Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications
Abstract
:1. Introduction
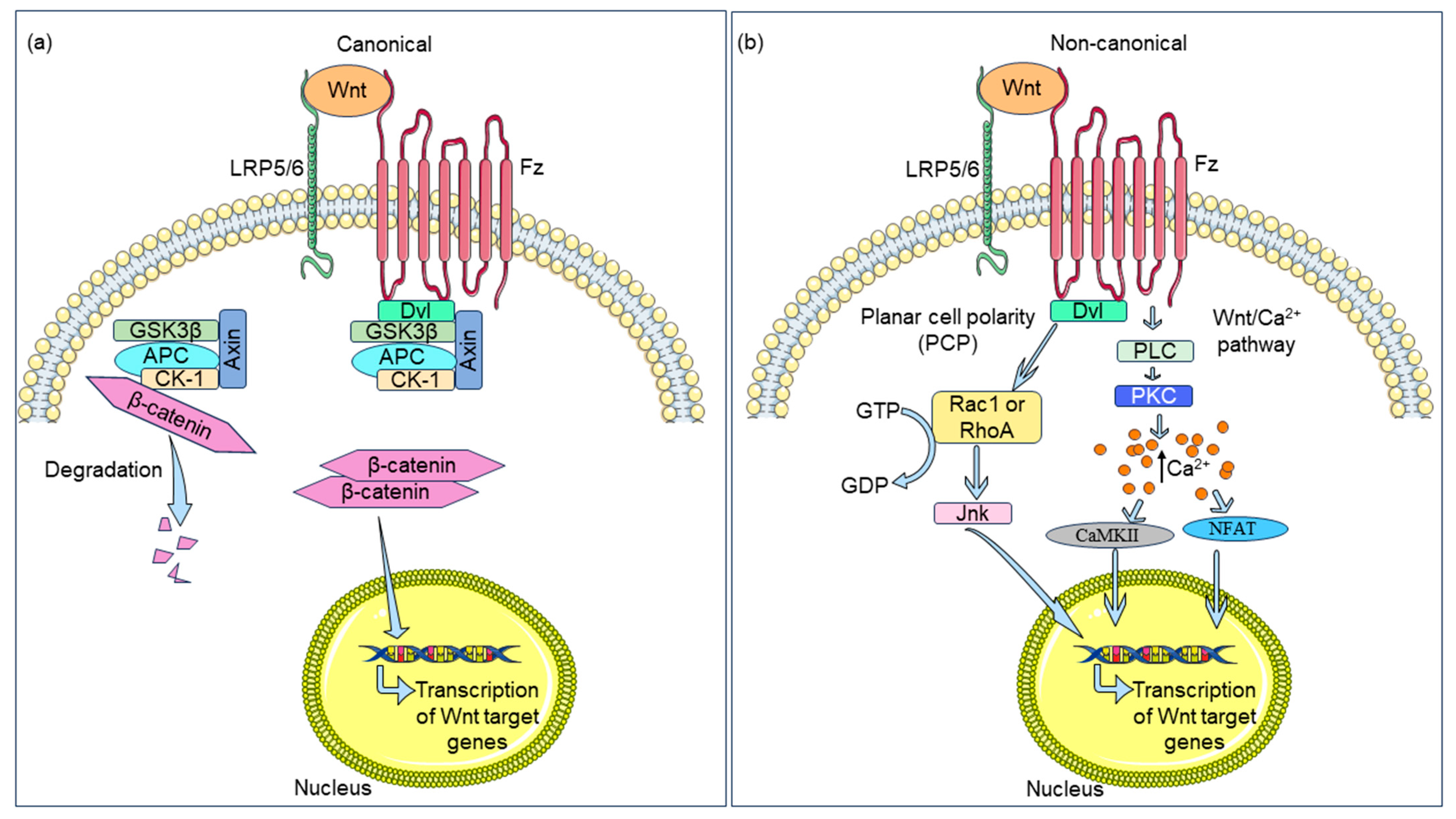
2. The Wnt Signaling Axis
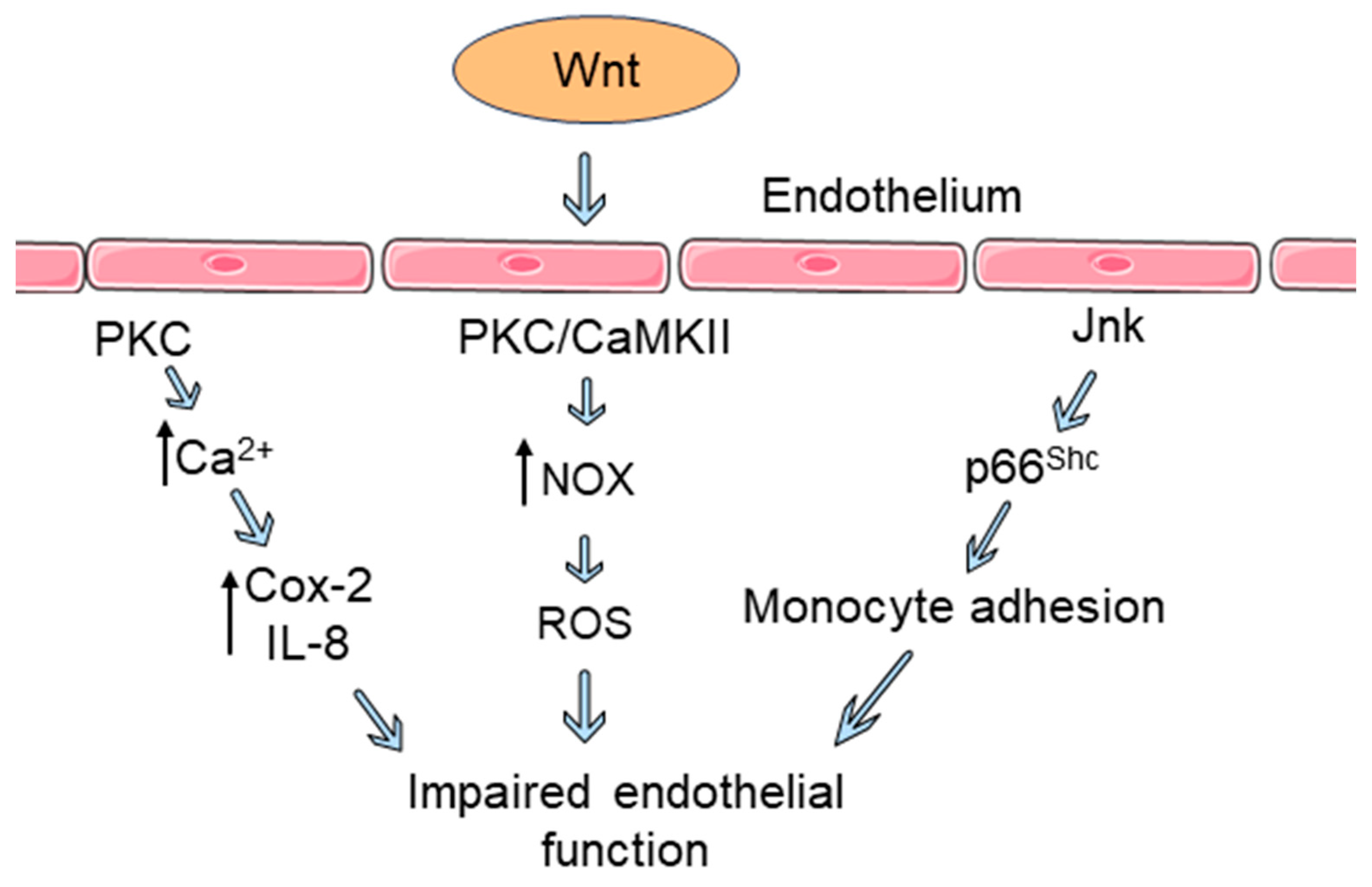
3. Wnt Signaling Influences Endothelial Dysfunction
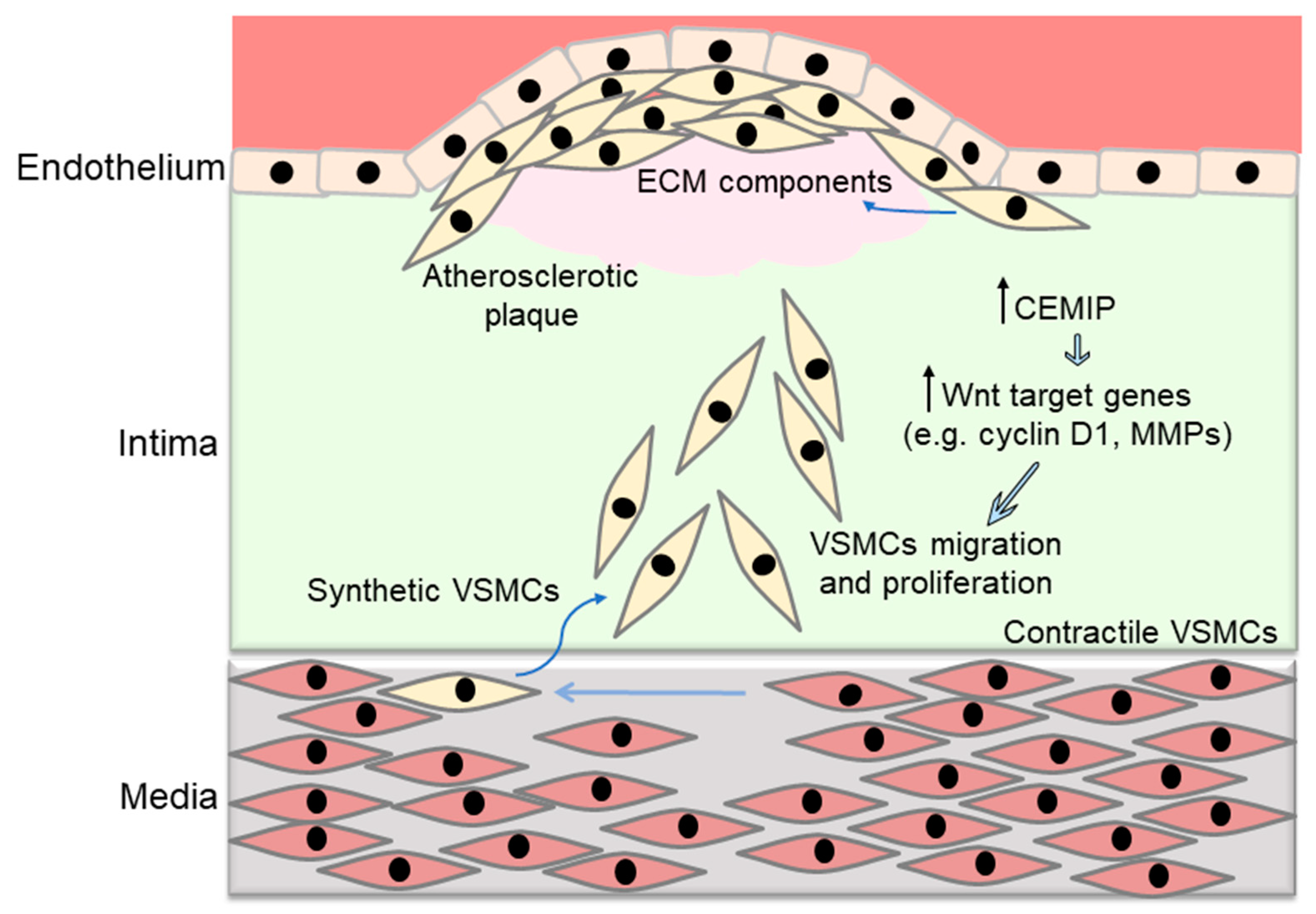
4. Wnt Pathway Regulates Vascular Smooth Muscle Cell Proliferation and Migration
5. Wnt Signaling in Macrophages
6. The Wnt Pathway in Cholesterol Storage and Trafficking
7. Targeting Wnt Pathways in Atherosclerosis: Therapeutic Opportunities and Challenges
7.1. Potential Strategies to Target Wnt Pathway
| Compound | Target(s) | Effect(s) |
|---|---|---|
| GNF-6231 | Wnt ligands | Temporary and systemic inhibition of the Wnt/β-catenin pathway in the infarcted heart and improvement of post-MI recovery [51] |
| XAV939 | Tankyrase | Inhibits nuclear translocation of β-catenin and suppresses proliferation, migration, and apoptosis of VSMCs [53] |
| Suppresses hyperactivation of the Wnt/β-catenin pathway in infarcted heart [54] | ||
| LGK974 | Wnt ligands | Decreases ECM deposition and collagen accumulation, restores endothelial GR levels, and suppresses the expression of inflammatory cytokines [55] |
| Pyrvinium | CK1α | Promotes wound repair, post-MI cardiac remodeling [64], and improves cardiac dysfunction [65] |
| SFRP4 | Interaction between β-catenin and CD24 | Decreases aortic lipid deposition, suppresses inflammatory cytokines, and downregulates Nox activity [68] |
| Reduces fibrotic scar size and ameliorates cardiac function after ischemic injury [67] | ||
| Sclerostin | Interaction between LRP5/6 and Fz | Provides protection against atherosclerosis and inflammation through reduction of aortic lipid deposition and downregulation of inflammatory cytokines |
7.2. Wnt Signaling Components as Therapeutic Targets
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blankesteijn, W.M.; Hermans, K.C. Wnt signaling in atherosclerosis. Eur. J. Pharmacol. 2015, 763, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Radhakrishnan, J.; Wang, H.; Mani, A.; Mani, M.-A.; Nelson-Williams, C.; Carew, K.S.; Mane, S.; Najmabadi, H.; Wu, D. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007, 315, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Salvi, F.; Bordicchia, M.; Guerra, F.; Battistoni, I.; Pagliariccio, G.; Carbonari, L.; Dessì-Fulgheri, P.; Rappelli, A. Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 150–156. [Google Scholar] [CrossRef]
- Christman, M.A.; Goetz, D.J.; Dickerson, E.; McCall, K.D.; Lewis, C.J.; Benencia, F.; Silver, M.J.; Kohn, L.D.; Malgor, R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2864–H2870. [Google Scholar] [CrossRef]
- Malgor, R.; Bhatt, P.M.; Connolly, B.A.; Jacoby, D.L.; Feldmann, K.J.; Silver, M.J.; Nakazawa, M.; McCall, K.D.; Goetz, D.J. Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic lesions. Inflamm. Res. 2014, 63, 277–285. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Peifer, M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harb. Perspect. Biol. 2009, 1, a002881. [Google Scholar] [CrossRef]
- Vikram, A.; Kim, Y.-R.; Kumar, S.; Naqvi, A.; Hoffman, T.A.; Kumar, A.; Miller Jr, F.J.; Kim, C.-S.; Irani, K. Canonical Wnt signaling induces vascular endothelial dysfunction via p66Shc-regulated reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2301–2309. [Google Scholar] [CrossRef]
- Dejana, E. The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef]
- Du, J.; Li, J. The role of Wnt signaling pathway in atherosclerosis and its relationship with angiogenesis. Exp. Ther. Med. 2018, 16, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Manukjan, N.; Ahmed, Z.; Fulton, D.; Blankesteijn, W.M.; Foulquier, S. A systematic review of WNT signaling in endothelial cell oligodendrocyte interactions: Potential relevance to cerebral small vessel disease. Cells 2020, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Boucher, P.; Matz, R.L.; Terrand, J. atherosclerosis: Gone with the Wnt? Atherosclerosis 2020, 301, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Polkinghorne, M.; Antoniades, C. Non-canonical WNT signalling in cardiovascular disease: Mechanisms and therapeutic implications. Nat. Rev. Cardiol. 2022, 19, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wadey, K.S.; Somos, A.; Leyden, G.; Blythe, H.; Chan, J.; Hutchinson, L.; Poole, A.; Frankow, A.; Johnson, J.L.; George, S.J. Pro-inflammatory role of Wnt/β-catenin signaling in endothelial dysfunction. Front. Cardiovasc. Med. 2023, 9, 1059124. [Google Scholar] [CrossRef]
- Farb, M.G.; Karki, S.; Park, S.-Y.; Saggese, S.M.; Carmine, B.; Hess, D.T.; Apovian, C.; Fetterman, J.L.; Bretón-Romero, R.; Hamburg, N.M. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc. Med. 2016, 21, 489–496. [Google Scholar] [CrossRef]
- Kühl, M.; Sheldahl, L.C.; Malbon, C.C.; Moon, R.T. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 2000, 275, 12701–12711. [Google Scholar] [CrossRef]
- Weerackoon, N.; Gunawardhana, K.L.; Mani, A. Wnt signaling cascades and their role in coronary artery health and disease. J. Cell. Signal. 2021, 2, 52. [Google Scholar]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.; Deb, A.; Blankesteijn, W.M. WNT signaling in cardiac and vascular disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Bretón-Romero, R.; Feng, B.; Holbrook, M.; Farb, M.G.; Fetterman, J.L.; Linder, E.A.; Berk, B.D.; Masaki, N.; Weisbrod, R.M.; Inagaki, E. Endothelial dysfunction in human diabetes is mediated by Wnt5a–JNK signaling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Kim, D.W.; Ha, Y.; Ihm, M.H.; Kim, H.; Song, K.; Lee, I. Wnt5a induces endothelial inflammation via β-catenin–independent signaling. J. Immunol. 2010, 185, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 causes hyperpermeability of vascular endothelial cells through Wnt5A signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef] [PubMed]
- Skaria, T.; Bachli, E.; Schoedon, G. Wnt5A/Ryk signaling critically affects barrier function in human vascular endothelial cells. Cell Adhes. Migr. 2017, 11, 24–38. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Ren, Y.-H.; Sun, Z.; Dong, J.; Yan, H.; Xu, Y.; Wang, D.W.; Zheng, G.-Y.; Du, J. Mutant LRP6 impairs endothelial cell functions associated with familial normolipidemic coronary artery disease. Int. J. Mol. Sci. 2016, 17, 1173. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.A.; Liebner, S.; Gerhardt, H. Vascular morphogenesis: A Wnt for every vessel? Curr. Opin. Genet. Dev. 2009, 19, 476–483. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.-P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Johnson, J.D.; Deem, T.L.; Ochi, A.; Wang, L.; Zheng, Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004, 378, 539–547. [Google Scholar] [CrossRef]
- Fontayne, A.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A.; El Benna, J. Phosphorylation of p47 p hox Sites by PKC α, βΙΙ, δ, and ζ: Effect on Binding to p22 p hox and on NADPH Oxidase Activation. Biochemistry 2002, 41, 7743–7750. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Popov, M.A.; Chegodaev, Y.S.; Postnov, A.Y.; Orekhov, A.N. Mechanisms of the Wnt Pathways as a Potential Target Pathway in Atherosclerosis. J. Lipid Atheroscler. 2023, 12, 223. [Google Scholar] [CrossRef]
- Tsaousi, A.; Williams, H.; Lyon, C.A.; Taylor, V.; Swain, A.; Johnson, J.L.; George, S.J. Wnt4/-Catenin Signaling Induces VSMC Proliferation and Is Associated with Intimal Thickening. Circ. Res. 2011, 108, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Mao, J.Q.; Yu, M.; Dong, L.Y.; Fan, Y.L.; Lv, Z.Q.; Xiao, M.D.; Yuan, Z.X. Hyperlipidemia induces vascular smooth muscle cell proliferation involving Wnt/β-catenin signaling. Cell Biol. Int. 2016, 40, 121–130. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.; Li, Y.; Kong, X.; Yang, K.; Li, Z.; Lao, W.; Li, J.; Zhong, J.; Zhang, S. Research on the biological mechanism and potential application of CEMIP. Front. Immunol. 2023, 14, 1222425. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, X.; Deng, X.; Huang, Y.; Tian, W. CEMIP regulates the proliferation and migration of vascular smooth muscle cells in atherosclerosis through the WNT–beta-catenin signaling pathway. Biochem. Cell Biol. 2020, 98, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ackers, I.; Szymanski, C.; Silver, M.J.; Malgor, R. Oxidized low-density lipoprotein induces WNT5A signaling activation in THP-1 derived macrophages and a human aortic vascular smooth muscle cell line. Front. Cardiovasc. Med. 2020, 7, 567837. [Google Scholar] [CrossRef]
- Marchand, A.; Atassi, F.; Gaaya, A.; Leprince, P.; Le Feuvre, C.; Soubrier, F.; Lompré, A.M.; Nadaud, S. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell 2011, 10, 220–232. [Google Scholar] [CrossRef]
- Eken, S.M.; Jin, H.; Chernogubova, E.; Li, Y.; Simon, N.; Sun, C.; Korzunowicz, G.; Busch, A.; Bäcklund, A.; Österholm, C. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ. Res. 2017, 120, 633–644. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Malgor, R. Wnt5a: A player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis 2014, 237, 155–162. [Google Scholar] [CrossRef]
- Shao, Y.; Zheng, Q.; Wang, W.; Xin, N.; Song, X.; Zhao, C. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 2016, 7, 67674. [Google Scholar] [CrossRef] [PubMed]
- Schaale, K.; Neumann, J.; Schneider, D.; Ehlers, S.; Reiling, N. Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur. J. Cell Biol. 2011, 90, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Borrell-Pages, M.; Romero, J.C.; Juan-Babot, O.; Badimon, L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur. Heart J. 2011, 32, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Vossio, S.; Vacca, F.; Snijder, B.; Larios, J.; Schaad, O.; Guex, N.; Kuznetsov, D.; Martin, O.; Chambon, M. Wnt directs the endosomal flux of LDL-derived cholesterol and lipid droplet homeostasis. EMBO Rep. 2015, 16, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Go, G.-w.; Srivastava, R.; Hernandez-Ono, A.; Gang, G.; Smith, S.B.; Booth, C.J.; Ginsberg, H.N.; Mani, A. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab. 2014, 19, 209–220. [Google Scholar] [CrossRef]
- Ye, Z.-J.; Go, G.-W.; Singh, R.; Liu, W.; Keramati, A.R.; Mani, A. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J. Biol. Chem. 2012, 287, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mani, S.; Davis, N.R.; Sarrafzadegan, N.; Kavathas, P.B.; Mani, A. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ. Res. 2008, 103, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, Z.F.; Moon, R.T.; Chien, A.J. Targeting Wnt pathways in disease. Cold Spring Harb. Perspect. Biol. 2012, 4, a008086. [Google Scholar] [CrossRef]
- Blagodatski, A.; Poteryaev, D.; Katanaev, V.L. Targeting the Wnt pathways for therapies. Mol. Cell. Ther. 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Gay, A.; Towler, D.A. Wnt signaling in cardiovascular disease: Opportunities and challenges. Curr. Opin. Lipidol. 2017, 28, 387. [Google Scholar] [CrossRef]
- Bastakoty, D.; Saraswati, S.; Joshi, P.; Atkinson, J.; Feoktistov, I.; Liu, J.; Harris, J.L.; Young, P.P. Temporary, systemic inhibition of the WNT/β-catenin pathway promotes regenerative cardiac repair following myocardial infarct. Cell Stem Cells Regen. Med. 2016, 2. [Google Scholar] [CrossRef]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Chen, L.; Zhuang, J.; Singh, S.; Wang, K.; Xiong, M.; Xu, D.; Chen, W.; Pang, J.; Xu, Y.; Li, X. XAV939 inhibits intima formation by decreasing vascular smooth muscle cell proliferation and migration through blocking Wnt signaling. J. Cardiovasc. Pharmacol. 2016, 68, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Segersvärd, H.; Siren, J.; Perttunen, S.; Immonen, K.; Kosonen, R.; Chen, Y.-C.; Tolva, J.; Laivuori, M.; Mäyränpää, M.I. Tankyrase inhibition attenuates cardiac dilatation and dysfunction in ischemic heart failure. Int. J. Mol. Sci. 2022, 23, 10059. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Liu, B.; Kanasaki, K.; Koya, D.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Loss of endothelial glucocorticoid receptor accelerates diabetic nephropathy. Nat. Commun. 2021, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mehta, S.; Srivastava, S.P.; Grabinska, K.; Zhang, X.; Wong, C.; Hedayat, A.; Perrotta, P.; Fernández-Hernando, C.; Sessa, W.C. Endothelial cell–glucocorticoid receptor interactions and regulation of Wnt signaling. JCI Insight 2020, 5, e131384. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; Nevler, A. Pyrvinium pamoate: Past, present, and future as an anti-cancer drug. Biomedicines 2022, 10, 3249. [Google Scholar] [CrossRef]
- Rodgers, S.J.; Ooms, L.M.; Mitchell, C.A. The FDA-Approved Drug Pyrvinium Selectively Targets ER+ Breast Cancer Cells with High INPP4B Expression. Cancers 2022, 15, 135. [Google Scholar] [CrossRef]
- Li, B.; Flaveny, C.A.; Giambelli, C.; Fei, D.L.; Han, L.; Hang, B.I.; Bai, F.; Pei, X.-H.; Nose, V.; Burlingame, O. Repurposing the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic agent for intestinal polyposis. PLoS ONE 2014, 9, e101969. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Uthe, F.-W.; Hüttenrauch, M.; Mühling, B.; Linnebacher, M.; Krummenast, F.; Germer, C.-T.; Thalheimer, A.; Otto, C. The impact of pyrvinium pamoate on colon cancer cell viability. Int. J. Color. Dis. 2014, 29, 1189–1198. [Google Scholar] [CrossRef]
- Xu, W.; Lacerda, L.; Debeb, B.G.; Atkinson, R.L.; Solley, T.N.; Li, L.; Orton, D.; McMurray, J.S.; Hang, B.I.; Lee, E. The antihelmintic drug pyrvinium pamoate targets aggressive breast cancer. PLoS ONE 2013, 8, e71508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lou, Y.; Zheng, X.; Wang, H.; Sun, J.; Dong, Q.; Han, B. Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des. Dev. Ther. 2015, 9, 2399–2407. [Google Scholar]
- Venugopal, C.; Hallett, R.; Vora, P.; Manoranjan, B.; Mahendram, S.; Qazi, M.A.; McFarlane, N.; Subapanditha, M.; Nolte, S.M.; Singh, M. Pyrvinium targets CD133 in human glioblastoma brain tumor–initiating cells. Clin. Cancer Res. 2015, 21, 5324–5337. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Alfaro, M.P.; Thorne, C.A.; Atkinson, J.; Lee, E.; Young, P.P. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS ONE 2010, 5, e15521. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Gupta, K.; Kumari, A.; Singh, G.; Pandey, S.; Singh, R. Wnt/β-catenin antagonist pyrvinium exerts cardioprotective effects in polymicrobial sepsis model by attenuating calcium dyshomeostasis and mitochondrial dysfunction. Cardiovasc. Toxicol. 2021, 21, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, J.; Du, Y.; Zhu, E.; Wang, Z.; Que, B.; Miao, H.; Shi, S.; Qin, X.; Zhao, Y. Human epicardial adipose tissue-derived and circulating secreted frizzled-related protein 4 (SFRP4) levels are increased in patients with coronary artery disease. Cardiovasc. Diabetol. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Matsushima, K.; Suyama, T.; Takenaka, C.; Nishishita, N.; Ikeda, K.; Ikada, Y.; Sawa, Y.; Jakt, L.M.; Mori, H.; Kawamata, S. Secreted frizzled related protein 4 reduces fibrosis scar size and ameliorates cardiac function after ischemic injury. Tissue Eng. Part A 2010, 16, 3329–3341. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Liang, Z.; Wang, M.; Hu, C.; Chang, C.; Shi, L.; Ji, Q.; Liu, L. Secreted frizzled-related protein 4 exerts anti-atherosclerotic effects by reducing inflammation and oxidative stress. Eur. J. Pharmacol. 2022, 923, 174901. [Google Scholar] [CrossRef]
- Guan, H.; Liu, T.; Liu, M.; Wang, X.; Shi, T.; Guo, F. SFRP4 Reduces Atherosclerosis Plaque Formation in ApoE Deficient Mice. Cardiol. Res. Pract. 2023, 2023, 8302289. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Seto, S.-W.; Jose, R.J.; Li, J.; Morton, S.K.; Biros, E.; Wang, Y.; Nsengiyumva, V.; Lindeman, J.H.; Loots, G.G. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II–induced aortic aneurysm and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Laeremans, H.; Hackeng, T.M.; van Zandvoort, M.A.; Thijssen, V.L.; Janssen, B.J.; Ottenheijm, H.C.; Smits, J.F.; Blankesteijn, W.M. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation 2011, 124, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Uitterdijk, A.; Hermans, K.C.; de Wijs-Meijler, D.P.; Daskalopoulos, E.P.; Reiss, I.K.; Duncker, D.J.; Blankesteijn, W.M.; Merkus, D. UM206, a selective Frizzled antagonist, attenuates adverse remodeling after myocardial infarction in swine. Lab. Investig. 2016, 96, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Sanna, F.; Margaritis, M.; Badi, I.; Akawi, N.; Herdman, L.; Coutinho, P.; Fagan, H.; Antonopoulos, A.S.; Oikonomou, E.K. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl. Med. 2019, 11, eaav5055. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, H.; Shen, E.; Wan, L.; Arnold, J.M.O.; Peng, T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 2010, 59, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Vila-Petroff, M.; Salas, M.A.; Said, M.; Valverde, C.A.; Sapia, L.; Portiansky, E.; Hajjar, R.J.; Kranias, E.G.; Mundiña-Weilenmann, C.; Mattiazzi, A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia–reperfusion injury. Cardiovasc. Res. 2007, 73, 689–698. [Google Scholar] [CrossRef]
- He, Q.; Cheng, J.; Wang, Y. Chronic CaMKII inhibition reverses cardiac function and cardiac reserve in HF mice. Life Sci. 2019, 219, 122–128. [Google Scholar] [CrossRef]
- Oh, J.; Riek, A.E.; Zhang, R.M.; Williams, S.A.; Darwech, I.; Bernal-Mizrachi, C. Deletion of JNK2 prevents vitamin-D-deficiency-induced hypertension and atherosclerosis in mice. J. Steroid Biochem. Mol. Biol. 2018, 177, 179–186. [Google Scholar] [CrossRef]
- Kwok, K.H.; Cheng, K.K.; Hoo, R.L.; Ye, D.; Xu, A.; Lam, K.S. Adipose-specific inactivation of JNK alleviates atherosclerosis in apoE-deficient mice. Clin. Sci. 2016, 130, 2087–2100. [Google Scholar] [CrossRef]
- Willems, E.; Spiering, S.; Davidovics, H.; Lanier, M.; Xia, Z.; Dawson, M.; Cashman, J.; Mercola, M. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell–derived mesoderm. Circ. Res. 2011, 109, 360–364. [Google Scholar] [CrossRef]
- Zhang, Q.; Major, M.B.; Takanashi, S.; Camp, N.D.; Nishiya, N.; Peters, E.C.; Ginsberg, M.H.; Jian, X.; Randazzo, P.A.; Schultz, P.G. Small-molecule synergist of the Wnt/β-catenin signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 7444–7448. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-W.; Tadjuidje, E.; White, J.; Wells, J.; Mayhew, C.; Wylie, C.; Heasman, J. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr. Biol. 2009, 19, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afroz, R.; Goodwin, J.E. Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications. Biomedicines 2024, 12, 276. https://doi.org/10.3390/biomedicines12020276
Afroz R, Goodwin JE. Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications. Biomedicines. 2024; 12(2):276. https://doi.org/10.3390/biomedicines12020276
Chicago/Turabian StyleAfroz, Rizwana, and Julie E. Goodwin. 2024. "Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications" Biomedicines 12, no. 2: 276. https://doi.org/10.3390/biomedicines12020276
APA StyleAfroz, R., & Goodwin, J. E. (2024). Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications. Biomedicines, 12(2), 276. https://doi.org/10.3390/biomedicines12020276





