1. Introduction
Nitrous oxide (N
2O) abuse induces peripheral neurological damage through a spectrum of clinical presentations, ranging from mild sensory disturbances to severe motor deficits. Recent advances in our understanding of these conditions have elucidated the critical role of cobalamin (vitamin B12) metabolism in maintaining the integrity of the peripheral nervous system. Cobalamin is a necessary cofactor for key enzymatic reactions within the nervous tissue. Deficiencies in cobalamin, whether due to dietary insufficiency, malabsorption, or interference by environmental toxins, such as nitrous oxide, can disrupt these pathways. Nitrous oxide (N
2O), a colorless and non-flammable gas, is extensively utilized in medical settings for its established analgesic, anesthetic, and anxiolytic properties. [
1]. Commonly referred to as “laughing gas” or “whippits”, nitrous oxide is recognized for its transient recreational effects, including euphoria, altered perception, and a sense of dissociation, which typically subside within a few minutes. [
2]. However, the misuse of this substance has escalated significantly in recent years, positioning it as one of the most commonly abused psychoactive agents among young individuals across Europe [
3].
The pervasive recreational utilization of nitrous oxide and the increase in consumption rates, both quantitatively and frequency-wise, have led to an uptick in chronic toxicity cases in recent years. There has been an exponential rise in hospitalizations due to neurological complications induced by N
2O, such as acute or subacute tetraparesis, which are often attributed to N
2O-induced neuropathy and/or myelopathy. N
2O-induced neuropathy typically presents as a length-dependent sensorimotor axonal neuropathy characterized by profound motor axonal loss predominantly affecting the lower limb [
4,
5,
6,
7]. Furthermore, subacute demyelinating patterns have been observed, demonstrating clinical and electrophysiological features that may occasionally mimic those of Guillain–Barré syndrome, also called acute inflammatory demyelinating polyradiculoneuropathy (AIDP) [
8,
9]. The distinction is important not only for diagnosis but also for prognosis. We know that demyelinating lesions often have a better prognosis given the potential for remyelination (especially if the causal aspect is stopped) while axonal loss is permanent. In the case of axonal loss, motor recovery is only possible by terminal reinnervation (and sensory recovery rather than by neuroplasticity), mechanisms slower and more limited than remyelination.
Thus, axonal damage is more severe. However, the initial clinical severity assessed by clinical examination scores does not make it possible to distinguish between these two pathophysiological processes. Only electroneuromyography allows this distinction. In primary demyelinating disorders, we also know that there can be secondary damage, and when the axonal damage is too severe, the signs of demyelination can no longer be evaluated. Thus, demyelinating damage could also occur earlier in N2O-induced neuropathies.
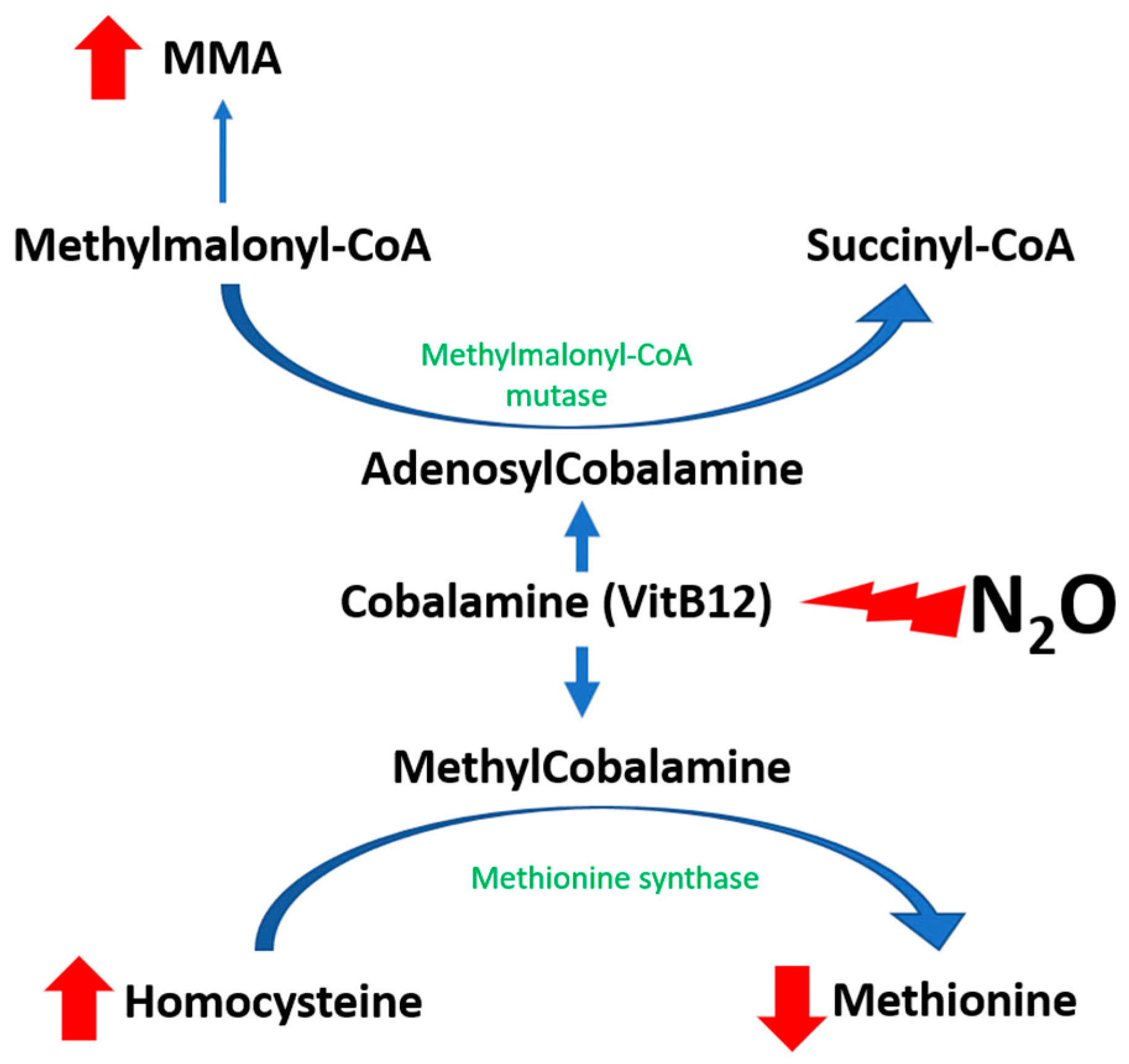
The pathophysiology of N
2O-induced toxicity is linked to the functional inhibition of vitamin B12, also known as cobalamin (
Figure 1). Nitrous oxide acts as a potent oxidizing agent, leading to the oxidation of the cobalt ion (Co+) within cobalamin(I), converting it to the Co3+ state and subsequently generating cobalamin (II), which is incapable of accepting methyl groups [
10]. This leads to a decrease in available methylcobalamin, a necessary cofactor for the enzyme methionine synthase. As a result, there is a subsequent decrease in the activity of methionine synthase, which plays a pivotal role in catalyzing the transformation of homocysteine into methionine [
11]. The authors propose that the accrual of cobalamin (II), which also acts as a cofactor for methionine synthase reductase (MTRR), promotes the enhancement of MTRR activity [
12]. This enzyme-mediated process facilitates the conversion of cobalamin (II) to cobalamin (III) via S-adenosylmethionine, thereby increasing the production of homocysteine [
1].
Related to the two previously described mechanisms, patients who have consumed N
2O typically exhibit markedly elevated levels of homocysteine. Plasma homocysteine levels are highly sensitive and serve as an indicator of recent N
2O exposure, given their rapid increase following consumption [
13]. Nonetheless, this biomarker is not exclusive to N
2O intoxication; elevated levels may also occur in instances of vitamin B9 or B12 deficiencies, renal insufficiency, or hereditary metabolic disorders.
The impact of nitrous oxide (N
2O) on the activity of methylmalonyl-CoA mutase, an enzyme facilitating the conversion of methylmalonic acid (MMA) to succinyl-CoA, remains a subject of ongoing debate. It has been hypothesized that the oxidation of cobalamin(I) by N
2O may induce a generalized cobalamin deficit, encompassing a reduction in adenosylcobalamin—a cofactor essential for methylmalonyl-CoA mutase function. Although elevations in MMA levels are not consistently observed and, thus, do not serve as a reliable marker for N
2O misuse, they are more specifically indicative of disturbances in vitamin B12-dependent pathways. Moreover, there appears to be a correlation between increased MMA levels and the clinical severity of N
2O intoxication [
13].
While the characteristics of neurological complications associated with nitrous oxide (N2O) exposure are extensively documented, the relationship between distinct peripheral neurological patterns and their underlying pathophysiological processes remains to be elucidated. This study aimed to improve understanding of N2O pathophysiology by examining metabolic alteration in patients presenting with axonal versus demyelinating patterns of N2O-induced neuropathy and myelopathy. By providing an in-depth analysis of the biochemical pathways that contribute to the observed electrophysiological variations, this research could potentially inform the development of targeted therapeutic strategies.
2. Materials and Methods
2.1. Patient Selection and Data Collection
Patients with N2O abuse (last declared N2O consumption is less than 1 week ago) treated in the CHU de Lille (from the Emergency Unit or general practice consultations) between March 2020 and April 2023, who underwent electromyography in our referral center, were included in the study. The study protocol was assigned the declaration number DEC21-356. In accordance with the French National Data Protection Commission regulation, written informed consent was not required for this non-interventional retrospective study. The data collected were extracted from hospital medical records. The exclusion criteria were as follows: patients with inherited metabolic disorders, renal insufficiency, folate deficiency, total vitamin B12 > 5 ng/mL (possible self-medication), and last declared N2O consumption more than 1 week ago.
2.2. Clinical Data
Clinical features collected included the presence of sensory symptoms, motor weakness (if present, affecting the lower and/or upper limbs, distal and/or proximal), gait abnormality, and deep tendon reflexes (classified as normal, absent for Achilles reflexes, absent in the lower limbs, or absent in all four limbs). The patients were then classified according to their clinical phenotypes: length-dependent vs. non-length-dependent, sensory or sensorimotor, and the functional evaluation carried out with the peripheral neuropathy disability (PND) (ranging from I, indicating sensory disturbances with preserved walking capability, to IV, representing confinement to a wheelchair or being bedridden,
Table 1). Concerning neurophysiological data, the criteria of Hadden et al. were used to define patients with demyelinating criteria [
14]. As usual, patients with no demyelinating criteria were classified as “axonal” concerning the group of neuropathy. However, as stated in the introduction, it is possible for a patient with a demyelinating pattern to also have an axonal loss, defined as a decrease in amplitude in the nerve conduction studies (defining sensory axonal loss and/or motor axonal loss).
2.3. Biological Measurements
Concurrent with clinical evaluation, quantitative determinations of vitamin B12, B9 (folate), homocysteine, methylmalonic acid (MMA), and methionine were conducted for each patient to ensure temporal consistency between biochemical and clinical assessments. For homocysteine and MMA, blood samples were collected in EDTA tubes. Plasma was aliquoted and then frozen and stored at −20 °C until analysis. Homocysteine was measured by high-performance liquid chromatography on UFLC XR (Shimadzu, Kyoto, Japan) associated with tandem mass spectrometry using MRM on API 3200 Q TRAP (AB SCIEX, Framingham, MA, USA). MMA was measured by ultra-performance liquid chromatography associated with tandem mass spectrometry, using UPLC I-CLASS/XEVO TQXS1 (Waters Corp., Milford, MA, USA). Plasma homocysteine increase is defined as >15 µmol/L and plasma MMA increase is defined as >0.4 µmol/L.
Vitamin B9 and B12 were measured using blood samples collected in a clot activator tube (CAT). Their concentration was determined by chemiluminescent immunoassay on a UNICEL DXI 800 (Beckman Coulter Inc., Brea, CA, USA). Serum total vitamin B12 deficiency was defined as <0.2 ng/mL, and serum folate deficiency was defined as <3.1 ng/mL.
2.4. Electrophysiological Data (EDX)
Electrophysiological assessments were systematically carried out by a certified neurophysiologist for all participants, utilizing the NATUS (Middleton, WI, USA) Dantec Keypoint apparatus (center of reference of neuromuscular disorders). A comprehensive sensory and motor nerve conduction study was performed, including the fibular and median nerves for motor conduction, while the sural and median nerves were assessed for sensory conduction. The inclusion of additional nerve conduction studies was at the discretion of the examining neurophysiologist.
For each participant, electromyographic examinations were reviewed to ascertain compliance with Hadden’s criteria for acute inflammatory demyelinating polyneuropathy (AIDP) [
14], as well as for axonal and demyelinating neuropathies in accordance with the classification system proposed by Albers [
15]. According to Albers, a reduced distal motor amplitude <80% of the norm on at least 2 nerves defines an axonal pattern, whereas a prolonged distal motor latency >120% of the upper limit of the norm, and/or proximal/distal motor amplitude ratio and/or motor conduction velocity < 80% of the norm, on at least 2 nerves, defines a demyelinating pattern. F-wave latency was not used in the classification for demyelinating ENMG because it was not systematically studied.
AIDP criteria were used to determine the groups as “demyelinating” and “axonal” for further comparisons.
2.5. Statistical Analyses
Statistical analyses were performed using JASP Team (2020) (JASP, Amsterdam, The Netherlands) and JASP (Version 0.18.1) (JASP. Group comparisons were performed using the independent samples t-test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. Statistical significance was defined at a p-value threshold of <0.05.
4. Discussion
The primary objective of this study was to enhance our understanding of the pathophysiological mechanisms underlying nitrous oxide (N2O) exposure and its effects on peripheral neurology. Our research specifically aimed to delineate the relationship between distinct neurological patterns induced by N2O exposure and their biochemical correlates. We focused on patients presenting with axonal versus demyelinating patterns of N2O-induced neuropathy and myelopathy, examining metabolic changes and their impact on the peripheral nervous system. A critical aspect of this study was the investigation of cobalamin (vitamin B12) metabolism, essential for the integrity of the nervous system and its disruption due to N2O exposure. This included analyzing elevated plasma homocysteine levels, indicative of recent N2O exposure, and the effect of N2O on methylmalonyl-CoA mutase activity.
The study aimed to provide a detailed analysis of the clinical and electrophysiological features of N2O-induced neurological damage, with a focus on understanding the biochemical pathways that contribute to these conditions. By exploring these pathways, the research sought to inform the development of targeted therapeutic strategies for N2O-induced neuropathy and myelopathy. This endeavor is particularly critical given the increasing prevalence of N2O misuse and the need for a deeper understanding of the pathophysiological basis for the varied neurological manifestations associated with its abuse.
From a clinical perspective, similar to other series in the literature, we primarily observe a sensorimotor axonal electromyographic profile with a motor or predominantly motor pattern that is length-dependent. Demyelination signs are found in 34–40% of patients, according to our criteria, most often presenting as mixed axonal–myelinic involvement (secondary axonal loss). In our cohort, patients with demyelinating features exhibit significantly higher PND scores. These demyelinating mechanisms may play a role in more subacute neuropathy, characterized by rapidly evolving symptoms and leading to greater functional impairment at diagnosis. However, a better prognosis may be expected in cases with higher motor amplitude in the common fibular and tibial nerves. A previous published study indicated a correlation between longer exposure duration and more prolonged symptoms with lower motor amplitudes. Further follow-up studies of these patients are needed, as there is a keen interest in understanding their outcomes in subgroups [
7].
The demyelinating abnormalities observed in EMG are essentially motor-based. This is linked to a methodological pitfall because the criteria for demyelination are more debated for sensory nerves. We should propose more a more systematic study of sensory evoked potentials for ataxic patients with normal sensory amplitudes on the peripheral nerves to better study on the sensory radicular damage of these patients and to search for proximal demyelination (as for AIDP).
Concerning the patients with normal EDX, we can suppose that (i) the EDX was too early, (ii) the patient only had central damage, or (iii) the patient only had a small fiber neuropathy (not evaluated by EDX study). On the other hand, we have to highlight the frequency of axonal loss (89% of patients, 80% with motor one), explaining the poor prognosis of this neuropathy.
The biological characteristics of the patients included in the study present a pattern indicative of nitrous oxide-related metabolic disturbance. Elevated homocysteine and methylmalonic acid (MMA) levels were a consistent finding, reflecting the direct impact of nitrous oxide on the metabolism of cobalamins. Specifically, homocysteine concentrations were significantly above the normal range, with a mean level of 104 µmol/L, highlighting a substantial deviation from standard values. Meanwhile, the observed decrease in vitamin B12 levels among these patients aligns with the known effects of nitrous oxide on vitamin B12 inactivation and subsequent functional deficiency. Contrary to the alterations seen in homocysteine and MMA, the levels of folate (vitamin B9) and methionine remained within the normal range across the cohort. This suggests that the metabolic perturbations are specific to the pathways involving vitamin B12, rather than a broad impairment of the one-carbon metabolic network. Collectively, these biological characteristics provide valuable insights into the biochemical sequelae of nitrous oxide exposure and underscore the need for targeted metabolic evaluations in affected individuals.
No differences were also found between the levels of MMA (methylmalonic acid) and methionine, which may seem contradictory to certain previously published studies where MMA levels increased and methionine levels decreased with clinical severity [
13,
16,
17]. However, in these studies, general clinical involvement was assessed without sub-categorization according to types of neurological involvement. These results suggest that metabolic alterations in the monocarbon pathway do not condition the type of neurological involvement, necessitating the investigation of still unknown pathways, such as alterations in antioxidant defenses. Indeed, nitrous oxide is a potent oxidizing agent that can lead to significant oxidative stress, potentially causing peripheral neurological damage [
18]. The only metabolic difference found between the axonal and demyelinating groups is a lower homocysteine level in demyelinating patients. However, the role of homocysteine in the direct neurological toxicity of nitrous oxide intoxication remains unclear. The fact that homocysteine is found to be higher in axonal involvement, which has a poorer prognosis, could suggest its toxicity to certain types of neuronal cells. Nevertheless, the kinetics of homocysteine in intoxication are still poorly understood and appear to be more directly related to the kinetics of nitrous oxide rather than clinical severity [
17]. Kinetic studies on these markers are, therefore, necessary to better understand their implications.
The group with the demyelination criterion had a more severe clinical presentation on admission than the pure axonal group with nevertheless lower hyperhomocysteinemia. This is in good agreement with the fact that hyperhomocysteinemia reflects secondary axonal damage. This goes with the hypothesis of a primary demyelinating neuropathy: thus, patients who have a subacute attack consult earlier, as they are faced with more serious signs, than those who have a chronic attack, more progress more slowly but with a much worse prognosis because the axonal lesions are more severe. We could assume here that the subacute demyelinating pattern differences are the result of consumption (i.e more intense/impulsive consumption with rapid initial demyelination) or perhaps that there are associated inflammatory factors.
Our study has several limitations. Since our cohort consists solely of patients who underwent electromyography at our referral center, we predominantly report cases with severe N
2O-induced neurological pathologies (100% were hospitalized patients, 89% had gait disorders, and 30% had a PND score greater than IIIa). This may represent only the tip of the iceberg: a large survey involving over 240,000 individuals found that 17% of participants reported chronic use of N
2O, with 4.2% of recreational users experiencing persistent paresthesia or numbness in a stocking-glove pattern, indicative of a length-dependent axonal polyneuropathy [
16]. Investigating metabolic disorders in these patients with milder symptoms could be highly informative. Similar to most studies on this topic, our study is limited by a small patient cohort and a lack of long-term follow-up, attributed to the challenges in tracking patients who often refuse care.
This study provides preliminary data that underscore the necessity for further confirmation through research on a larger patient cohort. Additionally, our findings suggest the potential need for exploring the neurotoxic effects of homocysteine. The limited scale of our current investigation highlights the importance of expanding our research scope to not only validate these initial observations but also to delve deeper into understanding the intricate relationship between elevated homocysteine levels and the possible neurotoxic implications. Future studies with expanded cohorts are crucial for establishing definitive conclusions and enhancing our comprehension of homocysteine role in nitrous oxide abuse.