Abstract
Introduction: While most patients with iatrogenic tracheal stenosis (ITS) respond to endoscopic ablative procedures, approximately 15% experience a recalcitrant, recurring disease course that is resistant to conventional management. We aimed to explore genetic profiles of patients with recalcitrant ITS to understand underlying pathophysiology and identify novel therapeutic options. Methods: We collected 11 samples of granulation tissue from patients with ITS and performed RNA sequencing. We identified the top 10 most highly up- and down-regulated genes and cellular processes that these genes corresponded to. For the most highly dysregulated genes, we identified potential therapeutic options that favorably regulate their expression. Results: The dysregulations in gene expression corresponded to hyperkeratinization (upregulation of genes involved in keratin production and keratinocyte differentiation) and cellular proliferation (downregulation of cell cycle regulating and pro-apoptotic genes). Genes involved in retinoic acid (RA) metabolism and signaling were dysregulated in a pattern suggesting local cellular RA deficiency. Consequently, RA also emerged as the most promising potential therapeutic option for ITS, as it favorably regulated seven of the ten most highly dysregulated genes. Conclusion: This is the first study to characterize the role of hyperkeratinization and dysregulations in RA metabolism and signaling in the disease pathophysiology. Given the ability of RA to favorably regulate key genes involved in ITS, future studies must explore its efficacy as a potential therapeutic option for patients with recalcitrant ITS.
1. Introduction
Tracheal stenosis occurs most commonly as a sequela of iatrogenic causes such as prolonged intubation and tracheostomy [1]. While over 50% of patients experience some degree of tracheal insult after prolonged intubation [2], the incidence of iatrogenic tracheal stenosis (ITS) is reported to range from 6 to 21% [3]. ITS can be a devastating and chronic disease for patients to endure, necessitating long-term management, and can negatively impact both physical and psychosocial quality of life [4].
ITS is extremely challenging to manage, with surgical and endoscopic interventions being the mainstay of treatment. These include endoscopic balloon dilation, laser therapy, spray cryotherapy, tracheal stenting, and, less commonly, tracheal resection and reconstruction. However, surgery is limited by several contraindications, including previous surgery or mucosal inflammation beyond the area of resection, as well as by a patient’s general operability [5]. While endoscopic interventions provide a reasonable alternative to open surgery, relapse rates are higher [6]. Thus, patients often require routine intervention for symptom control. Several adjunctive medical therapies such as glucocorticoids and antibiotics have been explored to consolidate interventional management, but with limited success [7,8].
While the pathogenesis of ITS is not completely understood, literature suggests that underlying genetic susceptibilities and immune dysregulations in the setting of tracheal insult may lead to the unregulated hypergranulation seen in ITS. Molecules such as VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor receptor), FGF (fibroblast growth factor), and TGF-β (transforming growth factor-beta) have been identified as contributors to the disease process. Subsequently, these molecules have been therapeutic targets with medications such as pirfenidone, nintedanib, and rapamycin, which have shown some promising results in animal models [9]. Prior work with coronavirus disease 2019 (COVID-19)-associated tracheal stenosis (CATS) further corroborates the potential role for genetic and immune dysregulations in the development of tracheal hypergranulation [10]. In CATS, genes associated with anti-viral and anti-microbial functions was upregulated, such as CXCL11 (C-X-C motif chemokine ligand 11), CCL8 (C-C motif chemokine ligand 8), DEFB103A (defensin beta 103A), IFI6 (interferon alpha inducible protein 6), ACOD1 (aconitate decarboxylase 1), and DEFB4A (defensin beta 4A) [10]. Based on these dysregulations, several drugs with specific gene product targets have been suggested as potential therapies for CATS [10]. However, gene expression analysis of ITS granulation tissue is relatively nascent and an understanding of the molecular mechanisms behind ITS may hold promise in terms of novel treatment and preventive options. Thus, we aimed to investigate patterns of gene expression in the tracheal granulation tissue of patients with ITS and identify potential therapeutic options based on genetic profiles.
2. Materials and Methods
This study was conducted between July 2019–July 2021 at Nuvance Health in Connecticut, USA, after receiving ethical approval from the institutional review board (ID: 2019-19).
2.1. Patient Enrollment and Sample Collection
Eight adult patients (age ≥ 18 years) presenting to clinics for management of severe, recalcitrant ITS, as visualized by upper airway endoscopy, were included in this study after informed consent was obtained. Inclusion criteria included over 70% tracheal lumen occlusion and at least two recurrences which required cryotherapy/balloon dilation procedures. Patient data collected included demographics, baseline health status, and clinical history.
To understand active disease mechanisms in ITS (prior to potentially irreversible tissue scarring), we examined transcriptomic dysregulation in granulation tissue. Tracheal tissue biopsies of granulation tissue were collected at the time of endoscopic intervention, which consisted of balloon dilation and spray cryotherapy. If enrolled patients returned to clinics requiring reintervention for re-stenosis, they were reapproached for collection of additional samples. Additional biopsies were collected from consenting patients, with these being considered as unique samples for analysis.
2.2. RNA Sequencing of Samples
Frozen tissue samples were sent to Azenta Life Sciences for RNA extraction, processing, and RNA sequencing. cDNA libraries were generated using Illumina preparation kits with Poly(A) selection. Sequencing was conducted using the Illumina HiSeq® platform configured to the following settings: 2 × 150 bp, ~350 M paired-end reads, single index, per lane with ≥80% of bases ≥Q30. Analysis was performed using the vendor standard RNA-seq analysis.
2.3. Normal Control
Publicly accessible genomic data for normal tracheal tissue was sourced from the public data repositories of the National Center for Biotechnology Information (NCBI: SRR16760102) and the European Nucleotide Archive (ENA: ERR 2022844).
2.4. Data Analysis
CLC Genomics Workbench by QIAGEN was used for analysis of the sequenced data. Trimmed reads were aligned and annotated with Ensembl 91: December 2017 (GRCh38.p10). Differential gene expressions were explored between tissue samples of ITS and normal control data. Gene expression was considered significant if the FDR (false discovery rate) p-value was <0.5 and the fold change (ratio of value in specimen to value in normal control) was >1.5. The top 10 most highly upregulated and downregulated genes were identified by calculating a change coefficient that accounted for both FDR p-value and fold change, as follows: |change coefficient| = −log2 FDR p-value × log2 fold change. The protein class of the gene products and their relevant functions were retrieved from the Human Protein Atlas [11], an open-source repository containing data on all proteins coded by the human genome. For each of these pathologically dysregulated genes, we queried the Human Portal of the Rat Genome Database [12] to identify potential therapeutic options that could inhibit pathologically upregulated gene products or promote pathologically downregulated gene products. We prioritized drugs with FDA approval for use in humans.
Gene Ontology (GO) and KEGG (Kyoto Encyclopedia of Genes and Genomes) functional enrichment were applied via DAVID (Database for Annotation, Visualization, and Integrated Discovery) for significantly upregulated and downregulated genes separately. Functional annotations, enriched GO terms, and pathways identified were noted. Only pathways and processes with a Benjamini p-value <0.05 were considered significant.
3. Results
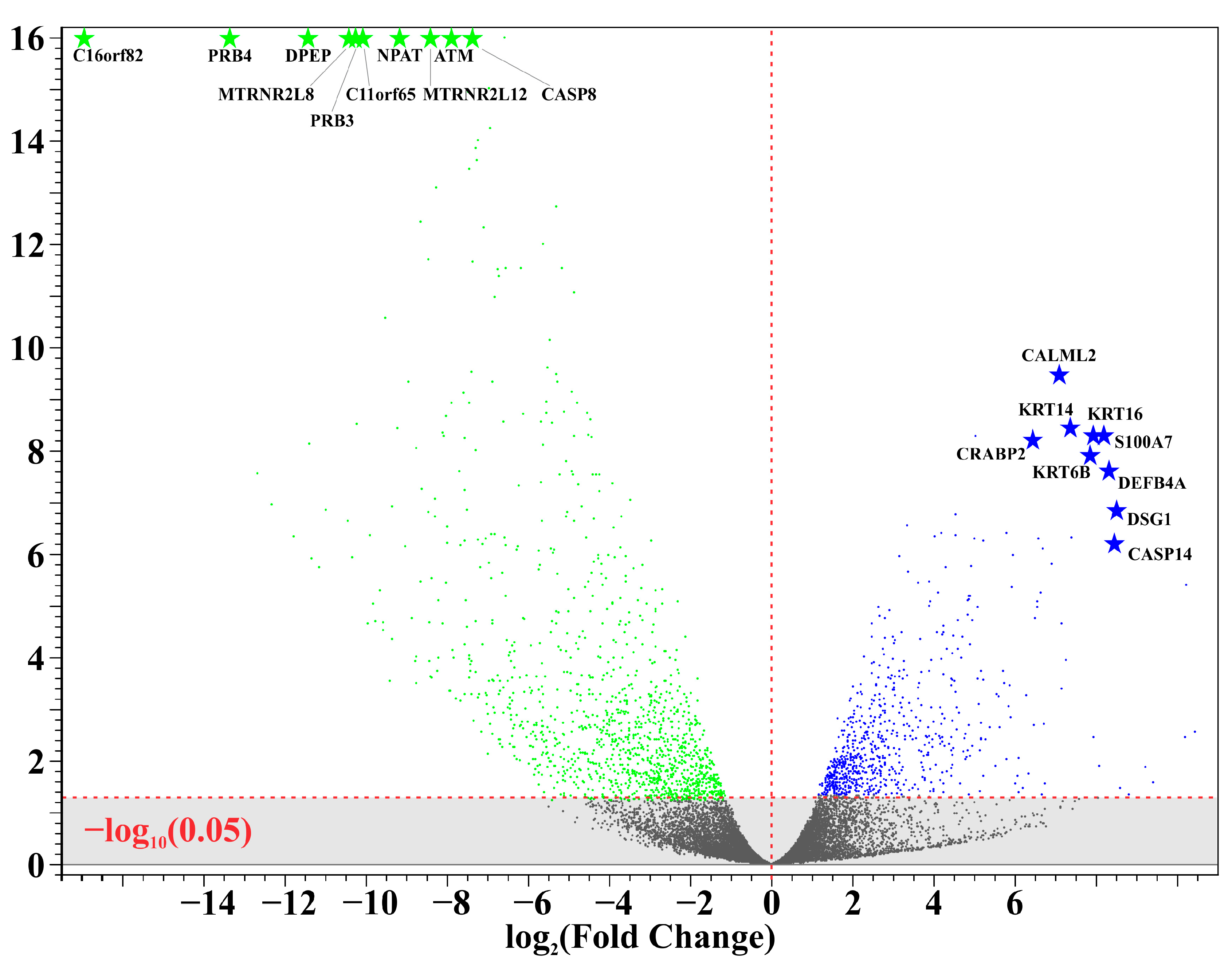
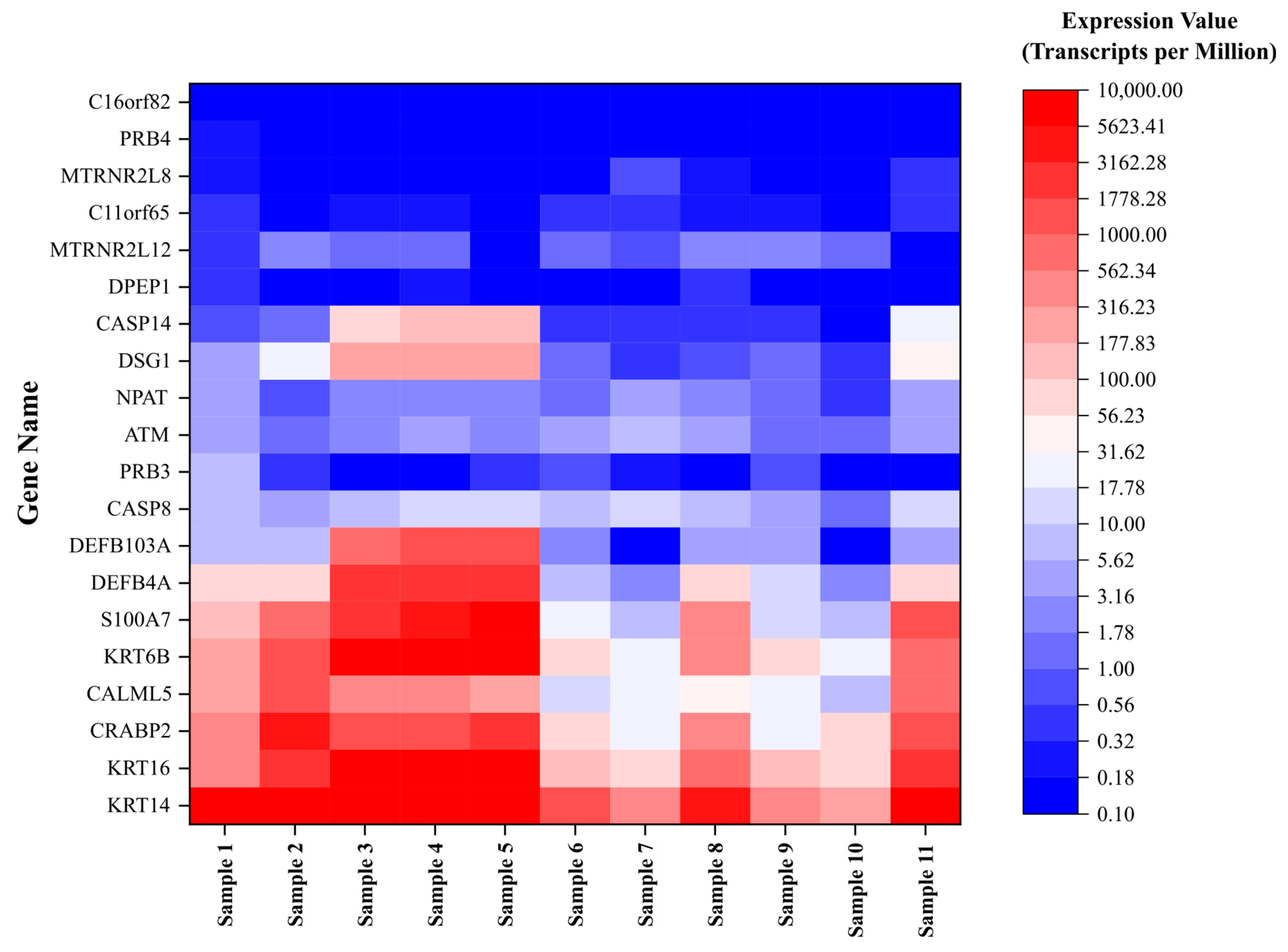
A total of 11 unique samples from the 8 patients were sequenced for analysis. Table 1 shows the ten most upregulated and downregulated genes (by change coefficient) in our ITS tissue samples. Amongst the upregulated genes, the greatest fold changes were seen in DEFB103A (fold increase: 10.24), DSG1 (8.52), and CASP14 (8.47). Amongst the downregulated genes, the greatest fold decreases were seen in C16orf82 (fold decrease: 125,487.85), PRB4 (10,386.22), and DPEP1 (2730.08). Figure 1 shows a volcano plot depicting the differential expression of all sequenced genes in ITS tissue samples compared to the normal controls. Figure 2 shows a heatmap depicting expression of the most highly upregulated and downregulated genes in each individual tissue sample. The KRT16, KRT14, and KRT6B were most consistently upregulated across samples, while C16orf82, PRB4, and MTRNR2L8 were most consistently downregulated.

Table 1.
Dysregulated genes in iatrogenic tracheal stenosis. ATM: Ataxia–Telangiectasia Mutated; C16orf82: Chromosome 16 open reading frame 82; C11orf65: Chromosome 11 open reading frame 65; CALML5: Calmodulin-like 5; CASP14: Caspase 14; CASP8: Caspase 8; CRABP2: Cellular Retinoic Acid Binding Protein 2; DEFB103A: Defensin beta 103A; DEFB4A: Defensin beta 4A; DPEP1: Dipeptidase 1; DSG1: Desmoglein 1; KRT16: Keratin 16; KRT14: Keratin 14; KRT6B: Keratin 6B; PRB4: Proline Rich Protein BstNI Subfamily 4; MTRNR2L8: Mitochondrially Encoded 16S RRNA-like 8; MTRNR2L12: Mitochondrially Encoded 16S RRNA-like 12; NPAT: Nuclear Protein, Coactivator Of Histone Transcription; PRB3: Proline Rich Protein BstNI Subfamily 3; S100A7: S100 Calcium Binding Protein A7.
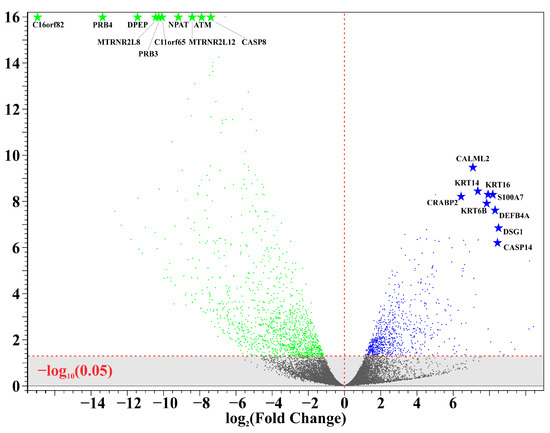
Figure 1.
Differential gene expression in iatrogenic tracheal stenosis.
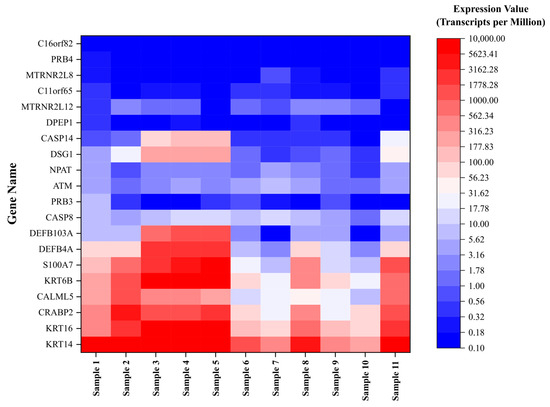
Figure 2.
Expression of dysregulated genes in iatrogenic tracheal stenosis.
Biological pathway analysis revealed that the changes in gene expression corresponded to increased keratinization and cell cycle progression/mitosis/cell division. The dysregulated gene expression was identified as most similar to various diseases of the epidermis, including palmoplantar keratoderma, ectodermal dysplasia, ichthyosis, hypotrichosis, and epidermolysis bullosa. The relevant functions of the most highly dysregulated genes, as shown in Table 2, reinforce the similarities to epidermal disease. Most of the upregulated genes have roles in keratinocyte differentiation (CALML5, S100A7, KRT16, KRT6B, KRT14 and CASP14) or chronic inflammation (S100A7, KRT16, DEFB4A, DEFB103A, and NPAT), while most of the downregulated genes have roles in cell cycle regulation and apoptosis (C11orf65, MTRNR2L8, PRB3, NPAT, MTRNR2L12, ATM, and CASP8). Retinoic acid (RA)-mediated cell signaling also appeared to be dysregulated, with ATM (a serine/threonine kinase that mediates gene expression of RA-dependent genes) being highly downregulated and CRABP2 (an RA cytosol-to-nuclear shuttle protein) being highly upregulated.

Table 2.
Potential regulatory agents for dysregulated genes. ATM: Ataxia–Telangiectasia Mutated; C16orf82: Chromosome 16 open reading frame 82; C11orf65: Chromosome 11 open reading frame 65; CALML5: Calmodulin-like 5; CASP14: Caspase 14; CASP8: Caspase 8; CRABP2: Cellular Retinoic Acid Binding Protein 2; DEFB103A: Defensin beta 103A; DEFB4A: Defensin beta 4A; DPEP1: Dipeptidase 1; DSG1: Desmoglein 1; KRT16: Keratin 16; KRT14: Keratin 14; KRT6B: Keratin 6B; PRB4: Proline Rich Protein BstNI Subfamily 4; MTRNR2L8: Mitochondrially Encoded 16S RRNA-like 8; MTRNR2L12: Mitochondrially Encoded 16S RRNA-like 12; NPAT: Nuclear Protein, Coactivator Of Histone Transcription; PRB3: Proline Rich Protein BstNI Subfamily 3; S100A7: S100 Calcium Binding Protein A7.
Table 2 also displays drugs that regulate the expression of the most highly dysregulated genes. Since no single drug adequately regulated all the dysregulated genes, we sought to identify FDA-approved drugs that regulated the greatest number of genes. Members of the retinoid family appeared to regulate seven of the upregulated (CALML5, S100A7, KRT16, DEFB4A, KRT14, DSG1 and CRABP2) and two of the downregulated genes (ATM and CASP8). Valproic acid regulated three of the upregulated (CALML5, DEFB103A, and CRABP2) and five of the downregulated genes (C16orf82, DPEP1, NPAT, ATM and CASP8).
4. Discussion
Our study reports patterns of gene expression in ITS. RNA sequencing analysis indicated an upregulation of genes and pathways involved in keratinization and chronic inflammation, and downregulation of genes involved in cell cycle regulation. Our results also indicated cellular non-responsiveness to retinoic acid. Subsequently, retinoic acid also emerged as a promising potential therapeutic option for the management of ITS.
To the best of our knowledge, our study is the first to demonstrate the role of dysregulated keratinization in the development of ITS. Keratinization is a process that occurs predominantly in the skin, whereby keratinocytes migrate from the stratum basalis to the stratum corneum to form the keratinized, stratified surface epithelium of the skin. Keratinocytes produce and secrete keratin, a protein that forms intermediate filaments serving structural and barrier-forming roles in the skin. Keratin is also produced by non-skin epithelial cells in the body, such as the cells lining the lumens of the aerodigestive system, where it is involved in cell signaling, cell transport, and maintaining cytoskeletal integrity [13]. However, abnormally increased keratin production (hyperkeratinization) may rarely occur as a result of sustained cellular damage to aerodigestive epithelial surfaces. In the oral cavity, keratinization may occur in reactive lesions (e.g., frictional keratosis, nicotine stomatitis, and hairy leukoplakia), immune-mediated lesions (e.g., lichen planus, and discoid lupus erythematosus), pre-neoplastic and neoplastic diseases (e.g., leukoplakia, proliferative verrucous leukoplakia, and squamous cell carcinoma), and infections (e.g., squamous cell papilloma, verruca vulgaris, and condyloma acuminatum). Esophageal hyperkeratinization may be an uncommon manifestation of chronic gastric acid-induced epithelial damage seen in gastroesophageal reflux disease (GERD) [14,15]. Similarly, tracheobronchial hyperkeratinization has been reported as a possible result of chronic exposure to cigarette smoke or other inhaled environmental pollutants [16]. Squamous metaplasia of the mucociliary airway epithelium, which lends itself to keratinization, has also been described as a potential sequela of high-pressure airway cuffs [17]. Hyperkeratinization processes result in significant alterations to the gross appearance (skin-like lesions) and function of normal epithelial surfaces. In the trachea, this aberrant hyperkeratotic, hyperproliferative tissue can occlude the airway lumen and result in airway stenosis.
The tissue samples in our study also showed evidence of chronic inflammation and dysregulation of cell-cycle and apoptotic pathways. These findings are supported by the previous literature that demonstrates chronic inflammatory processes orchestrated by T-helper 2 cells, M2 macrophages, and fibroblasts [18]. Key molecular players that have been described include TGF-β [19,20], and interleukins-4 (IL-4), -6 (IL-6), and -17 (IL-17) [18]. We found the DEFB103A (defensin β 103A) and DEFB4A (defensin β 4A) genes to be highly upregulated in our samples. β defensins are a family of antimicrobial proteins that provide innate immunity at the aerodigestive mucosal surfaces, and their dysregulated expression may account for persistent chemotactic recruitment of immune cells and chronic inflammation [21]. Cell-cycle dysregulation has also been previously described as a key pathophysiological mechanism driving the hyperproliferative processes observed in ITS [22].
In our search for potential therapeutic for tracheal stenosis, retinoic acid emerged as the top candidate given its ability to regulate several of the most highly dysregulated genes. Retinoic acid is well-known for its role in maintaining epithelial integrity and promoting mucosal immunity [23]. Topical retinoic acid can improve the regeneration of a mucociliary respiratory epithelium after iatrogenic mucosal injury [24]. The genetic alterations in our study point towards a potential loss of adequate cellular response to retinoic acid. ATM (ataxia–telangiectasia mutated) is a serine threonine kinase that is involved in several intracellular processes, including mediating cellular responses to retinoic acid [25]. It follows that the upregulation of CRABP2 (cellular retinoic acid binding protein 2; a cytosol-to-nucleus shuttle protein for retinoic acid) was present as a compensatory mechanism for the cellular non-responsiveness to retinoic acid. Interestingly, retinoic acid itself may help promote the kinase activity of ATM [26]. These findings provide further support for our case for retinoic acid as a therapeutic agent for ITS. Retinoic acid has been used to treat disorders of hyperkeratinization of the skin by virtue of its ability to favorably regulate keratin expression (including KRT6B, KRT14, and KRT16 [27], which were amongst the most highly dysregulated genes in our samples). Moreover, retinoic acid has also been shown to downregulate overexpression of DSG1 (desmoglein-1), which was noted in our samples, and thus promote dissolution of excessive inter-cellular adhesions [28]. Thus, the regulatory acceptance of retinoic acid and its ability to favorably modulate multiple dysregulated pathways substantiate its position as a prime candidate for future testing in ITS disease models.
Our findings of retinoic acid as a promising potential therapeutic agent for the prevention and treatment of tracheal stenosis has exciting implications beyond its use for ITS. Our previous work shows that COVID-19 has the potential to contribute towards the pathophysiology of tracheal stenosis in patients undergoing airway interventions [10]. Interestingly, even with this COVID-19-associated tracheal stenosis, several relevant retinoic acid-related dysregulations are evident. CRABP1 (cellular retinoic acid-binding protein 1: inhibits retinoic acid’s activity) is significantly downregulated, while CRABP2 and RBP1 (retinol-binding protein 1 facilitates enzymatic conversion of retinol to retinoic acid) are significantly upregulated. Enzymatic conversion of retinoid precursors to physiologically active retinoic acid was also suppressed, as evidenced by significant downregulations in RALDH2/ALDH1A2 [10].
Finally, the tracheal stenosis-mitigating effects of retinoic acid can be extended to more experimental applications, such as tracheal graft bioengineering. While these investigational grafts were shown to remain patent and effective beyond the immediate postoperative period, their permanent success was precluded by the development of tracheal granulation tissue causing stenosis and luminal occlusion [29,30,31,32,33,34]. Molecular analysis of the tracheal granulation tissue from the stenotic grafts in the porcine pre-clinical models revealed dysregulations in cellular pathways including inflammation and extracellular matrix remodeling [35]. Further work in this field may reveal a role for retinoic acid to assist in the mitigation and tracheal stenosis in the field of airway bioengineering and transplantation.
This study has limitations. First, this study presented data from only 11 samples. Second, our work discussed the biological plausibility of potential therapeutic options, and future work is required to evaluate actual benefits of these drugs. Third, our work was restricted to genomic analysis; complementary proteomic and histologic analysis would further validate our conclusions.
5. Conclusions
Our study demonstrates patterns of gene expression in ITS that indicate an upregulation of genes and pathways involved in keratinization and chronic inflammation, and downregulation of genes involved in cell cycle regulation. Our results also indicated cellular non-responsiveness to retinoic acid due to dysregulations in RA metabolism and signaling. Given the ability of RA to favorably regulate key genes involved in ITS, future studies must explore its efficacy as a potential therapeutic option for patients with recalcitrant ITS.
Author Contributions
Conceptualization, R.S.M., J.W. and F.Y.B.; data curation, J.W.; formal analysis, R.S.M., J.W. and J.L.; funding acquisition, F.Y.B.; investigation, R.S.M., J.W., A.H.A.S., R.S.L. and F.Y.B.; methodology, R.S.M., J.W., A.H.A.S., R.S.L. and F.Y.B.; project administration, F.Y.B.; resources, F.Y.B.; software, J.W.; supervision, F.Y.B.; validation, R.S.M., J.W. and J.L.; visualization, R.S.M. and J.L.; writing—original draft, R.S.M. and J.W.; writing—review and editing, R.S.M., J.W., B.J., J.L., K.P., M.J.L., S.S.R., A.H.A.S., R.S.L. and F.Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding. Faiz Y. Bhora has consulting relationships with the following, that are unrelated to this study: AstraZeneca, Genentech, Biodesix, Johnson & Johnson/Ethicon, Boston Scientific, and Medtronic. The other authors have no relevant financial disclosures.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Nuvance Health (protocol code: 2019-19 in June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. (Dataset contains confidential genetic data from patients).
Conflicts of Interest
Faiz Bhora has consulting relationships with the following, which are unrelated to this study: AstraZeneca, Genentech, Biodesix, Johnson & Johnson/Ethicon, Boston Scientific, and Medtronic. The other authors declare no conflicts of interest.
References
- Gelbard, A.; Francis, D.O.; Sandulache, V.C.; Simmons, J.C.; Donovan, D.T.; Ongkasuwan, J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015, 125, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.R.; Kimura, K.S.; Campbell, B.R.; Lowery, A.S.; Wootten, C.T.; Garrett, C.G.; Francis, D.O.; Hillel, A.T.; Du, L.; Casey, J.D.; et al. Incidence and Outcomes of Acute Laryngeal Injury After Prolonged Mechanical Ventilation. Crit. Care Med. 2019, 47, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Farzanegan, R.; Farzanegan, B.; Zangi, M.; Eraghi, M.G.; Noorbakhsh, S.; Tabarestani, N.D.; Shadmehr, M.B. Incidence Rate of Post-Intubation Tracheal Stenosis in Patients Admitted to Five Intensive Care Units in Iran. Iran. Red Crescent Med. J. 2016, 18, e37574. [Google Scholar] [CrossRef] [PubMed]
- Bibas, B.J.; Cardoso, P.F.G.; Salati, M.; Minamoto, H.; Tamagno, M.F.L.; Terra, R.M.; Pêgo-Fernandes, P.M. Health-related quality of life evaluation in patients with non-surgical benign tracheal stenosis. J. Thorac. Dis. 2018, 10, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- Siciliani, A.; Rendina, E.A.; Ibrahim, M. State of the art in tracheal surgery: A brief literature review. Multidiscip. Respir. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Mandour, M.; Remacle, M.; Van de Heyning, P.; Elwany, S.; Tantawy, A.; Gaafar, A. Chronic subglottic and tracheal stenosis: Endoscopic management vs. surgical reconstruction. European archives of oto-rhino-laryngology: Official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2003, 260, 374–380. [Google Scholar]
- Almanzar, A.; Danckers, M. Laryngotracheal Stenosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hosna, A.; ul Rasool, M.H.; Noff, N.C.; Makhoul, K.; Miller, D.; Umar, Z.; Ghallab, M.; Hasan, R.; Ashfaq, S.; Parikh, A.; et al. Cryotherapy for the Treatment of Tracheal Stenosis: A Systematic Review. Cureus 2023, 15, e41012. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, A.; Tonelli, R.; Andreani, A.; Cappiello, G.F.; Fermi, M.; Trentacosti, F.; Castaniere, I.; Fantini, R.; Tabbì, L.; Andrisani, D.; et al. Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults. Int. J. Mol. Sci. 2022, 23, 2421. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.S.; Weber, J.; Poulikidis, K.; Shetawi, A.H.A.; Latif, M.J.; Razi, S.S.; Lebovics, R.S.; Bhora, F.Y. Gene expression profiles in COVID-19-associated tracheal stenosis indicate persistent anti-viral response and dysregulated retinol metabolism. BMC Res. Notes 2024, 17, 140. [Google Scholar] [CrossRef]
- Uhl, M.; Fagerberg, L.; Hallstr, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar]
- Vedi, M.; Smith, J.R.; Thomas Hayman, G.; Tutaj, M.; Brodie, K.C.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Kaldunski, M.L.; Lamers, L.; et al. 2022 updates to the Rat Genome Database: A Findable, Accessible, Interoperable, and Reusable (FAIR) resource. Genetics 2023, 224, iyad042. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, M.M.; Kanojia, D. Keratins: Markers of cell differentiation or regulators of cell differentiation? J. Biosci. 2007, 32, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kisloff, B.; McGrath, K.M.; Davison, J.M. Esophageal Hyperkeratosis in a Healthy Adult. Clin. Gastroenterol. Hepatol. 2011, 9, A20. [Google Scholar] [CrossRef] [PubMed]
- Taggart, M.W.; Rashid, A.; Ross, W.A.; Abraham, S.C. Oesophageal hyperkeratosis: Clinicopathological associations. Histopathology 2013, 63, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Isolated Tracheobronchial Hyperkeratosis. CHEST 2016, 149, A425. [Google Scholar] [CrossRef]
- Paegle, R.D.; Bernhard, W.N. Squamous metaplasia of tracheal epithelium associated with high-volume, low pressure airway cuffs. Anesth. Analg. 1975, 54, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Hillel, A.T. Inflammatory pathways in the pathogenesis of iatrogenic laryngotracheal stenosis: What do we know? Transl. Cancer Res. 2020, 9, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, K.A.; Miller, F.; April, M.M.; Gruber, B.L. Growth factors in subglottic stenosis. Ann. Otol. Rhinol. Laryngol. 1996, 105, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Velehorschi, V.; Obertrifter, B.; Macha, H.N.; Linder, A.; Freitag, L. High-level expression of matrix-associated transforming growth factor-beta1 in benign airway stenosis. CHEST 2006, 129, 1298–1304. [Google Scholar] [CrossRef]
- Jarczak, J.; Kościuczuk, E.M.; Lisowski, P.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Defensins: Natural component of human innate immunity. Hum. Immunol. 2013, 74, 1069–1079. [Google Scholar] [CrossRef]
- Li, X.Z.; Wang, Z.C.; Qiu, Y.; Ma, S.X.; Meng, L.B.; Wu, W.H.; Zhang, P.; Yang, W.; Song, W.P.; Huang, L. Bioinformatics analysis and verification of gene targets for benign tracheal stenosis. Mol. Genet. Genom. Med. 2020, 8, e1245. [Google Scholar] [CrossRef] [PubMed]
- McCullough, F.S.; Northrop-Clewes, C.A.; Thurnham, D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999, 58, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.M.; Wang, C.T.; Chen, Y.W.; Huang, T.W. Reduction of adhesions and antrostomy stenosis with topical vitamin A after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2015, 29, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Kawaguchi, M.; Suzuki, M.; Jung, C.G.; Asai, K.; Shibamoto, Y.; Lavin, M.F.; Khanna, K.K.; Miura, Y. The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress. Dis. Models Mech. 2010, 3, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.D.; Sun, Y.; Price, B.D. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J. Biol. Chem. 2007, 282, 16577–16584. [Google Scholar] [CrossRef]
- Törmä, H. Regulation of keratin expression by retinoids. Derm.-Endocrinol. 2011, 3, 136–140. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, S.E.; Chang, J.Y.; Kim, S.C. Retinoid Induces the Degradation of Corneodesmosomes and Downregulation of Corneodesmosomal Cadherins: Implications on the Mechanism of Retinoid-induced Desquamation. Ann. Dermatol. 2011, 23, 439–447. [Google Scholar] [CrossRef]
- Al-Ayoubi, A.M.; Rehmani, S.S.; Sinclair, C.F.; Lebovics, R.S.; Bhora, F.Y. Reconstruction of anterior tracheal defects using a bioengineered graft in a porcine model. Ann. Thorac. Surg. 2017, 103, 381–389. [Google Scholar] [CrossRef]
- Al Shetawi, A.H.; Weber, J.; Baig, M.Z.; Muslim, Z.; Bhora, F. Advances in tracheal reconstruction and tissue engineering. Plast. Aesthetic Res. 2021, 8, 38. [Google Scholar] [CrossRef]
- Bhora, F.Y.; Lewis, E.E.; Rehmani, S.S.; Ayub, A.; Raad, W.; Al-Ayoubi, A.M.; Lebovics, R.S. Circumferential three-dimensional–printed tracheal grafts: Research model feasibility and early results. Ann. Thorac. Surg. 2017, 104, 958–963. [Google Scholar] [CrossRef]
- Rehmani, S.S.; Al-Ayoubi, A.M.; Ayub, A.; Barsky, M.; Lewis, E.; Flores, R.; Lebovics, R.; Bhora, F.Y. Three-dimensional-printed bioengineered tracheal grafts: Preclinical results and potential for human use. Ann. Thorac. Surg. 2017, 104, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.F.; Rehmani, S.S.; Baig, M.Z.; Jadoon, Y.; Bhora, F.Y. Successes and failures in tracheal bioengineering: Lessons learned. Ann. Thorac. Surg. 2021, 112, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.F.; Rehmani, S.S.; Baig, M.Z.; Lebovics, R.; Raad, W.; Connery, C.; Bhora, F.Y. Novel composite trachea grafts using 3-dimensional printing. JTCVS Open 2021, 5, 152–160. [Google Scholar] [CrossRef]
- Weber, J.; Martins, R.S.; Muslim, Z.; Baig, M.Z.; Poulikidis, K.; Bhora, F.Y. Anastomotic stenosis of bioengineered trachea grafts is driven by transforming growth factor β1-induced signaling, proinflammatory macrophages, and delayed epithelialization. JTCVS Open 2023, 15, 489–496. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).