of Treatment With Sodium-Glucose Cotransporter 2 Inhibitor on the Initiation of Continuous Positive Airway Pressure Therapy in Type 2 Diabetic Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. Res. 2021, 13, 497–501. [Google Scholar] [CrossRef]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sun, Q.; Bai, X.-Y.; Zhou, Y.-F.; Zhou, Q.-L.; Zhang, M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: A preliminary study. Nutr. Diabetes 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Karashima, S.; Kometani, M.; Oka, R.; Takeda, Y.; Sawamura, T.; Fujimoto, A.; Demura, M.; Wakayama, A.; Usukura, M.; et al. Effect of sodium glucose cotransporter 2 inhibitors on obstructive sleep apnea in patients with type 2 diabetes. Endocr. J. 2018, 65, 461–467. [Google Scholar] [CrossRef]
- Furukawa, S.; Miyake, T.; Senba, H.; Sakai, T.; Furukawa, E.; Yamamoto, S.; Niiya, T.; Matsuura, B.; Hiasa, Y.; Furukawa, S.; et al. The effectiveness of dapagliflozin for sleep-disordered breathing among Japanese patients with obesity and type 2 diabetes mellitus. Endocr. J. 2018, 65, 953–961. [Google Scholar] [CrossRef]
- Armentaro, G.; Pelaia, C.; Condoleo, V.; Severini, G.; Crudo, G.; De Marco, M.; Pastura, C.A.; Tallarico, V.; Pezzella, R.; Aiello, D.; et al. Effect of SGLT2-Inhibitors on Polygraphic Parameters in Elderly Patients Affected by Heart Failure, Type 2 Diabetes Mellitus, and Sleep Apnea. Biomedicines 2024, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Jering, K.; DE Boer, R.A.; Claggett, B.L.; Desai, A.S.; Hernandez, A.F.; Inzucchi, S.E.; Jhund, P.S.; Køber, L.; Kosiborod, M.N.; et al. Heart Failure, Investigator-Reported Sleep Apnea and Dapagliflozin: A Patient-Level Pooled Meta-Analysis of DAPA-HF and DELIVER. J. Card. Fail. 2024, 30, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef] [PubMed]

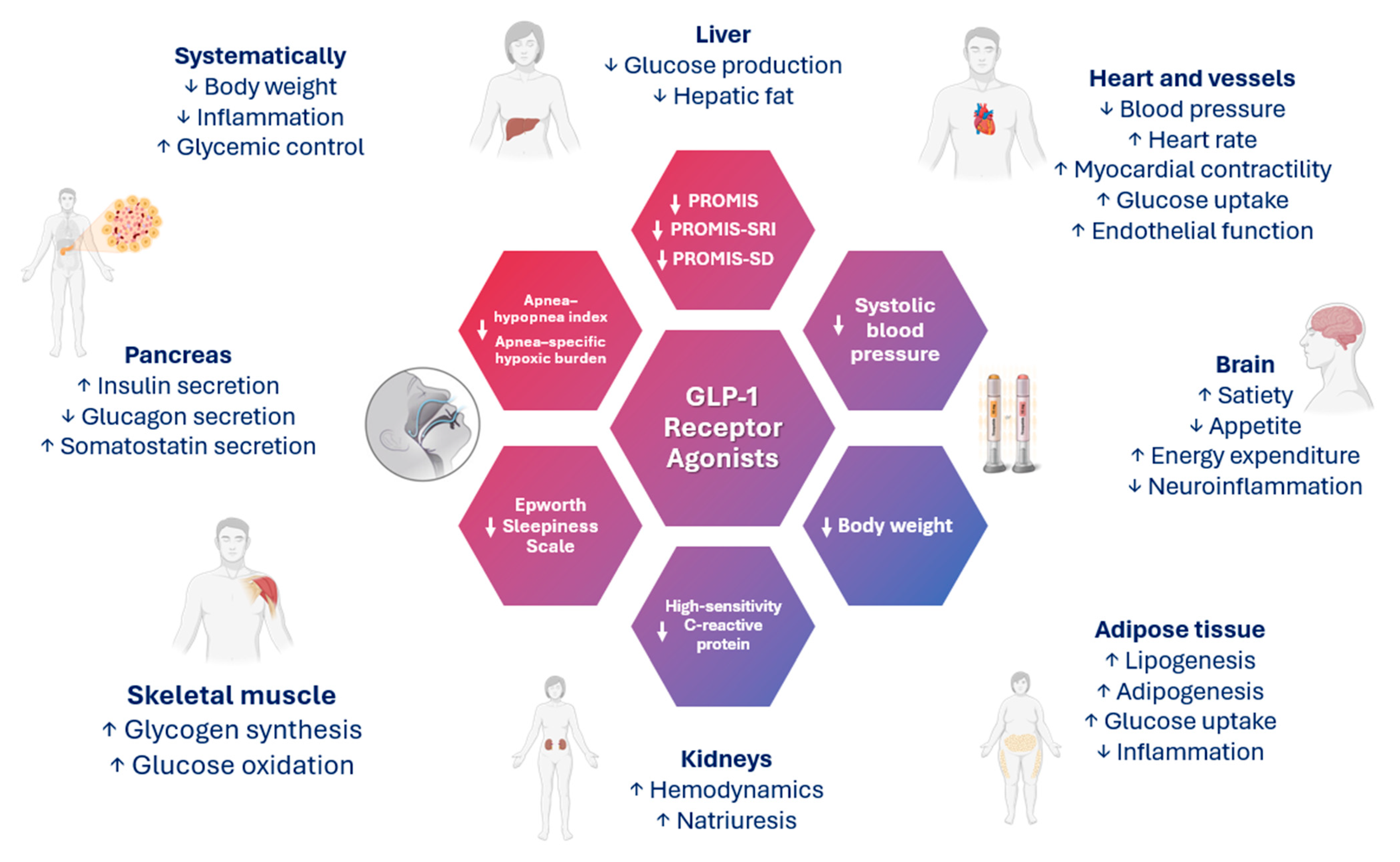
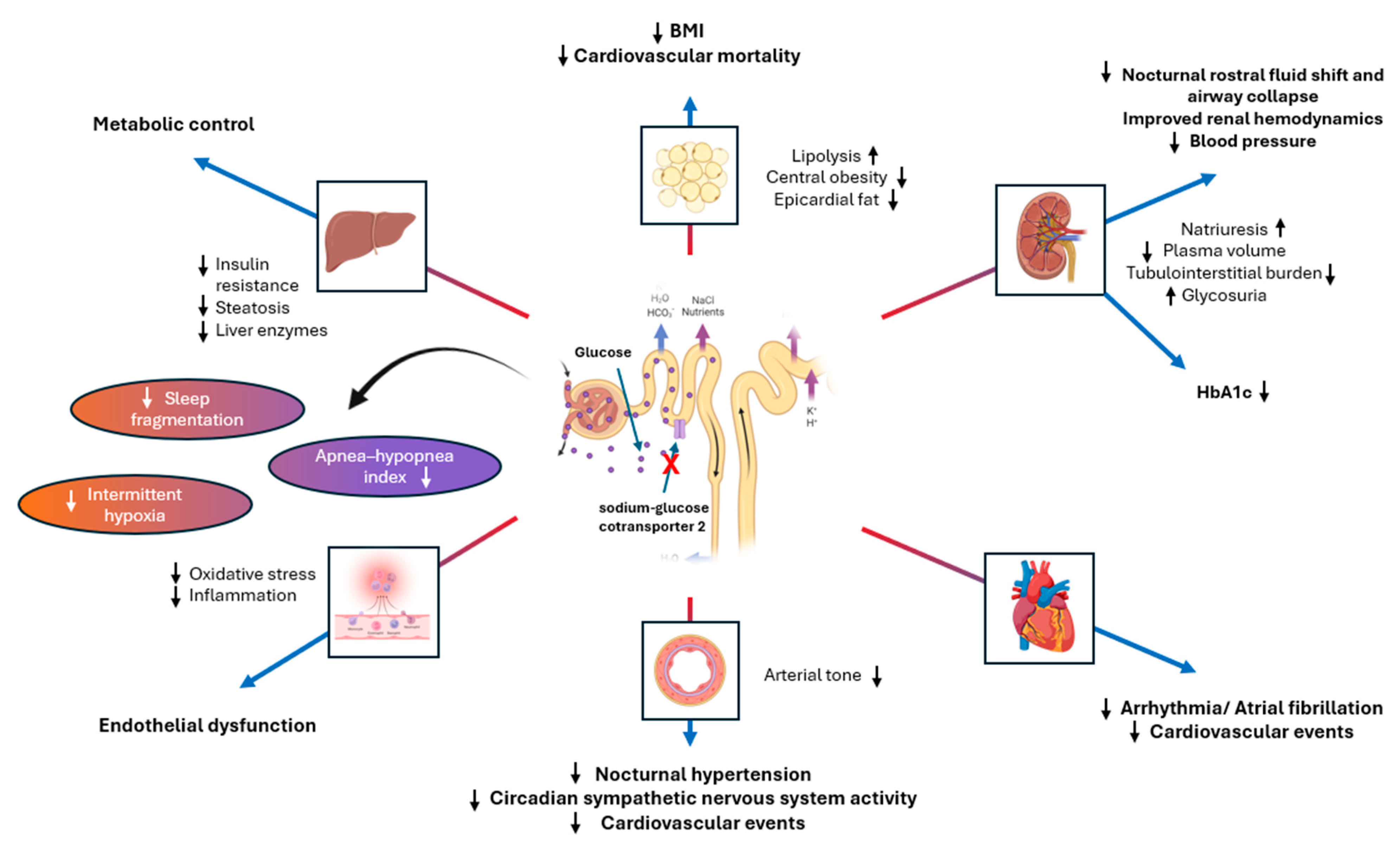
| Study (Year) | Cohort | Number of Patients | Age (Years); Men (%) | Hypoxic Burden (%min/h) | Follow-Up Duration | Outcome; Number of Events | Findings |
|---|---|---|---|---|---|---|---|
| Azarbarzin, 2019 [48] | MrOS | 2743 | 76.3 ± 5.5; 100 | 58.4 ± 52.9 | 10 ± 3.5 years | Cardiovascular mortality; 440 | High hypoxic burden was linked to increased cardiovascular mortality |
| SHHS | 5111 | 63.7 ± 10.9; 47.2 | 50.2 ± 57.3 | 10.9 ± 3.1 years | Cardiovascular mortality; 313 | ||
| Blanchard, 2021 [49] | Pays de la Loire Sleep Cohort | 3597 | 58 (48–67); 63 | Not reported | 5.9 (3.5–8.4) years | First incident stroke; 83 (70 ischemic, including TIA) | Increased hypoxic burden was associated with stroke |
| Azarbarzin, 2020 [50] | SHHS | 4881 | 63.6 ± 11.1; 45.6 | 62 ± 64.7 in men; 37 ± 39.4 in women | 10.4 ± 3.4 years | Incident heart failure; 543 | Increased hypoxic burden was associated with incident heart failure in men |
| MrOS | 2653 | 76.2 ± 5.4; 100 | 57.3 ± 53 | 8.8 ± 2.8 years | Incident heart failure; 145 | ||
| Kim, 2020 [51] | MESA | 2055 | 68.4 ± 9.1; 46 | 56.6 ± 65.9 | Cross-sectional | Systolic and diastolic blood pressure | Increased hypoxic burden was associated with higher diastolic blood pressure |
| Jackson, 2021 [52] | MESA | 1895 | 68.2 ± 9.1; 46 | 56.5 ± 65.1 | Cross-sectional | Prevalent moderate-to-severe chronic kidney disease | Increased hypoxic burden was associated with higher prevalence of moderate-to-severe chronic kidney disease |
| Trzepizur, 2022 [53] | Pays de la Loire Sleep Cohort | 5358 | 60 (51–69); 63.7 | 32 (13–71) | 78 (52–109) months | MACE; 592 | Increased hypoxic burden was associated with an increased risk of MACE |
| Author/Study | Study Design | Participants | Intervention | Primary Endpoint | Outcomes |
|---|---|---|---|---|---|
| Malhotra, 2024 [96] | SURMOUNT-OSA; Phase 3, double-blind, randomized, controlled trials | In Trial 1, 234 participants who were not undergoing CPAP treatment at baseline were enrolled, while Trial 2 included 235 participants who were already receiving CPAP therapy at the start of the study. Patients with baseline diabetes were excluded | Maximum tolerated dose of tirzepatide (10 mg or 15 mg) or placebo for 52 weeks | The change in the AHI (the number of apneas and hypopneas during an hour of sleep) from baseline | By week 52 in Trial 1, AHI decreased by an average of 25.3 events per hour (95% CI, −29.3 to −21.2) in the tirzepatide group compared to a reduction of 5.3 events per hour (95% CI, −9.4 to −1.1) with placebo, resulting in a treatment difference of −20.0 events per hour (95% CI, −25.8 to −14.2). In Trial 2, the AHI reduction with tirzepatide was 29.3 events per hour (95% CI, −33.2 to −25.4), while placebo showed a reduction of 5.5 events per hour (95% CI, −9.9 to −1.2), yielding a treatment difference of −23.8 events per hour (95% CI, −29.6 to −17.9). Tirzepatide significantly improved all prespecified secondary outcomes compared to placebo |
| O’Donnell, 2024 [97] | Randomized proof-of-concept study | 30 obese patients with newly diagnosed moderate to severe OSA. Those with T2D, heart failure, or unstable cardiovascular disease were excluded | CPAP (group A), liraglutide (group B) and combination therapy (group C) for 24 weeks | The change in the AHI and several cardiometabolic parameters | CPAP therapy, both alone and in combination, produced a significantly greater reduction in the AHI compared to liraglutide alone, with mean decreases of 45 and 43 events per hour, respectively, versus 12 events per hour (p < 0.05). While both liraglutide and combination therapy resulted in substantial weight loss, only CPAP monotherapy led to a significant reduction in vascular inflammation, as indicated by a decrease in the aortic wall target-to-background ratio (p = 0.010). This was accompanied by improvements in endothelial function and reductions in C-reactive protein levels. Additionally, low-attenuation coronary artery plaque volume, a marker of unstable plaque, decreased with both CPAP therapy and combination treatment, but no significant changes were observed with liraglutide monotherapy. |
| Jiang, 2023 [98] | Two-center, prospective randomized controlled trial | 90 patients with T2D and severe OSA | CPAP and drug treatment including liraglutide or CPAP and drug treatment without liraglutide) | Demographic and clinical characteristics, along with indices of sleep-disordered breathing and cardiac function, as well as adverse effects, were assessed and compared between the two groups both at baseline and after a 3-month follow-up | Liraglutide was associated with significant reductions in BMI, AHI, and mean systolic blood pressure compared to the control group (p < 0.05). Additionally, the liraglutide group exhibited a significantly higher minimum oxygen saturation after 3 months of follow-up (p < 0.05). There were no significant differences between the groups regarding the incidence of side effects (p > 0.05) |
| Sprung, 2020 [102] | Single-centered, open-labelled, prospective, phase 4 randomized controlled trial | 132 patients with newly diagnosed OSA (AHI ≥ 15 events/hour), and existing obesity and T2D | Participants will be randomly assigned in equal proportions to one of four treatment groups for a duration of 26 weeks: (i) liraglutide at a daily dose of 1.8 mg, (ii) liraglutide 1.8 mg daily combined with CPAP, (iii) CPAP alone as standard care, or (iv) a control group receiving no treatment | The change in OSA severity, determined by AHI | Ongoing |
| Blackman, 2016 [99] | Randomized, double-blind trial | Participants with obesity, without T2D who had moderate (AHI 15–29.9 events/h) or severe (AHI ≥ 30 events/h) OSA and were unwilling/unable to use CPAP | Liraglutide 3.0 mg (n = 180) or placebo (n = 179) for 32 weeks | The change in the AHI from baseline to week 32, assessed using the 2007 criteria recommended by the American Academy of Sleep Medicine. According to this definition, hypopnea events were scored based on a reduction of at least 30% in nasal pressure signal excursions from baseline, accompanied by a desaturation of at least 4% from the pre-event baseline | After 32 weeks, the reduction in the AHI was significantly greater with liraglutide compared to placebo,, resulting in an estimated treatment difference of −6.1 events per hour (p = 0.015). Liraglutide also led to a greater percentage of weight loss compared to placebo, with an estimated treatment difference of −4.2% (p < 0.0001). Post hoc analyses revealed a significant correlation between the extent of weight loss and improvements in OSA outcomes (p < 0.01 for all). Furthermore, liraglutide resulted in more pronounced reductions in both HbA1c and SBP compared to placebo. The safety profile of liraglutide at 3.0 mg was consistent with that observed at doses of 1.8 mg or lower. |
| Gomez-Peralta, 2015 [100] | Single-center retrospective study | 58 obese adult subjects with T2D | Liraglutide treatment at least 3 months before study inclusion | Epworth Sleepiness Scale (ESS), anthropometric parameters, glucose-control and several metabolic parameters | Significant reductions in the ESS scores were observed at both 1 month (−1.3 ± 2.8, p < 0.001) and 3 months (−1.5 ± 3.0, p < 0.001) following the initiation of liraglutide treatment. Additionally, after 3 months of liraglutide therapy, there were notable improvements in body weight (p < 0.001), BMI (p < 0.001), waist circumference (p < 0.001), and neck circumference (p < 0.005), as well as significant reductions in HbA1c (p < 0.001), mean blood glucose levels (p < 0.001), fasting plasma glucose (p < 0.001), triglycerides (p < 0.01), and total cholesterol (p < 0.001). |
| Idris, 2013 [101] | Placebo-controlled single-blind study | 80 obese patients with T2D excessive daytime sleepiness | Exenatide for 22 weeks (5 μg twice-daily dose by subcutaneous injection and increased to 10 μg twice daily within 4 weeks of treatment initiation) | Wakefulness and sleep latency test, Epworth score, driving performance, depression score, fasting glucose and HbA1c | Exenatide is linked to a substantial decrease in objective sleepiness among obese patients with T2D, regardless of their HbA1c levels. |
| Author/Study | Study Design | Participants | Intervention/Exposure | Primary Endpoint | Outcomes |
|---|---|---|---|---|---|
| Wojeck, 2022 [105] | Randomized controlled trial | Patients ≥ 40 years with T2D and atherosclerotic cardiovascular disease | Ertugliflozin (5 or 15 mg) or placebo | The composite of major adverse cardiovascular events |
|
| Kusunoki, 2021 [106] | Open label trial | 16 patients with T2D and OSA | Empagliflozin (10 mg), dapagliflozin (5 mg) and luseogliflozin (2.5 mg) along with CPAP therapy | Change in body weight, BMI, serum HbA1c level, lipid profile, liver function parameters, serum uric acid, and AHI |
|
| Neeland, 2020 [107] | Double-blind, placebo-controlled randomized trial (EMPA-REG OUTCOME) | 391 OSA patients with T2D and cardiovascular disease | Empagliflozin (10 and 25 mg) or placebo daily in addition to standard of care | The composite outcome 3P-MACE (death as a result of CV causes, nonfatal myocardial infarction, or nonfatal stroke), with secondary outcomes including hospitalization for heart failure, all-cause mortality, and incident or worsening nephropathy |
|
| Tang, 2019 [108] | Randomized controlled trial | 36 patients with newly-diagnosed T2D and OSA | Dapagliflozin and metformin versus glimepiride and metformin for 24 weeks | Changes in fasting plasma glucose (FPG), postprandial blood glucose (PPG), (HbA1c), fasting insulin levels, homeostasis model assessment of insulin resistance (HOMA-IR), lipid profile, BMI, blood pressure, AHI, minimum oxygen saturation (LSpO2), ESS score |
|
| Sawada, 2018 [109] | Retrospective cohort study | 18 patients with T2D and OSA | SGLT2 inhibitors | HbA1c, body weight, BMI, blood pressure and AHI were evaluated before and after SGLT2 inhibitors administration |
|
| Furukawa, 2018 [110] | Open-label, single-arm, multicentre trial | 30 patients with T2D and sleep-disordered breathing | Dapagliflozin (5 mg) once daily for 24 weeks | Change in at least five 3% oxygen desaturation index (ODI) events per hour |
|
| Armentaro, 2024 [111] | Observational cohort study | 514 consecutive elderly outpatients with heart failure, T2D and OSA not on CPAP therapy | SGLT2 inhibitors | Change in AHI |
|
| Butt, 2024 [112] | Patient-level pooled analysis of DAPA-HF and DELIVER trials | 11,005 patients with in HFrEF and HFmrEF/HFpEF | Dapagliflozin (10 mg) | A composite of worsening heart failure or cardiovascular death |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. https://doi.org/10.3390/biomedicines12112503
Karakasis P, Sagris M, Patoulias D, Koufakis T, Theofilis P, Klisic A, Fragakis N, El Tanani M, Rizzo M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines. 2024; 12(11):2503. https://doi.org/10.3390/biomedicines12112503
Chicago/Turabian StyleKarakasis, Paschalis, Marios Sagris, Dimitrios Patoulias, Theocharis Koufakis, Panagiotis Theofilis, Aleksandra Klisic, Nikolaos Fragakis, Mohamed El Tanani, and Manfredi Rizzo. 2024. "Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope?" Biomedicines 12, no. 11: 2503. https://doi.org/10.3390/biomedicines12112503
APA StyleKarakasis, P., Sagris, M., Patoulias, D., Koufakis, T., Theofilis, P., Klisic, A., Fragakis, N., El Tanani, M., & Rizzo, M. (2024). Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines, 12(11), 2503. https://doi.org/10.3390/biomedicines12112503









