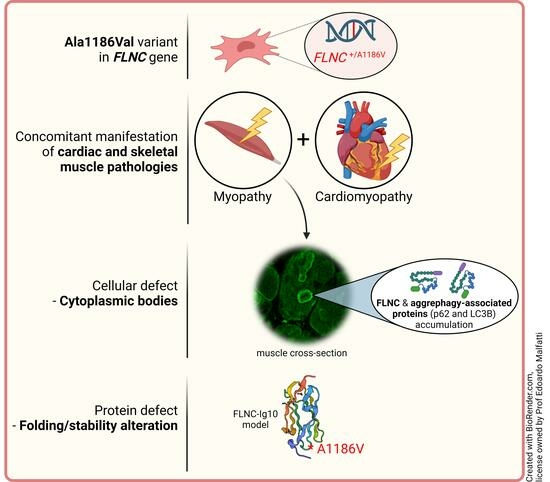
The FLNC Ala1186Val Variant Linked to Cytoplasmic Body Myopathy and Cardiomyopathy Causes Protein Instability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Targeted Gene Enrichment and Next-Generation Sequencing (NGS)
2.3. Bioinformatics Analysis
2.4. The Variant’s Interpretations
2.5. Protein Structure Analysis
2.6. Muscle Morphological and Protein Studies
2.6.1. Histochemistry and Immunohistochemistry
2.6.2. Immunofluorescence
2.6.3. Electron Microscopy
2.6.4. Western Blot
3. Results
3.1. Patients’ Characteristics
3.2. Muscle Biopsy Findings
3.3. Genetic Testing Results
3.4. Impact of the FLNC Variant in the Protein Tertiary Structure
3.5. FLNC Protein Expression in Muscle Biopsies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, T.G.; Chan, Y.-M.; Hack, A.A.; Brosius, M.; Rajala, M.; Lidov, H.G.W.; McNally, E.M.; Watkins, S.; Kunkel, L.M. Filamin 2 (FLN2): A Muscle-Specific Sarcoglycan Interacting Protein. J. Cell Biol. 2000, 148, 115–126. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, P.F.M.; Obermann, W.M.J.; Lemke, B.; Gautel, M.; Weber, K.; Fürst, D.O. Characterization of Muscle Filamin Isoforms Suggests a Possible Role of γ-Filamin/ABP-L in Sarcomeric Z-Disc Formation. Cell Motil. 2000, 45, 149–162. [Google Scholar] [CrossRef]
- van der Ven, P.F.M.; Wiesner, S.; Salmikangas, P.; Auerbach, D.; Himmel, M.; Kempa, S.; Hayeß, K.; Pacholsky, D.; Taivainen, A.; Schröder, R.; et al. Indications for a Novel Muscular Dystrophy Pathway: γ-Filamin, the Muscle-Specific Filamin Isoform, Interacts with Myotilin. J. Cell Biol. 2000, 151, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Nakamura, F. Structure and Function of Filamin C in the Muscle Z-Disc. Int. J. Mol. Sci. 2020, 21, 2696. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, M.; Gehmlich, K. Structural and Signaling Proteins in the Z-Disk and Their Role in Cardiomyopathies. Front. Physiol. 2023, 14, 1143858. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shi, A.; Lian, H.; Hu, S.; Nie, Y. Filamin C in Cardiomyopathy: From Physiological Roles to DNA Variants. Heart Fail. Rev. 2022, 27, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Vorgerd, M.; van der Ven, P.F.M.; Bruchertseifer, V.; Löwe, T.; Kley, R.A.; Schröder, R.; Lochmüller, H.; Himmel, M.; Koehler, K.; Fürst, D.O.; et al. A Mutation in the Dimerization Domain of Filamin C Causes a Novel Type of Autosomal Dominant Myofibrillar Myopathy. Am. J. Hum. Genet. 2005, 77, 297–304. [Google Scholar] [CrossRef]
- Duff, R.M.; Tay, V.; Hackman, P.; Ravenscroft, G.; McLean, C.; Kennedy, P.; Steinbach, A.; Schöffler, W.; van der Ven, P.F.M.; Fürst, D.O.; et al. Mutations in the N-Terminal Actin-Binding Domain of Filamin C Cause a Distal Myopathy. Am. J. Hum. Genet. 2011, 88, 729–740. [Google Scholar] [CrossRef]
- Kley, R.A.; Hellenbroich, Y.; van der Ven, P.F.M.; Fürst, D.O.; Huebner, A.; Bruchertseifer, V.; Peters, S.A.; Heyer, C.M.; Kirschner, J.; Schröder, R.; et al. Clinical and Morphological Phenotype of the Filamin Myopathy: A Study of 31 German Patients. Brain 2007, 130, 3250–3264. [Google Scholar] [CrossRef]
- Kiselev, A.; Vaz, R.; Knyazeva, A.; Khudiakov, A.; Tarnovskaya, S.; Liu, J.; Sergushichev, A.; Kazakov, S.; Frishman, D.; Smolina, N.; et al. De Novo Mutations in FLNC Leading to Early-Onset Restrictive Cardiomyopathy and Congenital Myopathy. Hum. Mutat. 2018, 39, 1161–1172. [Google Scholar] [CrossRef]
- Valdés-Mas, R.; Gutiérrez-Fernández, A.; Gómez, J.; Coto, E.; Astudillo, A.; Puente, D.A.; Reguero, J.R.; Álvarez, V.; Morís, C.; León, D.; et al. Mutations in Filamin C Cause a New Form of Familial Hypertrophic Cardiomyopathy. Nat. Commun. 2014, 5, 5326. [Google Scholar] [CrossRef] [PubMed]
- Golbus, J.R.; Puckelwartz, M.J.; Dellefave-Castillo, L.; Fahrenbach, J.P.; Nelakuditi, V.; Pesce, L.L.; Pytel, P.; McNally, E.M. Targeted Analysis of Whole Genome Sequence Data to Diagnose Genetic Cardiomyopathy. Circ. Cardiovasc. Genet. 2014, 7, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Ferrier, R.A.; Hamilton, S.J.; Greenway, S.C.; Brundler, M.-A.; Yu, W.; Gibson, W.T.; McKinnon, M.L.; McGillivray, B.; Alvarez, N.; et al. Mutations in FLNC Are Associated with Familial Restrictive Cardiomyopathy. Hum. Mutat. 2016, 37, 269–279. [Google Scholar] [CrossRef]
- Krahn, M.; Biancalana, V.; Cerino, M.; Perrin, A.; Michel-Calemard, L.; Nectoux, J.; Leturcq, F.; Bouchet-Séraphin, C.; Acquaviva-Bourdain, C.; Campana-Salort, E.; et al. A National French Consensus on Gene Lists for the Diagnosis of Myopathies Using Next-Generation Sequencing. Eur. J. Hum. Genet. 2019, 27, 349–352. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Page, R.C.; Clark, J.G.; Misra, S. Structure of Filamin A Immunoglobulin-like Repeat 10 from Homo Sapiens. Acta Cryst. F 2011, 67, 871–876. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Muravyev, A.; Vershinina, T.; Tesner, P.; Sjoberg, G.; Fomicheva, Y.; Čajbiková, N.N.; Kozyreva, A.; Zhuk, S.; Mamaeva, E.; Tarnovskaya, S.; et al. Rare Clinical Phenotype of Filaminopathy Presenting as Restrictive Cardiomyopathy and Myopathy in Childhood. Orphanet J. Rare Dis. 2022, 17, 358. [Google Scholar] [CrossRef]
- Baban, A.; Alesi, V.; Magliozzi, M.; Parlapiano, G.; Genovese, S.; Cicenia, M.; Loddo, S.; Lodato, V.; Di Chiara, L.; Fattori, F.; et al. Cardiovascular Involvement in Pediatric FLNC Variants: A Case Series of Fourteen Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 332. [Google Scholar] [CrossRef]
- Juntas Morales, R.; Perrin, A.; Solé, G.; Lacourt, D.; Pegeot, H.; Walther-Louvier, U.; Cintas, P.; Cances, C.; Espil, C.; Theze, C.; et al. An Integrated Clinical-Biological Approach to Identify Interindividual Variability and Atypical Phenotype-Genotype Correlations in Myopathies: Experience on A Cohort of 156 Families. Genes 2021, 12, 1199. [Google Scholar] [CrossRef]
- Matsumura, T.; Inoue, K.; Toyooka, K.; Inoue, M.; Iida, A.; Saito, Y.; Nishikawa, T.; Moriuchi, K.; Beck, G.; Nishino, I.; et al. Clinical Trajectory of a Patient with Filaminopathy Who Developed Arrhythmogenic Cardiomyopathy, Myofibrillar Myopathy, and Multiorgan Tumors. Neuromuscul. Disord. 2021, 31, 1282–1286. [Google Scholar] [CrossRef]
- Ghaoui, R.; Cooper, S.T.; Lek, M.; Jones, K.; Corbett, A.; Reddel, S.W.; Needham, M.; Liang, C.; Waddell, L.B.; Nicholson, G.; et al. Use of Whole-Exome Sequencing for Diagnosis of Limb-Girdle Muscular Dystrophy: Outcomes and Lessons Learned. JAMA Neurol. 2015, 72, 1424–1432. [Google Scholar] [CrossRef]
- Xiao, F.; Wei, Q.; Wu, B.; Liu, X.; Mading, A.; Yang, L.; Li, Y.; Liu, F.; Pan, X.; Wang, H. Clinical Exome Sequencing Revealed That FLNC Variants Contribute to the Early Diagnosis of Cardiomyopathies in Infant Patients. Transl. Pediatr. 2020, 9, 213–233. [Google Scholar] [CrossRef]
- Ravenscroft, G.; Clayton, J.S.; Faiz, F.; Sivadorai, P.; Milnes, D.; Cincotta, R.; Moon, P.; Kamien, B.; Edwards, M.; Delatycki, M.; et al. Neurogenetic Fetal Akinesia and Arthrogryposis: Genetics, Expanding Genotype-Phenotypes and Functional Genomics. J. Med. Genet. 2021, 58, 609–618. [Google Scholar] [CrossRef]
- Kley, R.A.; Serdaroglu-Oflazer, P.; Leber, Y.; Odgerel, Z.; van der Ven, P.F.M.; Olivé, M.; Ferrer, I.; Onipe, A.; Mihaylov, M.; Bilbao, J.M.; et al. Pathophysiology of Protein Aggregation and Extended Phenotyping in Filaminopathy. Brain 2012, 135, 2642–2660. [Google Scholar] [CrossRef]
- Kley, R.A.; Leber, Y.; Schrank, B.; Zhuge, H.; Orfanos, Z.; Kostan, J.; Onipe, A.; Sellung, D.; Güttsches, A.K.; Eggers, B.; et al. FLNC-Associated Myofibrillar Myopathy: New Clinical, Functional, and Proteomic Data. Neurol. Genet. 2021, 7, e590. [Google Scholar] [CrossRef]
- Evangelista, T.; Lornage, X.; Carlier, P.G.; Bassez, G.; Brochier, G.; Chanut, A.; Lacène, E.; Bui, M.-T.; Metay, C.; Oppermann, U.; et al. A Heterozygous Mutation in the Filamin C Gene Causes an Unusual Nemaline Myopathy With Ring Fibers. J. Neuropathol. Exp. Neurol. 2020, 79, 908–914. [Google Scholar] [CrossRef]
- de Stricker Borch, J.; Eisum, A.-S.V.; Krag, T.; Vissing, J. Expanding the Phenotype of Filamin-C-Related Myofibrillar Myopathy. Clin. Neurol. Neurosurg. 2019, 176, 30–33. [Google Scholar] [CrossRef]
- Fürst, D.O.; Goldfarb, L.G.; Kley, R.A.; Vorgerd, M.; Olivé, M.; van der Ven, P.F.M. Filamin C-Related Myopathies: Pathology and Mechanisms. Acta Neuropathol. 2013, 125, 33–46. [Google Scholar] [CrossRef]
- Schuelke, M.; Schwarz, M.; Stenzel, W.; Goebel, H.H. Cytoplasmic Body Myopathy Revisited. Neuromuscul. Disord. 2018, 28, 969–971. [Google Scholar] [CrossRef]
- Donkervoort, S.; Chan, S.H.S.; Hayes, L.H.; Bradley, N.; Nguyen, D.; Leach, M.E.; Mohassel, P.; Hu, Y.; Thangarajh, M.; Bharucha-Goebel, D.; et al. Cytoplasmic Body Pathology in Severe ACTA1-Related Myopathy in the Absence of Typical Nemaline Rods. Neuromuscul. Disord. 2017, 27, 531–536. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Pamphlett, R.; Burke, D.; Wills, E.J.; Kiernan, M.C. Cytoplasmic Body Myopathy Masquerading as Motor Neuron Disease. Muscle Nerve 2004, 30, 667–672. [Google Scholar] [CrossRef]
- Osborn, M.; Goebel, H.H. The Cytoplasmic Bodies in a Congenital Myopathy Can Be Stained with Antibodies to Desmin, the Muscle-Specific Intermediate Filament Protein. Acta Neuropathol. 1983, 62, 149–152. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.; van Hessem, L.; Jongbloed, J.; de Walle, H.; Capetanaki, Y.; van der Kooi, A.; van Langen, I.; van den Berg, M.; van Tintelen, J. Desmin-Related Myopathy. Clin. Genet. 2011, 80, 354–366. [Google Scholar] [CrossRef]
- Goebel, H.H. Desmin-Related Neuromuscular Disorders. Muscle Nerve 1995, 18, 1306–1320. [Google Scholar] [CrossRef]
- Fichna, J.P.; Potulska-Chromik, A.; Miszta, P.; Redowicz, M.J.; Kaminska, A.M.; Zekanowski, C.; Filipek, S. A Novel Dominant D109A CRYAB Mutation in a Family with Myofibrillar Myopathy Affects αB-Crystallin Structure. BBA Clin. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Brodehl, A.; Gaertner-Rommel, A.; Klauke, B.; Grewe, S.A.; Schirmer, I.; Peterschröder, A.; Faber, L.; Vorgerd, M.; Gummert, J.; Anselmetti, D.; et al. The Novel αB-Crystallin (CRYAB) Mutation p.D109G Causes Restrictive Cardiomyopathy. Hum. Mutat. 2017, 38, 947–952. [Google Scholar] [CrossRef]
- Gibertini, S.; Ruggieri, A.; Cheli, M.; Maggi, L. Protein Aggregates and Aggrephagy in Myopathies. Int. J. Mol. Sci. 2023, 24, 8456. [Google Scholar] [CrossRef]
- Sjekloća, L.; Pudas, R.; Sjöblom, B.; Konarev, P.; Carugo, O.; Rybin, V.; Kiema, T.-R.; Svergun, D.; Ylänne, J.; Carugo, K.D. Crystal Structure of Human Filamin C Domain 23 and Small Angle Scattering Model for Filamin C 23–24 Dimer. J. Mol. Biol. 2007, 368, 1011–1023. [Google Scholar] [CrossRef]
- Pudas, R.; Kiema, T.-R.; Butler, P.J.G.; Stewart, M.; Ylänne, J. Structural Basis for Vertebrate Filamin Dimerization. Structure 2005, 13, 111–119. [Google Scholar] [CrossRef]
- Sethi, R.; Seppälä, J.; Tossavainen, H.; Ylilauri, M.; Ruskamo, S.; Pentikäinen, O.T.; Pentikäinen, U.; Permi, P.; Ylänne, J. A Novel Structural Unit in the N-Terminal Region of Filamins. J. Biol. Chem. 2014, 289, 8588–8598. [Google Scholar] [CrossRef]
- Sethi, R.; Ylänne, J. Small-Angle X-Ray Scattering Reveals Compact Domain-Domain Interactions in the N-Terminal Region of Filamin C. PLoS ONE 2014, 9, e107457. [Google Scholar] [CrossRef]
- Suphamungmee, W.; Nakamura, F.; Hartwig, J.H.; Lehman, W. Electron Microscopy and 3D Reconstruction Reveals Filamin Ig Domain Binding to F-Actin. J. Mol. Biol. 2012, 424, 248–256. [Google Scholar] [CrossRef]








| Ref | N° | Sexe | Status | Pathology | Onset | Neuromuscular Involvement | CK Levels | Cardiac Involv. | Resp. Involv. | Outcome | Anapath. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscular | Cardiac | Muscle weakness | Arthrogryposis | Scoliosis | EMG | Presence of cytoplasmic bodies | |||||||||
| [25] | P26 | - | heteroz. | Myopathy | 38 y | Proximal, mild distal | - | - | - | Increased | - | - | - | Yes | |
| [10] | P25 | M | heteroz., de novo | RCM + myopathy | Birth | 1.4 y | Proximal | Yes | Yes | Myopathic | Increased CK 1.5–4 | RCM | Respiratory infection at 2.5 y | Death at 2.5 y | - |
| P16 | F | heteroz. | RCM + myopathy | 1st year | 3 y | Proximal | - | - | Myopathic | Increased CK 1.2 | RCM | - | Death at 9 y | - | |
| P22 | F | heteroz., de novo | RCM + myopathy | Birth | 15 y | Proximal | Yes | Yes | - | Normal | RCM | Pulmonary hypertension | Ht at 19 y | Yes | |
| [26] | P1 | M | heteroz., de novo | RCM | 30 mo | - | - | - | - | Normal | RCM | Pulmonary hypertension | Last follow up at 40 mo | - | |
| [23] | I298 | M | heteroz., de novo | CM + myopathy | - | - | - | Yes | - | Normal | CM | Restrictive respiratory syndrome | Alive at 38 y | Yes | |
| [24] | - | F | heteroz., de novo | HCM, MFM, multiorgan tumors | 30 y | 6 y | Proximal | - | Yes | Normal | Increased | HCM | FVC 24.5% | Alive at 41 y | Yes |
| [27] | D15-1576 | F | heteroz., de novo | Arthrogryposis | - | - | Yes | Yes | - | - | - | - | - | - | |
| [22] | P7 | M | heteroz., de novo | RCM + mucoskeletal involvement | 3.5y | - | Yes | Yes | - | Increased | RCM | - | Ht waiting list | - | |
| P8 | M | heteroz., from father | RCM + mucoskeletal involvement | 1y | - | - | Yes | - | Increased | RCM | - | Ht waiting list | - | ||
| [21] | P4 | M | heteroz., de novo | RCM + myopathy | 1st year | 1 y | - | - | - | Sensory polyneuropathy in the legs and arms | Increased CK 1.32 | RCM | No | - | - |
| P8 | M | heteroz., de novo | RCM + myopathy | Birth | 3.5 y | Proximal | Yes | Yes | Normal | Increased CK 2.8 | RCM | No | ICD | - | |
| P9 | M | heteroz., de novo | RCM + myopathy | Birth | 2 y | - | Yes | - | - | Increased CK 3.8 | RCM | No | - | - | |
| THIS STUDY | P1 | M | heteroz., de novo | RCM + myopathy | Birth | 13 y | Global | Yes | Yes | Myopathic | Normal | RCM | Restrictive respiratory syndrome | Alive at 17 y | Yes |
| P2 | M | heteroz. | HCM + myopathy | 2 y | 39 y | Global | - | Yes | - | Increased | HCM | Restrictive respiratory syndrome | Alive at 43 y | Yes | |
| P3 | F | heteroz. | HCM + myopathy | 3–4 y | 29 y | Axial and proximal | - | Yes | - | Increased | HCM | Restrictive respiratory syndrome | Alive at 34 y | Yes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onnée, M.; Bénézit, A.; Bastu, S.; Nadaj-Pakleza, A.; Lannes, B.; Ader, F.; Thèze, C.; Cintas, P.; Cances, C.; Carlier, R.-Y.; et al. The FLNC Ala1186Val Variant Linked to Cytoplasmic Body Myopathy and Cardiomyopathy Causes Protein Instability. Biomedicines 2024, 12, 322. https://doi.org/10.3390/biomedicines12020322
Onnée M, Bénézit A, Bastu S, Nadaj-Pakleza A, Lannes B, Ader F, Thèze C, Cintas P, Cances C, Carlier R-Y, et al. The FLNC Ala1186Val Variant Linked to Cytoplasmic Body Myopathy and Cardiomyopathy Causes Protein Instability. Biomedicines. 2024; 12(2):322. https://doi.org/10.3390/biomedicines12020322
Chicago/Turabian StyleOnnée, Marion, Audrey Bénézit, Sultan Bastu, Aleksandra Nadaj-Pakleza, Béatrice Lannes, Flavie Ader, Corinne Thèze, Pascal Cintas, Claude Cances, Robert-Yves Carlier, and et al. 2024. "The FLNC Ala1186Val Variant Linked to Cytoplasmic Body Myopathy and Cardiomyopathy Causes Protein Instability" Biomedicines 12, no. 2: 322. https://doi.org/10.3390/biomedicines12020322
APA StyleOnnée, M., Bénézit, A., Bastu, S., Nadaj-Pakleza, A., Lannes, B., Ader, F., Thèze, C., Cintas, P., Cances, C., Carlier, R.-Y., Metay, C., Cossée, M., & Malfatti, E. (2024). The FLNC Ala1186Val Variant Linked to Cytoplasmic Body Myopathy and Cardiomyopathy Causes Protein Instability. Biomedicines, 12(2), 322. https://doi.org/10.3390/biomedicines12020322