Transcutaneous Auricular Vagus Nerve Stimulation to Improve Emotional State
Abstract
:1. Introduction
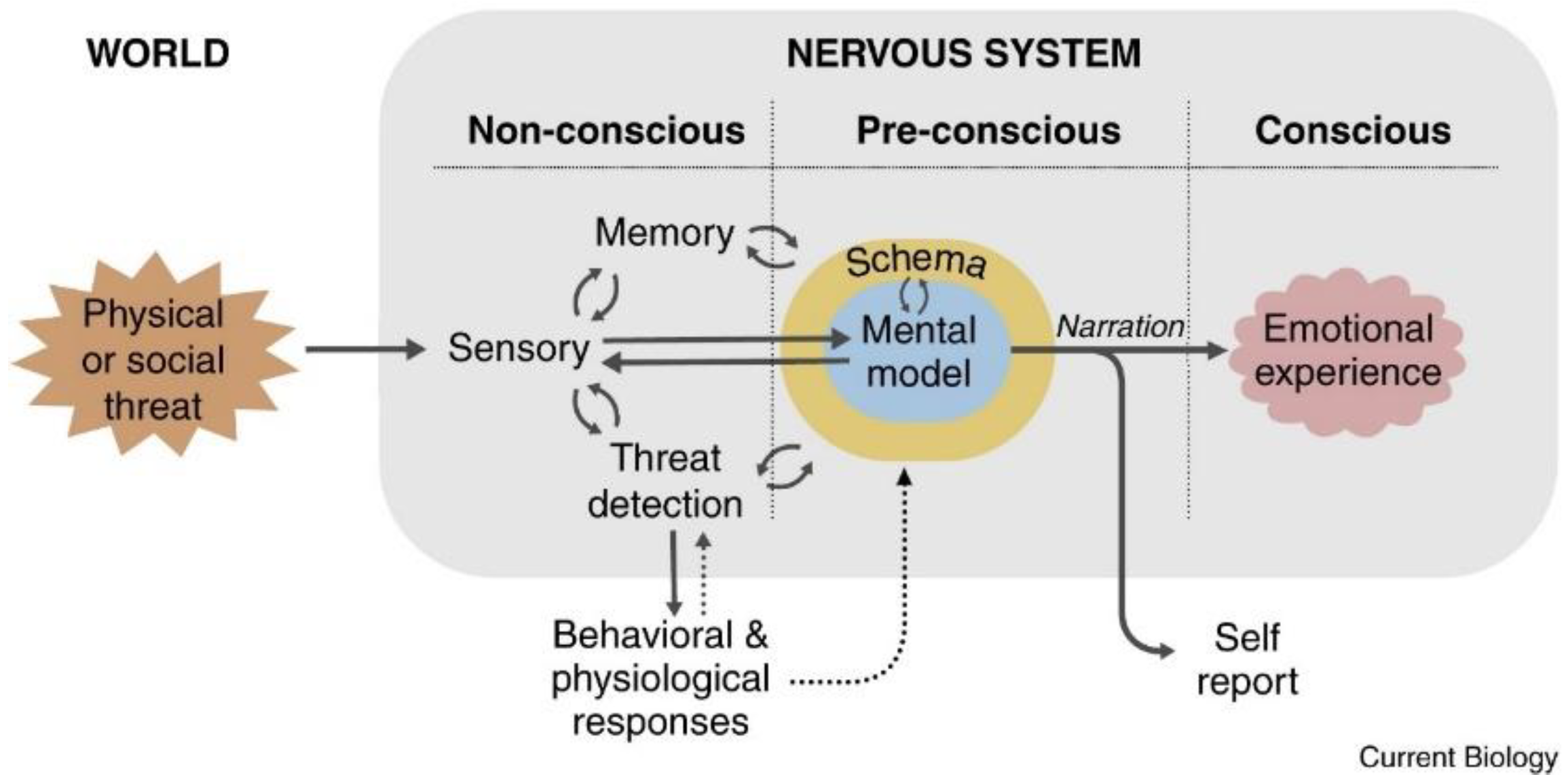
2. The LeDoux Proposal (2021) [11]
3. Theory of Constructed Emotion
4. Polyvagal Theory
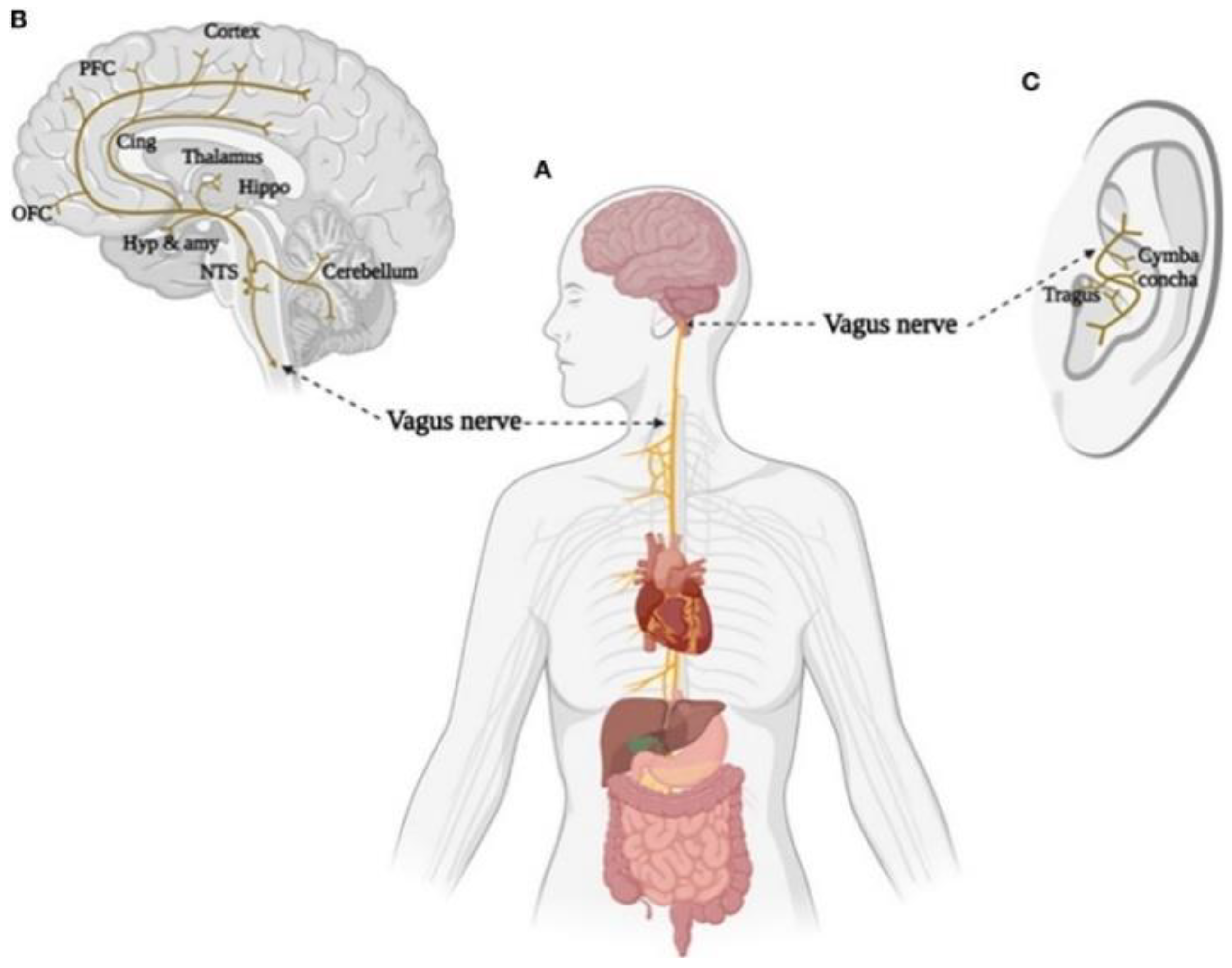
5. Transcutaneous Auricular Vagus Nerve Stimulation
6. Discussion and Future Research
7. Conclusions
Funding
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Disease and Injuries Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the global burden of mental disorders and their economic value. EclinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef]
- Hagihara, K.M.; Bukalo, O.; Zeller, M.; Aksoy-Aksel, A.; Karalis, N.; Limoges, A.; Rigg, T.; Campbell, T.; Mendez, A.; Weinholtz, C.; et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nat. Int. Wkly. J. Sci. 2021, 594, 403–407. [Google Scholar] [CrossRef]
- Kenwood, M.M.; Kalin, N.H.; Barbas, H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacol. Intersect. Brain Behav. Ther. 2021, 47, 260–275. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, C.; Li, G.; Wang, Y.; Liu, P.H.; Liu, Z.; Sun, N.; Zhang, K. Functional connectivity of the prefrontal cortex and amygdala is related to depression status in major depressive disorder. J. Affect. Disord. 2020, 274, 897–902. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Murray, G.; Ray, S. Circadian biology to advance therapeutics for mood disorders. Trends Pharmacol. Sci. 2023, 44, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Watson, S.; Friston, K.J. What is mood? a computational perspective. Psychol. Med. 2018, 48, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Kaltenboeck, A.; Harmer, C.J. Cognitive emotional processing across mood disorders. CNS Spectr. 2019, 24, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Aranberri Ruiz, A. Emotional experience and its biological underpinnings: Improving emotional well-being through vagal tone. Papeles Psicólogo Psychol. Pap. 2023, 44, 95. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Zwir, I. Genetics of human character and temperament. eLS 2022, 3, 1–20. [Google Scholar] [CrossRef]
- LeDoux, J.E. What emotions might be like in other animals. Curr. Biol. 2021, 31, R824–R829. [Google Scholar] [CrossRef]
- Barrett, L.F. The theory of constructed emotion: An active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 2017, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001, 42, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The vagal paradox: A polyvagal solution. Compr. Psychoneuroendocrinol. 2023, 16, 100200. [Google Scholar] [CrossRef]
- LeDoux, J. Rethinking the emotional brain. Neuron 2012, 73, 653–676. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Chin, R.; Chang, S.W.C.; Holmes, A.J. Beyond cortex: The evolution of the human brain. Psychol. Rev. 2023, 130, 285–307. [Google Scholar] [CrossRef]
- Brabec, J.; Rulseh, A.; Hoyt, B.; Vizek, M.; Horinek, D.; Hort, J.; Petrovicky, P. Volumetry of the human amygdala—An anatomical study. Psychiatry Res. Neuroimaging 2010, 182, 67–72. [Google Scholar] [CrossRef]
- Pape, H.-C.; Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010, 90, 419. [Google Scholar] [CrossRef]
- Tyszka, J.M.; Pauli, W.M. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum. Brain Mapp. 2016, 37, 3979–3998. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Quirk, G.J. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Coming to terms with fear. Proc. Natl. Acad. Sci. USA 2014, 111, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Barger, N.; Hanson, K.L.; Teffer, K.; Schenker-Ahmed, N.M.; Semendeferi, K. Evidence for evolutionary specialization in human limbic structures. Front. Hum. Neurosci. 2014, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jian, L.; Qiu, H.; Zhang, C.; Cheng, S.; Ji, J.; Li, T.; Wang, Y.; Li, J.; Li, K. Understanding complex functional wiring patterns in major depressive disorder through brain functional connectome. Transl. Psychiatry 2021, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Haris, E.M.; Bryant, R.A.; Williamson, T.; Korgaonkar, M.S. Functional connectivity of amygdala subnuclei in ptsd: A narrative review. Mol. Psychiatry 2023, 28, 3581–3594. [Google Scholar] [CrossRef] [PubMed]
- Henigsberg, N.; Kalember, P.; Petrović, Z.K.; Šečić, A. Neuroimaging research in posttraumatic stress disorder—Focus on amygdala, hippocampus and prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 37–42. [Google Scholar] [CrossRef]
- Millan, M.J. Agomelatine for the treatment of generalized anxiety disorder: Focus on its distinctive mechanism of action. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221105128. [Google Scholar] [CrossRef]
- Hohwy, J. New directions in predictive processing. Mind Lang. 2020, 35, 209–223. [Google Scholar] [CrossRef]
- Shipp, S.; Adams, R.A.; Friston, K.J. Reflections on agranular architecture: Predictive coding in the motor cortex. Trends Neurosci. 2013, 36, 706. [Google Scholar] [CrossRef]
- Barrett, L.F. In search of emotions. Curr. Biol. 2019, 29, 142. [Google Scholar] [CrossRef]
- Barrett, L.F. Context reconsidered: Complex signal ensembles, relational meaning, and population thinking in psychological science. Am. Psychol. 2022, 77, 894. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Finlay, B.L. Concepts, goals and the control of survival-related behaviors. Curr. Opin. Behav. Sci. 2018, 24, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How Do You Feel?: An Interoceptive Moment with Your Neurobiological Self; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Kurtin, D.L.; Giunchiglia, V.; Vohryzek, J.; Cabral, J.; Skeldon, A.C.; Violante, I.R. Moving from phenomenological to predictive modelling: Progress and pitfalls of modelling brain stimulation in-silico. Neuroimage 2023, 272, 120042. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Zhang, J.; Touroutoglou, A.; Chanes, L.; Xia, C.; Simmons, W.K.; Feldman Barrett, L. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 2017, 1, 0069. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Bort, C.; Wendt, J.; Weymar, M. The role of interoceptive sensibility and emotional conceptualization for the experience of emotions. Front. Psychol. 2021, 12, 712418. [Google Scholar] [CrossRef]
- Kuppens, P.; Tuerlinckx, F.; Russell, J.A.; Barrett, L.F. The relation between valence and arousal in subjective experience. Psychol. Bull. 2013, 139, 917–940. [Google Scholar] [CrossRef]
- Katsumi, Y.; Kamona, N.; Zhang, J.; Bunce, J.G.; Hutchinson, J.B.; Yarossi, M.; Barrett, L.F. Functional connectivity gradients as a common neural architecture for predictive processing in the human brain. BioRxiv 2021. [Google Scholar] [CrossRef]
- Katsumi, Y.; Theriault, J.E.; Quigley, K.S.; Barrett, L.F. Allostasis as a core feature of hierarchical gradients in the human brain. Netw. Neurosci. 2022, 6, 1010–1031. [Google Scholar] [CrossRef]
- Barrett, L.F.; Satpute, A.B. Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 2013, 23, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Press, C.; Kok, P.; Yon, D. The perceptual prediction paradox. Trends Cogn. Sci. 2020, 24, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Touroutoglou, A.; Bliss-Moreau, E.; Zhang, J.; Mantini, D.; Vanduffel, W.; Dickerson, B.C.; Barrett, L.F. A ventral salience network in the macaque brain. Neuroimage 2016, 132, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.D.; Kang, J.; Johnson, T.D.; Nichols, T.E.; Satpute, A.B.; Barrett, L.F.; Diedrichsen, J. A bayesian model of category-specific emotional brain responses. PLoS Comput. Biol. 2015, 11, e1004066. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. Basic Clin. 2000, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Neuhuber, W.L.; Berthoud, H.-R. Functional anatomy of the vagus system—Emphasis on the somato-visceral interface. Auton. Neurosci. Basic Clin. 2021, 236, 102887. [Google Scholar] [CrossRef]
- Gourine, A.V.; Machhada, A.; Trapp, S.; Spyer, K.M. Cardiac vagal preganglionic neurones: An update. Auton. Neurosci. Basic Clin. 2016, 199, 24–28. [Google Scholar] [CrossRef]
- Porges, S.W. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Ann. N. Y. Acad. Sci. 1997, 807, 62–77. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Ruffoli, R.; Giorgi, F.S.; Pizzanelli, C.; Murri, L.; Paparelli, A.; Fornai, F. The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat. 2011, 42, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Silberstein, S.D. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache 2016, 56, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, I.; Velde, H.M.; Robe, P.A.J.T.; Stokroos, R.J.; Smit, A.L. Tinnitus treatment by vagus nerve stimulation: A systematic review. PLoS ONE 2021, 16, 0247221. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Thayer, J.F. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef] [PubMed]
- De Smet, S.; Ottaviani, C.; Verkuil, B.; Kappen, M.; Baeken, C. Effects of non-invasive vagus nerve stimulation on cognitive and autonomic correlates of perseverative cognition. Psychophysiology 2023, 60, e14250. [Google Scholar] [CrossRef] [PubMed]
- Goggins, E.; Mitani, S.; Tanaka, S. Clinical perspectives on vagus nerve stimulation: Present and future. Clin. Sci. 2022, 136, 695–709. [Google Scholar] [CrossRef]
- Warren, C.M.; Tona, K.D.; Ouwerkerk, L.; van Paridon, J.; Poletiek, F.; van Steenbergen, H.; Bosch, J.A.; Nieuwenhuis, S. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 2019, 12, 635–642. [Google Scholar] [CrossRef]
- Assenza, G.; Campana, C.; Colicchio, G.; Tombini, M.; Assenza, F.; Di Pino, G.; Di Lazzaro, V. Transcutaneous and invasive vagal nerve stimulations engage the same neural pathways: In-vivo human evidence. Brain Stimul. 2017, 10, 853–854. [Google Scholar] [CrossRef]
- Peuker, E.T.; Filler, T.J. The nerve supply of the human auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- Kiyokawa, J.; Yamaguchi, K.; Okada, R.; Maehara, T.; Akita, K. Origin, course and distribution of the nerves to the posterosuperior wall of the external acoustic meatus. Anat Sci Int. 2014, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tubbs, R.S.; Satoh, S.; Zomorodi, A.R.; Liedtke, W.; Labidi, M.; Friedman, A.H.; Fukushima, T. Isolated Deep Ear Canal Pain: Possible Role of Auricular Branch of Vagus Nerve-Case Illustrations with Cadaveric Correlation. World Neurosurg. 2016, 96, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Strzelczyk, A.; Finisguerra, A.; Gourine, A.V.; Gharabaghi, A.; Hasan, A.; Burger, A.M.; Jaramillo, A.M.; Mertens, A.; Majid, A.; et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (version 2020). Front. Hum. Neurosci. 2021, 14, 568051. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, P.; Silva, M.E.D.; Carvalho, T.C.D.; Cordeiro, Q.; Brunoni, A.R.; Fregni, F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: A systematic review. Arquivos Neuro-Psiquiatria 2014, 72, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Sellaro, R.; de Gelder, B.; Finisguerra, A.; Colzato, L.S. Transcutaneous vagus nerve stimulation (tvns) enhances recognition of emotions in faces but not bodies. Cortex 2018, 99, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Vonck, K.; Raedt, R.; Naulaerts, J.; De, V.F.; Thiery, E.; Van, R.D.; Miatton, M.; Boon, P.; Aldenkamp, B. Vagus nerve stimulation. 25 years later! what do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Yan, Q.; Ma, Y.; Fang, J.; Yang, Y. Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front. Neurol. 2022, 13, 1015175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.-Y.; Wang, D.; Wu, M.-Z.; He, J.-K.; Zhang, J.-L.; Zhao, B.; Hou, L.-W.; Wang, J.-Y.; Wang, L.; et al. Transcutaneous auricular vagus nerve stimulation: From concept to application. Neurosci. Bull. 2020, 37, 853–862. [Google Scholar] [CrossRef]
- Pu-Wei, H.; Hsin-Cheng, H.; Yi-Wen, L.; Nou-Ying, T.; Chin-Yi, C.; Ching-Liang, H. The history, mechanism, and clinical application of auricular therapy in traditional chinese medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 495684. [Google Scholar] [CrossRef]
- Kong, J.; Fang, J.; Park, J.; Li, S.; Rong, P. Treating depression with transcutaneous auricular vagus nerve stimulation: State of the art and future perspectives. Front. Psychiatry 2018, 9, 20. [Google Scholar] [CrossRef]
- Ferstl, M.; Teckentrup, V.; Lin, W.M.; Kräutlein, F.; Kühnel, A.; Klaus, J.; Martin, W.; Kroemer, N.B. Non-invasive vagus nerve stimulation boosts mood recovery after effort exertion. Psychol. Med. 2022, 52, 3029–3039. [Google Scholar] [CrossRef]
- Ventureyra, E.C.G. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. Childs Nerv. Syst. 2000, 16, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical review of transcutaneous vagus nerve stimulation: Challenges for translation to clinical practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Carreno, F.R.; Frazer, A. The allure of transcutaneous vagus nerve stimulation as a novel therapeutic modality. Biol. Psychiatry 2016, 79, 260–261. [Google Scholar] [CrossRef]
- Daban, C.; Martinez-Aran, A.; Cruz, N.; Vieta, E. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. a systematic review. J. Affect. Disord. 2008, 110, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, A.; Urechie, V.; Shiffer, D.; Rigo, S.; Minonzio, M.; Cairo, B.; Smith, E.C.; Okamoto, L.E.; Barbic, F.; Bisoglio, A.; et al. Transdermal auricular vagus stimulation for the treatment of postural tachycardia syndrome. Auton. Neurosci. Basic Clin. 2021, 236, 102886. [Google Scholar] [CrossRef]
- Nicholson, W.C.; Kempf, M.-C.; Moneyham, L.; Vance, D.E. The potential role of vagus-nerve stimulation in the treatment of hiv-associated depression: A review of literature. Neuropsychiatr. Dis. Treat. 2017, 13, 1677–1689. [Google Scholar] [CrossRef]
- Seiden, D.; Corbett, S.A.; Lachman, E. Lachman’s Case Studies in Anatomy, 5th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Kim, A.Y.; Marduy, A.; de Melo, P.S.; Gianlorenco, A.C.; Kim, C.K.; Choi, H.; Song, J.-J.; Fregni, F. Safety of transcutaneous auricular vagus nerve stimulation (tavns): A systematic review and meta-analysis. Sci. Rep. 2022, 12, 22055. [Google Scholar] [CrossRef]
- Barbella, G.; Cocco, I.; Freri, E.; Marotta, G.; Visani, E.; Franceschetti, S.; Casazza, M. Transcutaneous vagal nerve stimulatio (t-vns): An adjunctive treatment option for refractory epilepsy. Seizure: Eur. J. Epilepsy 2018, 60, 115–119. [Google Scholar] [CrossRef]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hillenbrand, B.; Hirsch, M.; Last, C.; Lerche, H.; et al. Transcutaneous vagus nerve stimulation (tvns) for treatment of drug-resistant epilepsy: A randomized, double-blind clinical trial (cmpse02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef]
- Badran, B.W.; Mithoefer, O.J.; Summer, C.E.; LaBate, N.T.; Glusman, C.E.; Badran, A.W.; DeVries, W.H.; Summers, P.M.; Austelle, C.W.; McTeague, L.M.; et al. Short trains of transcutaneous auricular vagus nerve stimulation (tavns) have parameter-specific effects on heart rate. Brain Stimul. 2018, 11, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; D’Agostini, M.; Verkuil, B.; Van Diest, I. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology 2020, 57, e13571. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.; Day, D.; Leung, H.; Laud, P.J.; Ali, A.; Lindert, R.; Majid, A. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. 2018, 11, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Picq, C.; Sinniger, V.; Mayol, J.F.; Clarençon, D. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 2013, 25, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, X.; Zhou, M.; Kendrick, K.M.; Zhao, W. Therapeutic applications of transcutaneous auricular vagus nerve stimulation with potential for application in neurodevelopmental or other pediatric disorders. Front. Endocrinol. 2022, 13, 1000758. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: Fmri evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Komisaruk, B.R.; Frangos, E. Vagus nerve afferent stimulation: Projection into the brain, reflexive physiological, perceptual, and behavioral responses, and clinical relevance. Auton. Neurosci. Basic Clin. 2022, 237, 102908. [Google Scholar] [CrossRef]
- Banks, S.J.; Eddy, K.T.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007, 2, 303–312. [Google Scholar] [CrossRef]
- Berboth, S.; Morawetz, C. Amygdala-prefrontal connectivity during emotion regulation: A meta-analysis of psychophysiological interactions. Neuropsychologia 2021, 153, 107767. [Google Scholar] [CrossRef]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—An ale meta-analysis and macm analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; Baeken, C.; Van Schuerbeek, P.; Luypaert, R.; De Raedt, R. Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: An event related fmri study. Biol. Psychol. 2013, 92, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Messina, I.; Grecucci, A.; Viviani, R. Neurobiological models of emotion regulation: A meta-analysis of neuroimaging studies of acceptance as an emotion regulation strategy. Soc. Cogn. Affect. Neurosci. 2021, 16, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhen, R. How do physical and emotional abuse affect depression and problematic behaviors in adolescents? the roles of emotional regulation and anger. Child Abus. Negl. 2022, 129, 105641. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Ruffoli, R.; Giorgi, F.S.; Paparelli, A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur. J. Neurosci. 2011, 33, 2169–2178. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic neuropeptide brain protection: Focus on oxytocin. J. Clin. Med. 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- D’Agostini, M.; Burger, A.M.; Franssen, M.; Perkovic, A.; Claes, S.; von Leupoldt, A.; Murphy, P.R.; Van Diest, I. Short bursts of transcutaneous auricular vagus nerve stimulation enhance evoked pupil dilation as a function of stimulation parameters. Cortex 2023, 159, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Manta, S.; Dong, J.; Debonnel, G.; Blier, P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci. Jpn 2009, 34, 272–280. [Google Scholar] [PubMed]
- Wienke, C.; Grueschow, M.; Haghikia, A.; Zaehle, T. Phasic, Event-Related Transcutaneous Auricular Vagus Nerve Stimulation Modifies Behavioral, Pupillary, and Low-Frequency Oscillatory Power Responses. J. Neurosci. 2023, 43, 6306–6319. [Google Scholar] [CrossRef]
- Giraudier, M.; Ventura-Bort, C.; Burger, A.M.; Claes, N.; D’Agostini, M.; Fischer, R.; Franssen, M.; Kaess, M.; Koenig, J.; Liepelt, R.; et al. Evidence for a modulating effect of transcutaneous auricular vagus nerve stimulation (tavns) on salivary alpha-amylase as indirect noradrenergic marker: A pooled mega-analysis. Brain Stimul. 2022, 15, 1378–1388. [Google Scholar] [CrossRef]
- Lloyd, B.; Wurm, F.; de Kleijn, R.; Nieuwenhuis, S. Short-term transcutaneous vagus nerve stimulation increases pupil size but does not affect eeg alpha power: A replication of Sharon et al. (2021, Journal of Neuroscience). Brain Stimul. 2023, 16, 1001–1008. [Google Scholar] [CrossRef]
- Ventura-Bort, C.; Wirkner, J.; Genheimer, H.; Wendt, J.; Hamm, A.O.; Weymar, M. Effects of transcutaneous vagus nerve stimulation (tvns) on the p300 and alpha-amylase level: A pilot study. Front. Hum. Neurosci. 2018, 12, 202. [Google Scholar] [CrossRef]
- Burger, A.M.; Van der Does, W.; Brosschot, J.F.; Verkuil, B. From ear to eye? No effect of transcutaneous vagus nerve stimulation on human pupil dilation: A report of three studies. Biol. Psychol. 2020, 152, 107863. [Google Scholar] [CrossRef]
- Keute, M.; Demirezen, M.; Graf, A.; Mueller, N.G.; Zaehle, T. No modulation of pupil size and event-related pupil response by transcutaneous auricular vagus nerve stimulation (tavns). Sci. Rep. 2019, 9, 11452. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut microbe to brain signaling: What happens in vagus. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B.; et al. A neural circuit for gut-induced reward. Cell 2018, 175, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, 1219. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.; Morales, J.A.; Bitzer-Quintero, O. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef]
- Venegas, D.P.; De, L.F.M.K.; Landskron, G.; Hermoso, M.A.; Dijkstra, G.; Faber, K.N.; Gonzalez, M.J.; Quera, R.; Harmsen, H.J.M. Short chain fatty acids (scfas)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Egorova, N.; Rong, P.; Liu, J.; Hong, Y.; Fan, Y.; Wang, X.; Wang, H.; Yu, Y.; Ma, Y.; et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. Neuroimage Clin. 2017, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm. 2013, 120, 821–827. [Google Scholar] [CrossRef]
- Rong, P.-J.; Fang, J.-L.; Wang, L.-P.; Meng, H.; Liu, J.; Ma, Y.; Ben, H.; Li, L.; Liu, R.-P.; Huang, Z.-X.; et al. Transcutaneous vagus nerve stimulation for the treatment of depression: A study protocol for a double blinded randomized clinical trial. BMC Complement. Alternat. Med. Off. J. Int. Soc. Complement. Med. Res. 2012, 12, 255. [Google Scholar] [CrossRef]
- Lamb, D.G.; Porges, E.C.; Lewis, G.F.; Williamson, J.B. Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: Preliminary evidence. Front. Med. 2017, 4, 124. [Google Scholar] [CrossRef]
- Bottari, S.A.; Lamb, D.G.; Porges, E.C.; Murphy, A.J.; Tran, A.B.; Ferri, R.; Jaffee, M.S.; Davila, M.I.; Hartmann, S.; Baumert, M.; et al. Preliminary evidence of transcutaneous vagus nerve stimulation effects on sleep in veterans with post-traumatic stress disorder. J. Sleep Res. 2023, 33, e13891. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.M.; Shaam, P.; Williams, M.S.; McCann-Pineo, M.; Ryniker, L.; Debnath, S.; Zanos, T.P. Understanding mental health needs and gathering feedback on transcutaneous auricular vagus nerve stimulation as a potential PTSD treatment among 9/11 responders living with PTSD symptoms 20 years later: A qualitative approach. Int. J. Environ. Res. Public Health 2022, 19, 4847. [Google Scholar] [CrossRef]
- Sanchez-Perez, J.A.; Gazi, A.H.; Rahman, F.N.; Seith, A.; Saks, G.; Sundararaj, S.; Erbrick, R.; Harrison, A.B.; Nichols, C.J.; Modak, M.; et al. Transcutaneous auricular vagus nerve stimulation and median nerve stimulation reduce acute stress in young healthy adults: A single-blind sham-controlled crossover study. Front. Neurosci. 2023, 17, 1213982. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, D.; Rigoux, L.; Kuzmanovic, B.; Edwin Thanarajah, S.; Münte, T.F.; Fenselau, H.; Tittgemeyer, M. Technical note: Modulation of fmri brainstem responses by transcutaneous vagus nerve stimulation. Neuroimage 2021, 244, 118566. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Ritter, S.M.; Steenbergen, L. Transcutaneous vagus nerve stimulation (tvns) enhances divergent thinking. Neuropsychologia 2018, 111, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, B.J.; Immink, M.A.; Finisguerra, A.; Colzato, L.S. Transcutaneous vagus nerve stimulation (tvns) enhances response selection during sequential action. Front. Psychol. 2018, 9, 1159. [Google Scholar] [CrossRef]
- Szeska, C.; Richter, J.; Wendt, J.; Weymar, M.; Hamm, A.O. Promoting long-term inhibition of human fear responses by non-invasive transcutaneous vagus nerve stimulation during extinction training. Sci. Rep. 2020, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, J.; Wang, Z.; Rong, P.; Hong, Y.; Fan, Y.; Kong, J. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord. 2016, 205, 319–326. [Google Scholar] [CrossRef]
- Wu, C.; Liu, P.; Fu, H.; Chen, W.; Cui, S.; Lu, L.; Tang, C. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: A systematic review and meta-analysis. Medicine 2018, 97, e13845. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranberri Ruiz, A. Transcutaneous Auricular Vagus Nerve Stimulation to Improve Emotional State. Biomedicines 2024, 12, 407. https://doi.org/10.3390/biomedicines12020407
Aranberri Ruiz A. Transcutaneous Auricular Vagus Nerve Stimulation to Improve Emotional State. Biomedicines. 2024; 12(2):407. https://doi.org/10.3390/biomedicines12020407
Chicago/Turabian StyleAranberri Ruiz, Ainara. 2024. "Transcutaneous Auricular Vagus Nerve Stimulation to Improve Emotional State" Biomedicines 12, no. 2: 407. https://doi.org/10.3390/biomedicines12020407
APA StyleAranberri Ruiz, A. (2024). Transcutaneous Auricular Vagus Nerve Stimulation to Improve Emotional State. Biomedicines, 12(2), 407. https://doi.org/10.3390/biomedicines12020407




