Arecoline Induces ROS Accumulation, Transcription of Proinflammatory Factors, and Expression of KRT6 in Oral Epithelial Cells
Abstract
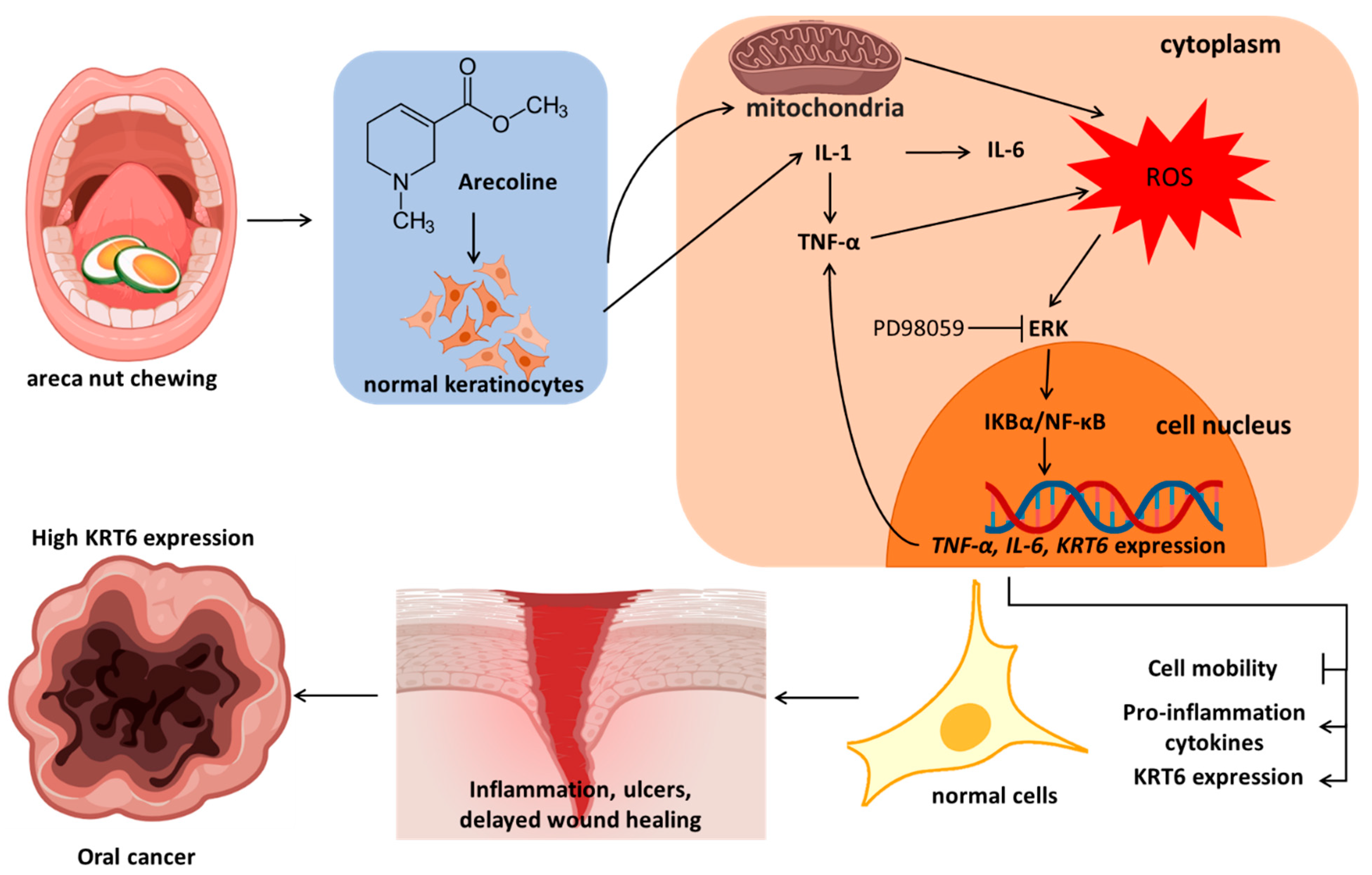
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Subjects
2.3. Cell Viability
2.4. Detection of Intracellular ROS
2.5. Western Blot Analysis
2.6. Reverse Transcription and Quantitative Real-Time PCR Analysis (RT-qPCR)
2.7. Immunocytochemistry Using Confocal Microscopy
2.8. Wound Healing Assay
2.9. Statistical Analysis
3. Results
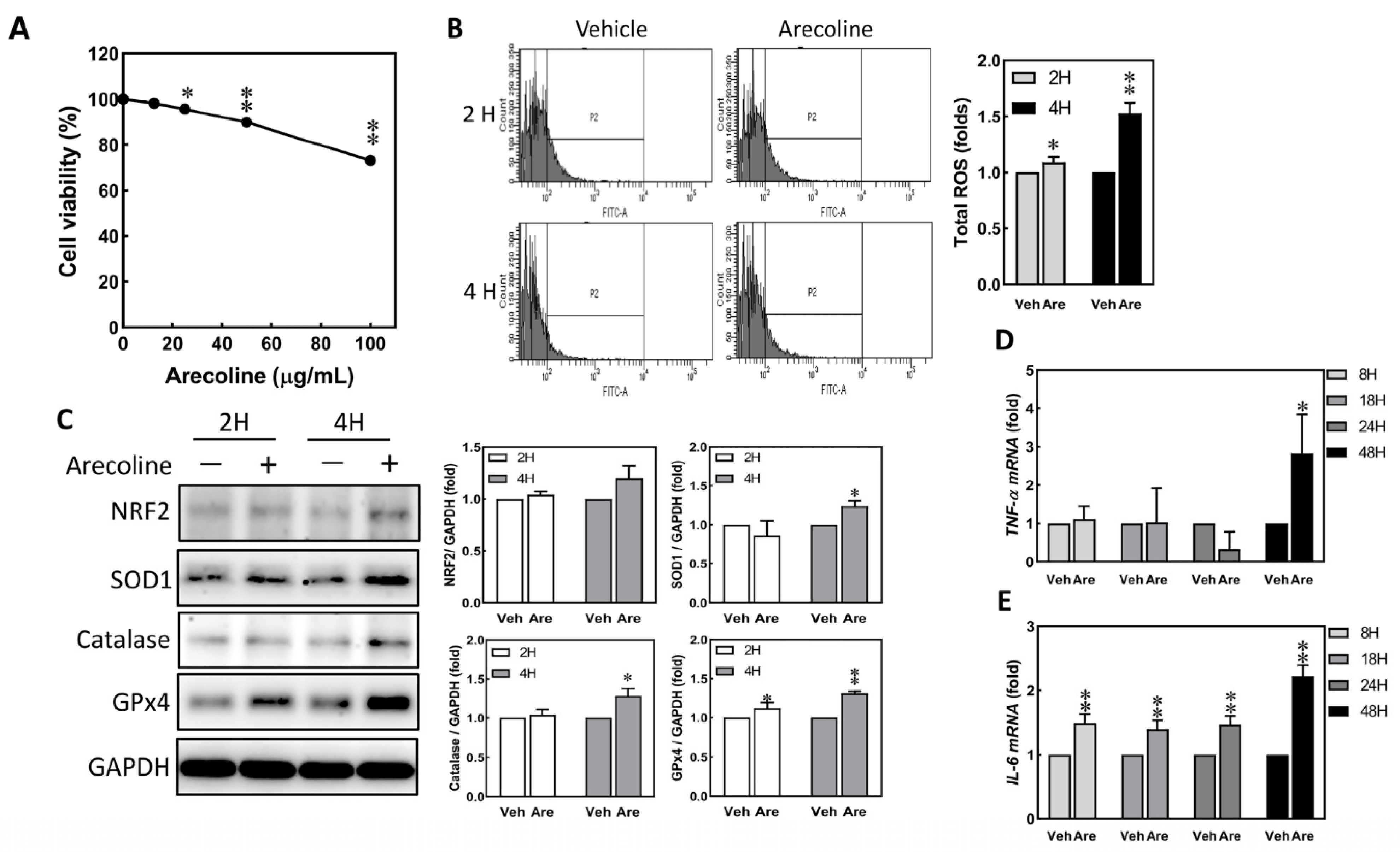
3.1. Arecoline Treatment Immediately Induced Up-Regulation of ROS and Proinflammatory Cytokines in S-G Cells
3.2. Arecoline Treatment Induced the Expression of KRT6 in S-G Cells
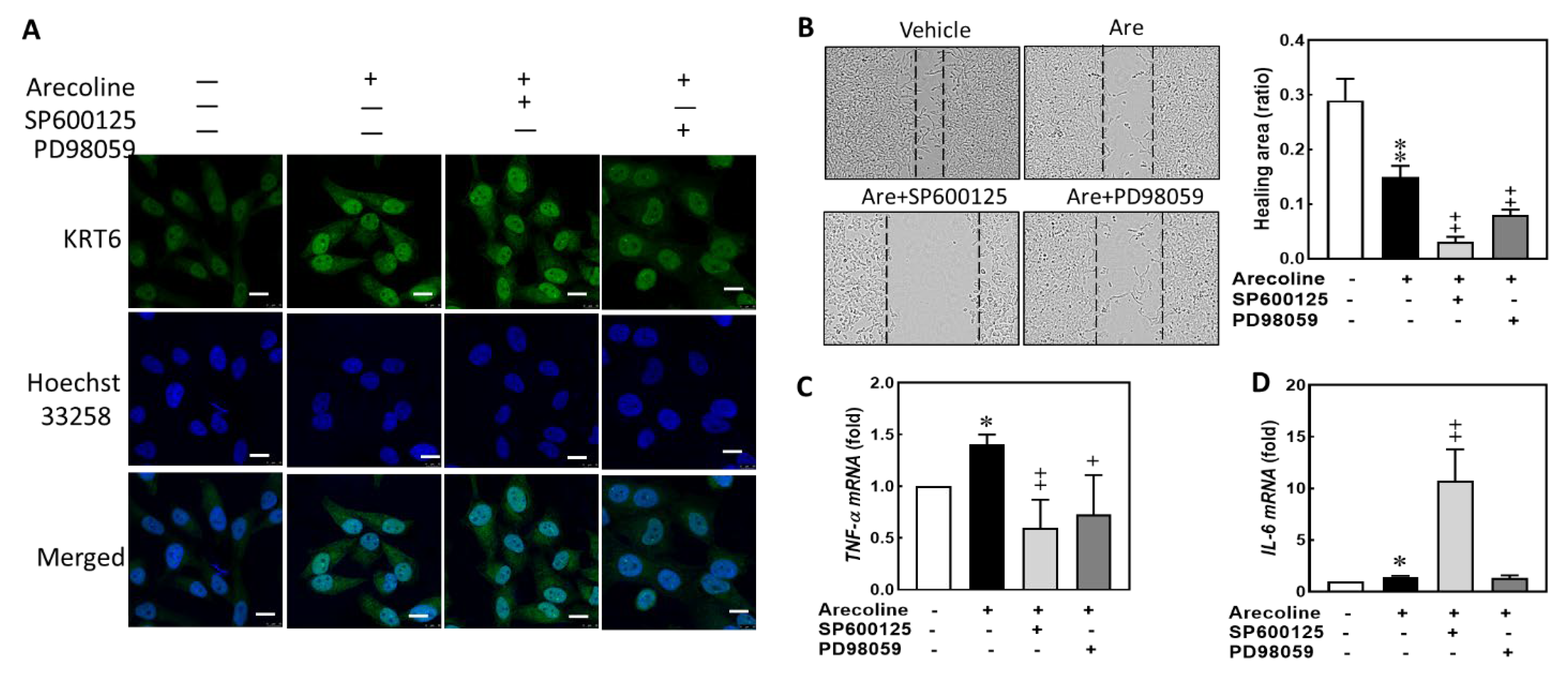
3.3. Arecoline Treatment Compromised the Abilities of Migration and Cell Proliferation in S-G Cells
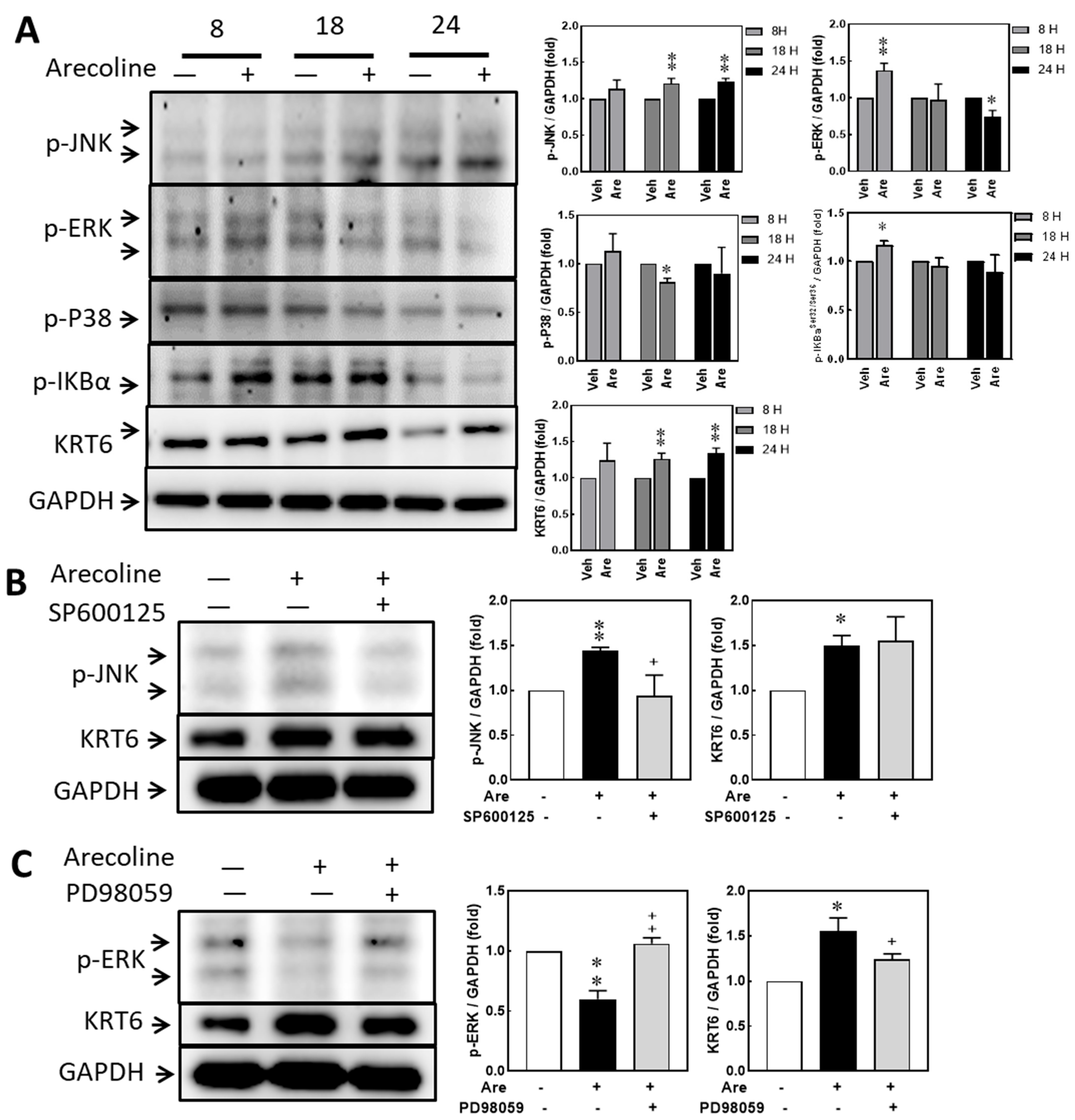
3.4. Arecoline Treatment Up-Regulated KRT6 Protein Expression via ERK–NFκB Signaling Pathway
3.5. Inhibiting JNK and MAPK/ERK Suppresses Arecoline-Induced TNF-α Transcription but Inhibiting JNK Enhances Arecoline-Induced IL-6 Transcription in the S-G Cells
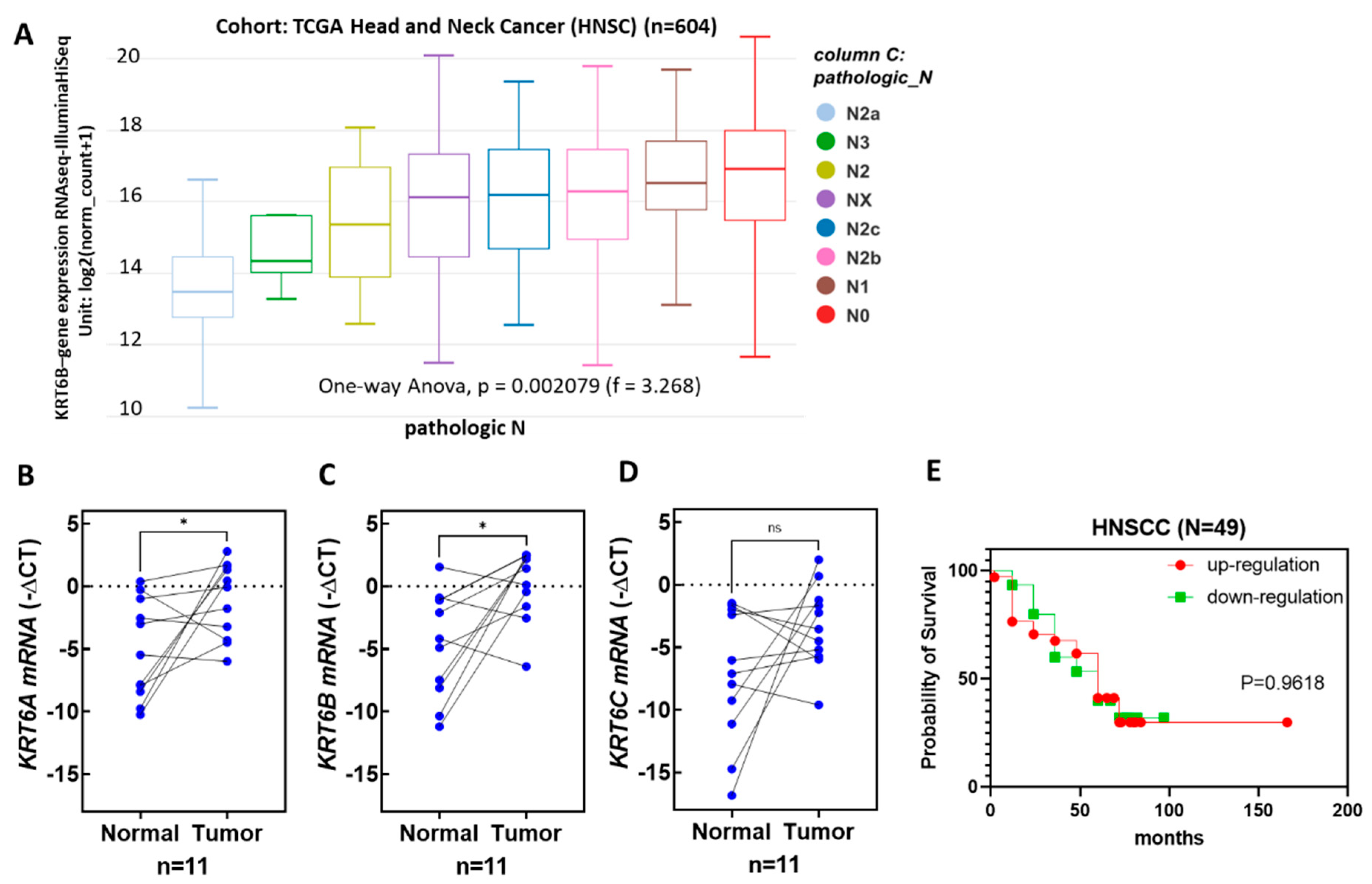
3.6. The Expression Levels of KRT6 Increased in the Tumor Specimens of Head and Neck Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IanMorison Orr, M.B. GLASG. Oral Cancer in Betel Nut Chewers in Travancore. Lancet 1933, 225, 575–580. [Google Scholar]
- Shanta, V.; Krishnamurthi, S. A study of aetiological factors in oral squamous cell carcinoma. Br. J. Cancer 1959, 13, 381–388. [Google Scholar] [CrossRef][Green Version]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral. Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Disease burden, risk factors, and trends of lip, oral cavity, pharyngeal cancers: A global analysis. Cancer Med. 2023, 12, 18153–18164. [Google Scholar] [CrossRef]
- Senevirathna, K.; Pradeep, R.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Illeperuma, R.; Warnakulasuriya, S.; Jayasinghe, R.D. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin. Pract. 2023, 13, 326–346. [Google Scholar] [CrossRef]
- Herzog, T.A.; Wilkens, L.R.; Badowski, G.; Mendez, A.J.P.; Franke, A.A.; Pokhrel, P.; Chennaux, J.S.N.; Tenorio, L.F.; Sotto, P.P.; Kawamoto, C.T.; et al. The Betel Nut Intervention Trial (BENIT)-A Randomized Clinical Trial for Areca Nut and Betel Quid Cessation: Primary Outcomes. Int. J. Environ. Res. Public. Health 2023, 20, 6622. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Feller, L.; Altini, M.; Lemmer, J. Inflammation in the context of oral cancer. Oral. Oncol. 2013, 49, 887–892. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Dewi, S.R.P. The Effect of Betel Quid Extract on Wound Healing Process in Male Wistar Rats (Rattus norvegicus L.). Asian J. Appl. Sci. 2019, 7, 788–797. [Google Scholar]
- Shieh, T.M.; Lin, S.C.; Liu, C.J.; Chang, S.S.; Ku, T.H.; Chang, K.W. Association of expression aberrances and genetic polymorphisms of lysyl oxidase with areca-associated oral tumorigenesis. Clin. Cancer Res. 2007, 13, 4378–4385. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chiu, K.C.; Wang, T.H.; Lan, W.C.; Tsai, B.H.; Wu, L.J.; Hsia, S.M.; Shieh, T.M. Effects of melatonin to arecoline-induced reactive oxygen species production and DNA damage in oral squamous cell carcinoma. J. Formos. Med. Assoc. 2021, 120, 668–678. [Google Scholar] [CrossRef]
- Li, Y.C.; Chang, J.T.; Chiu, C.; Lu, Y.C.; Li, Y.L.; Chiang, C.H.; You, G.R.; Lee, L.Y.; Cheng, A.J. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol. Carcinog. 2016, 55, 1012–1023. [Google Scholar] [CrossRef]
- Chang, M.C.; Chan, C.P.; Wang, W.T.; Chang, B.E.; Lee, J.J.; Tseng, S.K.; Yeung, S.Y.; Hahn, L.J.; Jeng, J.H. Toxicity of areca nut ingredients: Activation of CHK1/CHK2, induction of cell cycle arrest, and regulation of MMP-9 and TIMPs production in SAS epithelial cells. Head. Neck 2013, 35, 1295–1302. [Google Scholar] [CrossRef]
- Grellner, W.; Georg, T.; Wilske, J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci. Int. 2000, 113, 251–264. [Google Scholar] [CrossRef]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Investig. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef]
- Werner, S.; Breeden, M.; Hubner, G.; Greenhalgh, D.G.; Longaker, M.T. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J. Invest. Dermatol. 1994, 103, 469–473. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Simeonova, P.P.; Matheson, J.M.; Kommineni, C.; Guriel, J.L.; Sugawara, T.; Luster, M.I. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000, 14, 2525–2531. [Google Scholar] [CrossRef]
- Ko, A.M.; Tu, H.P.; Ko, Y.C. Systematic Review of Roles of Arecoline and Arecoline N-Oxide in Oral Cancer and Strategies to Block Carcinogenesis. Cells 2023, 12, 1208. [Google Scholar] [CrossRef]
- Jeng, J.H.; Wang, Y.J.; Chiang, B.L.; Lee, P.H.; Chan, C.P.; Ho, Y.S.; Wang, T.M.; Lee, J.J.; Hahn, L.J.; Chang, M.C. Roles of keratinocyte inflammation in oral cancer: Regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis 2003, 24, 1301–1315. [Google Scholar] [CrossRef]
- Wong, P.; Coulombe, P.A. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 2003, 163, 327–337. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Liu, H.B.; Parent, C.A.; Coulombe, P.A. Keratin 6 regulates collective keratinocyte migration by altering cell-cell and cell-matrix adhesion. J. Cell Biol. 2018, 217, 4314–4330. [Google Scholar] [CrossRef]
- Frohwitter, G.; Buerger, H.; PJ, V.A.N.D.; Korsching, E.; Kleinheinz, J.; Fillies, T. Cytokeratin and protein expression patterns in squamous cell carcinoma of the oral cavity provide evidence for two distinct pathogenetic pathways. Oncol. Lett. 2016, 12, 107–113. [Google Scholar] [CrossRef]
- Sesterhenn, A.M.; Mandic, R.; Dünne, A.A.; Werner, J.A. Cytokeratins 6 and 16 are frequently expressed in head and neck squamous cell carcinoma cell lines and fresh biopsies. Anticancer. Res. 2005, 25, 2675–2680. [Google Scholar]
- Ogunnigbagbe, O.; Bunick, C.G.; Kaur, K. Keratin 1 as a cell-surface receptor in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188664. [Google Scholar] [CrossRef]
- Smulow, J.B.; Glickman, I. An epithelial-like cell line in continuous culture from normal adult human gingiva. Proc. Soc. Exp. Biol. Med. 1966, 121, 1294–1296. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. A screening of growth inhibitory activity of Iranian medicinal plants on prostate cancer cell lines. Biomedicine 2018, 8, 8. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chen, C.C.; Kuo, Y.H.; Fuh, L.J.; Lan, W.C.; Wang, T.H.; Chiu, K.C.; Nguyen, T.V.; Hsia, S.M.; Shieh, T.M. Caffeic Acid Phenethyl Ester and Caffeamide Derivatives Suppress Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 9819. [Google Scholar] [CrossRef]
- Hsia, S.M.; Yu, C.C.; Shih, Y.H.; Chen, M.Y.; Wang, T.H.; Huang, Y.T.; Shieh, T.M. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck J. Sci. Spec. Head Neck 2016, 38, E360–E371. [Google Scholar] [CrossRef]
- Chang, J.F.; Hsieh, C.Y.; Liou, J.C.; Liu, S.H.; Hung, C.F.; Lu, K.C.; Lin, C.C.; Wu, C.C.; Ka, S.M.; Wen, L.L.; et al. Scavenging Intracellular ROS Attenuates p-Cresyl Sulfate-Triggered Osteogenesis through MAPK Signaling Pathway and NF-κB Activation in Human Arterial Smooth Muscle Cells. Toxins 2020, 12, 472. [Google Scholar] [CrossRef]
- Pal, R.; Shilagard, T.; Yang, J.; Villarreal, P.; Brown, T.; Qiu, S.; McCammon, S.; Resto, V.; Vargas, G. Remodeling of the Epithelial-Connective Tissue Interface in Oral Epithelial Dysplasia as Visualized by Noninvasive 3D Imaging. Cancer Res. 2016, 76, 4637–4647. [Google Scholar] [CrossRef]
- Wang, D.; Boerner, S.A.; Winkler, J.D.; LoRusso, P.M. Clinical experience of MEK inhibitors in cancer therapy. Biochim. Biophys. Acta 2007, 1773, 1248–1255. [Google Scholar] [CrossRef]
- Mansour, S.J.; Matten, W.T.; Hermann, A.S.; Candia, J.M.; Rong, S.; Fukasawa, K.; Vande Woude, G.F.; Ahn, N.G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 1994, 265, 966–970. [Google Scholar] [CrossRef]
- Rosin, M.P. The influence of pH on the convertogenic activity of plant phenolics. Mutat. Res. 1984, 135, 109–113. [Google Scholar] [CrossRef]
- Chen, P.H.; Huang, B.; Shieh, T.Y.; Wang, Y.H.; Chen, Y.K.; Wu, J.H.; Huang, J.H.; Chen, C.C.; Lee, K.W. The influence of monoamine oxidase variants on the risk of betel quid-associated oral and pharyngeal cancer. Sci. World J. 2014, 2014, 183548. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Tu, H.F.; Chen, M.Y.; Lai, J.C.; Chen, Y.L.; Wong, Y.W.; Yang, C.C.; Chen, H.Y.; Hsia, S.M.; Shih, Y.H.; Shieh, T.M. Arecoline-regulated ataxia telangiectasia mutated expression level in oral cancer progression. Head. Neck 2019, 41, 2525–2537. [Google Scholar] [CrossRef]
- Hernandez-Quintero, M.; Kuri-Harcuch, W.; Gonzalez Robles, A.; Castro-Munozledo, F. Interleukin-6 promotes human epidermal keratinocyte proliferation and keratin cytoskeleton reorganization in culture. Cell Tissue Res. 2006, 325, 77–90. [Google Scholar] [CrossRef]
- Wang, X.P.; Schunck, M.; Kallen, K.J.; Neumann, C.; Trautwein, C.; Rose-John, S.; Proksch, E. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J. Investig. Dermatol. 2004, 123, 124–131. [Google Scholar] [CrossRef]
- Chen, Q.; Jiao, J.; Wang, Y.; Mai, Z.; Ren, J.; He, S.; Li, X.; Chen, Z. Egr-1 mediates low-dose arecoline induced human oral mucosa fibroblast proliferation via transactivation of Wnt5a expression. BMC Mol. Cell Biol. 2020, 21, 80. [Google Scholar] [CrossRef]
- Xie, H.; Jing, R.; Liao, X.; Chen, H.; Xie, X.; Dai, H.; Pan, L. Arecoline promotes proliferation and migration of human HepG2 cells through activation of the PI3K/AKT/mTOR pathway. Hereditas 2022, 159, 29. [Google Scholar] [CrossRef]
- Petit-Frere, C.; Clingen, P.H.; Grewe, M.; Krutmann, J.; Roza, L.; Arlett, C.F.; Green, M.H. Induction of interleukin-6 production by ultraviolet radiation in normal human epidermal keratinocytes and in a human keratinocyte cell line is mediated by DNA damage. J. Investig. Dermatol. 1998, 111, 354–359. [Google Scholar] [CrossRef]
- Van Meter, M.; Simon, M.; Tombline, G.; May, A.; Morello, T.D.; Hubbard, B.P.; Bredbenner, K.; Park, R.; Sinclair, D.A.; Bohr, V.A.; et al. JNK Phosphorylates SIRT6 to Stimulate DNA Double-Strand Break Repair in Response to Oxidative Stress by Recruiting PARP1 to DNA Breaks. Cell Rep. 2016, 16, 2641–2650. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, D.J.; Lee, J.Y.; Kang, D.H.; Kwon, J.; Kang, S.W. Peroxiredoxin II restrains DNA damage-induced death in cancer cells by positively regulating JNK-dependent DNA repair. J. Biol. Chem. 2011, 286, 8394–8404. [Google Scholar] [CrossRef]
- Ho, M.; Thompson, B.; Fisk, J.N.; Nebert, D.W.; Bruford, E.A.; Vasiliou, V.; Bunick, C.G. Update of the keratin gene family: Evolution, tissue-specific expression patterns, and relevance to clinical disorders. Hum. Genom. 2022, 16, 1. [Google Scholar] [CrossRef]
- Ko, Y.C.; Huang, Y.L.; Lee, C.H.; Chen, M.J.; Lin, L.M.; Tsai, C.C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral. Pathol. Med. 1995, 24, 450–453. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Wu, K.J.; Chen, H.M.; Deng, Y.T. Arecoline activates latent transforming growth factor beta1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallate. J. Formos. Med. Assoc. 2018, 117, 527–534. [Google Scholar] [CrossRef]
- Cheng, Y.A.; Tsai, C.C. Nicotine- and arecoline-induced interleukin-1 secretion and intercellular adhesion molecular-1 expression in human oral epidermoid carcinoma cells in vitro. Arch. Oral Biol. 1999, 44, 843–851. [Google Scholar] [CrossRef]
- Kupper, T.S.; Groves, R.W. The interleukin-1 axis and cutaneous inflammation. J. Investig. Dermatol. 1995, 105 (Suppl. S1), 62S–66S. [Google Scholar] [CrossRef]
- Komine, M.; Rao, L.S.; Freedberg, I.M.; Simon, M.; Milisavljevic, V.; Blumenberg, M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J. Investig. Dermatol. 2001, 116, 330–338. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-H.; Shen, Y.-W.; Chen, H.-Y.; Chen, C.-C.; Lin, N.-C.; Shih, Y.-H.; Hsia, S.-M.; Chiu, K.-C.; Shieh, T.-M. Arecoline Induces ROS Accumulation, Transcription of Proinflammatory Factors, and Expression of KRT6 in Oral Epithelial Cells. Biomedicines 2024, 12, 412. https://doi.org/10.3390/biomedicines12020412
Wang T-H, Shen Y-W, Chen H-Y, Chen C-C, Lin N-C, Shih Y-H, Hsia S-M, Chiu K-C, Shieh T-M. Arecoline Induces ROS Accumulation, Transcription of Proinflammatory Factors, and Expression of KRT6 in Oral Epithelial Cells. Biomedicines. 2024; 12(2):412. https://doi.org/10.3390/biomedicines12020412
Chicago/Turabian StyleWang, Tong-Hong, Yen-Wen Shen, Hsin-Ying Chen, Chih-Chieh Chen, Nan-Chin Lin, Yin-Hwa Shih, Shih-Min Hsia, Kuo-Chou Chiu, and Tzong-Ming Shieh. 2024. "Arecoline Induces ROS Accumulation, Transcription of Proinflammatory Factors, and Expression of KRT6 in Oral Epithelial Cells" Biomedicines 12, no. 2: 412. https://doi.org/10.3390/biomedicines12020412
APA StyleWang, T.-H., Shen, Y.-W., Chen, H.-Y., Chen, C.-C., Lin, N.-C., Shih, Y.-H., Hsia, S.-M., Chiu, K.-C., & Shieh, T.-M. (2024). Arecoline Induces ROS Accumulation, Transcription of Proinflammatory Factors, and Expression of KRT6 in Oral Epithelial Cells. Biomedicines, 12(2), 412. https://doi.org/10.3390/biomedicines12020412









