Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis
Abstract
1. Introduction
2. Inflammatory Pathways in Sepsis
3. Overview of Quercetin and Its Natural Derivatives
4. Disease Management Roles of Quercetin and Its Natural Derivatives
4.1. Quercetin
4.2. Miquelianin
4.3. Reynoutrin
4.4. Rutin
4.5. Isoquercetin
4.6. Quercetin-3-O-Sambubioside
4.7. Quercitrin
4.8. Spiraeoside
4.9. Rhamnetin
4.10. Tamarixetin
4.11. Nepetin
4.12. Isorhamnetin
5. Oral Supplementations
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kempker, J.A.; Martin, G.S. A global accounting of sepsis. Lancet 2020, 395, 168–170. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yao, Y.; Yu, M.M.; Gao, Y.L.; Qi, A.L.; Jiang, T.Y.; Chen, Z.S.; Shou, S.T.; Chai, Y.F. Frequency and mortality of sepsis and septic shock in China: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 564. [Google Scholar] [CrossRef]
- Wen, R.; Liu, Y.P.; Tong, X.X.; Zhang, T.N.; Yang, N. Molecular mechanisms and functions of pyroptosis in sepsis and sepsis-associated organ dysfunction. Front. Cell Infect. Microbiol. 2022, 12, 962139. [Google Scholar] [CrossRef]
- Doganyigit, Z.; Eroglu, E.; Akyuz, E. Inflammatory mediators of cytokines and chemokines in sepsis: From bench to bedside. Hum. Exp. Toxicol. 2022, 41, 9603271221078871. [Google Scholar] [CrossRef]
- Cheema, H.A.; Sohail, A.; Fatima, A.; Shahid, A.; Shahzil, M.; Ur Rehman, M.E.; Awan, R.U.; Chinnam, S.; Nashwan, A.J. Quercetin for the treatment of COVID-19 patients: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2427. [Google Scholar] [CrossRef] [PubMed]
- Tianyu, Z.; Liying, G. Identifying the molecular targets and mechanisms of xuebijing injection for the treatment of COVID-19 via network parmacology and molecular docking. Bioengineered 2021, 12, 2274–2287. [Google Scholar] [CrossRef]
- Bozkurt, E.U.; Ozel, A.; Erol, M.; Tenekecigil, A.; Gayret, O.B.; Buke, O.; Tosun, V. Comparison of effects of quercetin and ascorbic acid on inflammatory cytokines and antioxidant biomarkers in infant rats using an experimental sepsis model. Bratisl. Lek. Listy 2023, 124, 768–773. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, X.; Chen, Q.; Wang, X.; Wu, Y.; Zhao, X. Quercetin inhibits the NOX2/ROS-mediated NF-kappaB/TXNIP signaling pathway to ameliorate pyroptosis of cardiomyocytes to relieve sepsis-induced cardiomyopathy. Toxicol. Appl. Pharmacol. 2023, 477, 116672. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Naeini, F.; Asghari Azar, V.; Hasanzadeh, M.; Ostadrahimi, A.; Niazkar, H.R.; Mobasseri, M.; Tutunchi, H. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine 2021, 86, 153567. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Weindl, G. Intracellular Lipopolysaccharide Sensing as a Potential Therapeutic Target for Sepsis. Trends Pharmacol. Sci. 2019, 40, 187–197. [Google Scholar] [CrossRef]
- Das, U.N. Infection, Inflammation, and Immunity in Sepsis. Biomolecules 2023, 13, 1332. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Lopes-Pires, M.E.; Frade-Guanaes, J.O.; Quinlan, G.J. Clotting Dysfunction in Sepsis: A Role for ROS and Potential for Therapeutic Intervention. Antioxidants 2021, 11, 88. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, S.H.; Liu, C.Y.; Gao, Y.L.; Meng, X.L.; Wei, W.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-kappaB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, X.; Liu, Y.; Chen, X.; Li, C.; Wang, L.; Zhao, W. Dexmedetomidine alleviates lung injury in sepsis mice through regulating P38 MAPK signaling pathway. Panminerva Med. 2021, 63, 563–564. [Google Scholar] [CrossRef]
- Su, J.; Guan, B.; Chen, K.; Feng, Z.; Guo, K.; Wang, X.; Xiao, J.; Chen, S.; Chen, W.; Chen, L.; et al. Fucoxanthin Attenuates Inflammation via Interferon Regulatory Factor 3 (IRF3) to Improve Sepsis. J. Agric. Food Chem. 2023, 71, 12497–12510. [Google Scholar] [CrossRef]
- Abdelnaser, M.; Alaaeldin, R.; Attya, M.E.; Fathy, M. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 397, 947–958. [Google Scholar] [CrossRef]
- Liu, S.; Pi, J.; Zhang, Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Bo, S.; Chang, S.K.; Chen, Y.; Sheng, Z.; Jiang, Y.; Yang, B. The structure characteristics, biosynthesis and health benefits of naturally occurring rare flavonoids. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives—A biochemical perspective. Food Chem. 2021, 334, 127508. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Li, L.; Cheng, J.; Lu, F.; Du, Y.; Xie, Y.; Zhou, C.; Zhang, J.; Feng, Y. Optimized HPLC extraction method of quercetin and berberine based on response surface analysis. RSC Adv. 2023, 13, 29427–29437. [Google Scholar] [CrossRef]
- Yin, H.; Ma, J.; Han, J.; Li, M.; Shang, J. Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-beta-D-glucuronide in rats by HPLC-MS. PeerJ 2019, 7, e6665. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Savadkouhi, N.; Ebrahimzadeh, M.A. Drug design strategies that aim to improve the low solubility and poor bioavailability conundrum in quercetin derivatives. Expert Opin. Drug Discov. 2023, 18, 1117–1132. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef]
- Gulec, K.; Demirel, M. Characterization and Antioxidant Activity of Quercetin/Methyl-beta-Cyclodextrin Complexes. Curr. Drug Deliv. 2016, 13, 444–451. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Ma, Z.X.; Zhang, R.Y.; Rui, W.J.; Wang, Z.Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/ BDNF signaling pathway. Behav. Brain Res. 2021, 406, 113245. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Lei, C.; Lei, X.; Zhu, X.; Yang, L.; Zhang, R. Quercetin exerts antidepressant and cardioprotective effects in estrogen receptor alpha-deficient female mice via BDNF-AKT/ERK1/2 signaling. J. Steroid Biochem. Mol. Biol. 2021, 206, 105795. [Google Scholar] [CrossRef]
- Gasmi, S.; Rouabhi, R.; Kebieche, M.; Boussekine, S.; Salmi, A.; Toualbia, N.; Taib, C.; Bouteraa, Z.; Chenikher, H.; Henine, S.; et al. Effects of Deltamethrin on striatum and hippocampus mitochondrial integrity and the protective role of Quercetin in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 16440–16457. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Luo, X.; Bao, X.; Weng, X.; Bai, X.; Feng, Y.; Huang, J.; Liu, S.; Jia, H.; Yu, B. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-kappaB and ROS/AMPK pathway. Life Sci. 2022, 291, 120064. [Google Scholar] [CrossRef]
- Chiang, S.C.C.; Owsley, E.; Panchal, N.; Chaturvedi, V.; Terrell, C.E.; Jordan, M.B.; Mehta, P.A.; Davies, S.M.; Akeno, N.; Booth, C.; et al. Quercetin ameliorates XIAP deficiency-associated hyperinflammation. Blood 2022, 140, 706–715. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Kim, K.S.; Kim, H.S.; Yu, K.N.; Sung, J.S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, W.; Yu, B.; Liang, H.; Mao, S.; Hu, X.; Feng, Y.; Xu, J.; Chu, L. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-kappaB signaling pathway. Biomed. Pharmacother. 2023, 168, 115653. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-kappaB axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Mamun, A.A.; Barreto, G.E.; Rashid, M.; Perveen, A.; Ashraf, G.M. Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 2020, 84, 106479. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, M.A.; Bejeshk, M.A.; Doustimotlagh, A.H.; Najafipour, H.; Eftekhari, M.; Mahmoodi, M.; Azizi, M.; Rostamabadi, F.; Pourghadamyari, H. The Alleviating Impacts of Quercetin on Inflammation and Oxidant-antioxidant Imbalance in Rats with Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2023, 22, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Ponczek, M.B.; Nowak, P. Polyphenol compounds belonging to flavonoids inhibit activity of coagulation factor X. Int. J. Biol. Macromol. 2014, 65, 129–135. [Google Scholar] [CrossRef]
- Pignatelli, P.; Di Santo, S.; Carnevale, R.; Violi, F. The polyphenols quercetin and catechin synergize in inhibiting platelet CD40L expression. Thromb. Haemost. 2005, 94, 888–889. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chen, S.R.; Ko, P.E.; Yang, M.Y.; Yu, M.H.; Wang, C.J.; Lee, H.J. Quercetin-3-O-beta-d-glucuronide in the Nuciferine Leaf Polyphenol Extract Promotes Neurogenesis Involving the Upregulation of the Tropomyosin Receptor Kinase (Trk) Receptor and AKT/Phosphoinositide 3-Kinase Signaling Pathway. J. Agric. Food Chem. 2023, 71, 15582–15592. [Google Scholar] [CrossRef]
- Yuan, Z.; Luan, G.; Wang, Z.; Hao, X.; Li, J.; Suo, Y.; Li, G.; Wang, H. Flavonoids from Potentilla parvifolia Fisch. and Their Neuroprotective Effects in Human Neuroblastoma SH-SY5Y Cells in vitro. Chem. Biodivers. 2017, 14, e1600487. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Jung, S.Y.; Kim, G.D.; Lee, S.Y.; Shin, H.S. Miquelianin Inhibits Allergic Responses in Mice by Suppressing CD4(+) T Cell Proliferation. Antioxidants 2021, 10, 1120. [Google Scholar] [CrossRef]
- Yang, W.; Tu, H.; Tang, K.; Huang, H.; Ou, S.; Wu, J. Reynoutrin Improves Ischemic Heart Failure in Rats Via Targeting S100A1. Front. Pharmacol. 2021, 12, 703962. [Google Scholar] [CrossRef]
- Sun, X.Y.; Li, L.J.; Dong, Q.X.; Zhu, J.; Huang, Y.R.; Hou, S.J.; Yu, X.L.; Liu, R.T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflammation 2021, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, W.; Ji, S.; Wang, J.; Luo, J.; Lu, B. Sophora japonica flowers and their main phytochemical, rutin, regulate chemically induced murine colitis in association with targeting the NF-kappaB signaling pathway and gut microbiota. Food Chem. 2022, 393, 133395. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Tan, C.; Meng, F.; Reece, E.A.; Zhao, Z. Modulation of nuclear factor-kappaB signaling and reduction of neural tube defects by quercetin-3-glucoside in embryos of diabetic mice. Am. J. Obstet. Gynecol. 2018, 219, 197.e1–197.e8. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, J.; Gong, Y.; Zhou, Z.; Wang, J. Isoquercetin alleviates sleep deprivation dependent hippocampal neurons damage by suppressing NLRP3-induced pyroptosis. Immunopharmacol. Immunotoxicol. 2022, 44, 766–772. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Z.; Lei, H.; Wu, F.; Chen, C.; Cao, Z.; Song, Y.; Zhang, C.; Zhou, J.; Lu, Y.; et al. Dietary Isoquercetin Reduces Hepatic Cholesterol and Triglyceride in NAFLD Mice by Modulating Bile Acid Metabolism via Intestinal FXR-FGF15 Signaling. J. Agric. Food Chem. 2023, 71, 7723–7733. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ma, J.L.; Chen, C.; Huang, P.; Ji, J.H.; Wu, D.; Ren, L.Q. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity through the AKT/Bcl-2 signaling pathway. Phytomedicine 2022, 94, 153815. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Zhang, R.; Liu, X.; Ma, C.; Cao, G.; Wei, Y.; Yang, P. Protective Effect of Hedyotis diffusa Willd. Ethanol Extract on Isoniazid-Induced Liver Injury in the Zebrafish Model. Drug Des. Devel Ther. 2022, 16, 1995–2015. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yin, W.; Zou, Z.; Zhang, C.; Sun, M.; Min, L.; Yang, L.; Kong, L. Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J. Adv. Res. 2021, 28, 255–267. [Google Scholar] [CrossRef]
- Xiong, W.; Yuan, Z.; Wang, T.; Wu, S.; Xiong, Y.; Yao, Y.; Yang, Y.; Wu, H. Quercitrin Attenuates Acetaminophen-Induced Acute Liver Injury by Maintaining Mitochondrial Complex I Activity. Front. Pharmacol. 2021, 12, 586010. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin Rapidly Alleviated Depression-like Behaviors in Lipopolysaccharide-Treated Mice: The Involvement of PI3K/AKT/NF-kappaB Signaling Suppression and CREB/BDNF Signaling Restoration in the Hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Cespedes-Acuna, C.L.; Oh, J.W. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem. Toxicol. 2021, 154, 112327. [Google Scholar] [CrossRef]
- Lee, H.; Krishnan, M.; Kim, M.; Yoon, Y.K.; Kim, Y. Rhamnetin, a Natural Flavonoid, Ameliorates Organ Damage in a Mouse Model of Carbapenem-Resistant Acinetobacter baumannii-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 12895. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Yang, H.; Cui, Y.; Wang, H.; Zhou, H.; Zhang, J.; Du, B.; Zhai, Q.; Wu, D.; et al. Tamarixetin protects against cardiac hypertrophy via inhibiting NFAT and AKT pathway. J. Mol. Histol. 2019, 50, 343–354. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.J.; Cho, J.; Gharbi, A.; Han, H.D.; Kang, T.H.; Kim, Y.; Lee, Y.; Park, W.S.; Jung, I.D.; et al. Tamarixetin Exhibits Anti-inflammatory Activity and Prevents Bacterial Sepsis by Increasing IL-10 Production. J. Nat. Prod. 2018, 81, 1435–1443. [Google Scholar] [CrossRef]
- Song, W.; Wang, B.; Sui, L.; Shi, Y.; Ren, X.; Wang, X.; Kong, X.; Hou, J.; Wang, L.; Wei, L.; et al. Tamarixetin Attenuated the Virulence of Staphylococcus aureus by Directly Targeting Caseinolytic Protease P. J. Nat. Prod. 2022, 85, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Ren, X.; Wang, L.; Kong, X.; Wang, X.; Chang, X.; Guo, X.; Shi, Y.; Guan, J.; Wang, T.; et al. Nepetin reduces virulence factors expression by targeting ClpP against MRSA-induced pneumonia infection. Virulence 2022, 13, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1beta induced inflammation via NF-kappaB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Kim, S.G.; Park, H.H.; Lee, E.; Lee, Y.J.; Jin, M.; Lee, E. Nepetin, a natural compound from Inulae flos, suppresses degranulation and eicosanoid generation through PLCgamma1 and Akt signaling pathways in mast cells. Arch. Pharm. Res. 2020, 43, 224–232. [Google Scholar] [CrossRef]
- Chu, B.; Chen, S.; Zheng, X.; Ye, J.; Cheng, X.; Zhang, L.; Guo, D.; Wang, P.; Hong, D.; Hong, Z. Nepetin inhibits osteoclastogenesis by inhibiting RANKL-induced activation of NF-kappaB and MAPK signalling pathway, and autophagy. J. Cell Mol. Med. 2020, 24, 14366–14380. [Google Scholar] [CrossRef]
- Saba, E.; Lee, Y.S.; Yang, W.K.; Lee, Y.Y.; Kim, M.; Woo, S.M.; Kim, K.; Kwon, Y.S.; Kim, T.H.; Kwak, D.; et al. Effects of a herbal formulation, KGC3P, and its individual component, nepetin, on coal fly dust-induced airway inflammation. Sci. Rep. 2020, 10, 14036. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, J.; Kim, B.R.; Jun, I.; Kim, T.I.; Namkung, W. Isorhamnetin Ameliorates Dry Eye Disease via CFTR Activation in Mice. Int. J. Mol. Sci. 2021, 22, 3954. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Wang, Y.; Ding, J.; Wang, R. Isorhamnetin Alleviates High Glucose-Aggravated Inflammatory Response and Apoptosis in Oxygen-Glucose Deprivation and Reoxygenation-Induced HT22 Hippocampal Neurons Through Akt/SIRT1/Nrf2/HO-1 Signaling Pathway. Inflammation 2021, 44, 1993–2005. [Google Scholar] [CrossRef]
- Liu, N.; Feng, J.; Lu, X.; Yao, Z.; Liu, Q.; Lv, Y.; Han, Y.; Deng, J.; Zhou, Y. Isorhamnetin Inhibits Liver Fibrosis by Reducing Autophagy and Inhibiting Extracellular Matrix Formation via the TGF-beta1/Smad3 and TGF-beta1/p38 MAPK Pathways. Mediat. Inflamm. 2019, 2019, 6175091. [Google Scholar] [CrossRef]
- Rodriguez, L.; Badimon, L.; Mendez, D.; Padro, T.; Vilahur, G.; Pena, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-hepatitis C virus activity and synergistic effect of Nymphaea alba extracts and bioactive constituents in liver infected cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, D.; Das, S.; Jha, N.K.; Pal, M.; Kolesarova, A.; Kesari, K.K.; Kalita, J.C.; Slama, P. Clinical Potential of Himalayan Herb Bergenia ligulata: An Evidence-Based Study. Molecules 2022, 27, 7039. [Google Scholar] [CrossRef]
- Eldin Elhawary, S.S.; Elmotyam, A.K.E.; Alsayed, D.K.; Zahran, E.M.; Fouad, M.A.; Sleem, A.A.; Elimam, H.; Rashed, M.H.; Hayallah, A.M.; Mohammed, A.F.; et al. Cytotoxic and anti-diabetic potential, metabolic profiling and insilico studies of Syzygium cumini (L.) Skeels belonging to family Myrtaceae. Nat. Prod. Res. 2022, 36, 1026–1030. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Petrova, N.V.; Shaldaeva, T.M.; Koval, V.V.; Chernonosov, A.A. Non-Targeted Screening of Metabolites in Aqueous-Ethanol Extract from Spiraea hypericifolia (Rosaceae) Using LC-HRMS. Int. J. Mol. Sci. 2023, 24, 13872. [Google Scholar] [CrossRef] [PubMed]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef]
- Pan, R.Y.; Ma, J.; Kong, X.X.; Wang, X.F.; Li, S.S.; Qi, X.L.; Yan, Y.H.; Cheng, J.; Liu, Q.; Jin, W.; et al. Sodium rutin ameliorates Alzheimer’s disease-like pathology by enhancing microglial amyloid-beta clearance. Sci. Adv. 2019, 5, eaau6328. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: A double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Chretien, M. Isoquercetin as an Anti-Covid-19 Medication: A Potential to Realize. Front. Pharmacol. 2022, 13, 830205. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yang, Y.; Duan, Y.; Li, C.; Gao, H.; Liu, H.; Cui, Q.; Guo, Z.; Liu, X.; Wang, Z. Quality Marker Discovery and Quality Evaluation of Eucommia ulmoides Pollen Using UPLC-QTOF-MS Combined with a DPPH-HPLC Antioxidant Activity Screening Method. Molecules 2023, 28, 5288. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Gainche, M.; Ogeron, C.; Ripoche, I.; Senejoux, F.; Cholet, J.; Decombat, C.; Delort, L.; Berthon, J.Y.; Saunier, E.; Caldefie Chezet, F.; et al. Xanthine Oxidase Inhibitors from Filipendula ulmaria (L.) Maxim. and Their Efficient Detections by HPTLC and HPLC Analyses. Molecules 2021, 26, 1939. [Google Scholar] [CrossRef]
- Savina, T.; Lisun, V.; Feduraev, P.; Skrypnik, L. Variation in Phenolic Compounds, Antioxidant and Antibacterial Activities of Extracts from Different Plant Organs of Meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules 2023, 28, 3512. [Google Scholar] [CrossRef]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The Marine Seagrass Halophila stipulacea as a Source of Bioactive Metabolites against Obesity and Biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef]
- Novo Belchor, M.; Hessel Gaeta, H.; Fabri Bittencourt Rodrigues, C.; Ramos da Cruz Costa, C.; de Oliveira Toyama, D.; Domingues Passero, L.F.; Dalastra Laurenti, M.; Hikari Toyama, M. Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules 2017, 22, 1441. [Google Scholar] [CrossRef]
- Shatta, M.A.; El-Derany, M.O.; Gibriel, A.A.; El-Mesallamy, H.O. Rhamnetin ameliorates non-alcoholic steatosis and hepatocellular carcinoma in vitro. Mol. Cell Biochem. 2023, 478, 1689–1704. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Musharraf, S.G.; Xiao, J.; Saeed, A.; Du, M.; Khatib, A.; Abdel-Daim, M.M.; et al. Exploring natural products-based cancer therapeutics derived from egyptian flora. J. Ethnopharmacol. 2021, 269, 113626. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, T.; Zhao, C.; Huang, X.; Du, W. Resistance of nepetin and its analogs on the fibril formation of human islet amyloid polypeptide. Int. J. Biol. Macromol. 2021, 166, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Vitale, J.A.; Belcaro, G.; Hu, S.; Feragalli, B.; Vinciguerra, G.; Cacchio, M.; Bonanni, E.; Giacomelli, L.; Eggenhoffner, R.; et al. Quercetin phytosome(R) in triathlon athletes: A pilot registry study. Minerva Med. 2018, 109, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Slighoua, M.; Amrati, F.E.; Chebaibi, M.; Mahdi, I.; Al Kamaly, O.; El Ouahdani, K.; Drioiche, A.; Saleh, A.; Bousta, D. Quercetin and Ferulic Acid Elicit Estrogenic Activities In Vivo and In Silico. Molecules 2023, 28, 5112. [Google Scholar] [CrossRef]
- Yamada, S.; Shirai, M.; Inaba, Y.; Takara, T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: A randomized, placebo-controlled, double-blind parallel-group study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4331–4345. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Owczarek-Januszkiewicz, A.; Magiera, A.; Olszewska, M.A. Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 14784. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Parravano, M.; Tedeschi, M.; Manca, D.; Costanzo, E.; Di Renzo, A.; Giorno, P.; Barbano, L.; Ziccardi, L.; Varano, M.; Parisi, V. Effects of Macuprev((R)) Supplementation in Age-Related Macular Degeneration: A Double-Blind Randomized Morpho-Functional Study Along 6 Months of Follow-Up. Adv. Ther. 2019, 36, 2493–2505. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Xu, L.; Bian, X.; Lian, W.; Yin, S.; Zhao, S.; Gao, W.; Guo, C.; Shi, T. Quercetin positively affects gene expression profiles and metabolic pathway of antibiotic-treated mouse gut microbiota. Front. Microbiol. 2022, 13, 983358. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Xu, W.; Han, J.; Liu, Q.Y.; Gao, L.F.; Wang, X.H.; Li, X.L. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer. Drugs 2020, 31, 684–692. [Google Scholar] [CrossRef]
- Khan, A.; Iqtadar, S.; Mumtaz, S.U.; Heinrich, M.; Pascual-Figal, D.A.; Livingstone, S.; Abaidullah, S. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial. Front. Pharmacol. 2022, 13, 898062. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Chang, Z.; Li, J.; Li, J. Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei. Antibiotics 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.N.; Wang, W.H.; Qu, Y.J.; Xue, G.L.; Wei, Z.Y.; Liu, J.Q.; Han, H.Y.; Zhang, S.; Song, P. Biosynthesis and evaluation of a novel highly water-soluble quercetin glycoside derivative. J. Asian Nat. Prod. Res. 2022, 24, 754–760. [Google Scholar] [CrossRef]
- Majumder, D.; Roychoudhry, S.; Kundu, S.; Dey, S.K.; Saha, C. Hydrophobic quercetin encapsulated hemoglobin nanoparticles: Formulation and spectroscopic characterization. J. Biomol. Struct. Dyn. 2022, 40, 9860–9869. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Ji, H.; Lin, Q.; Li, X.; Sang, S.; Julian McClements, D.; Chen, L.; Long, J.; Jiao, A.; et al. Physicochemical stability, antioxidant activity, and antimicrobial activity of quercetin-loaded zein nanoparticles coated with dextrin-modified anionic polysaccharides. Food Chem. 2023, 415, 135736. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tong, M.; Li, S.; Yu, X.; Hu, Z.; Zhang, Q.; Xu, R.; Wang, J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: A case of quercetin. Drug Dev. Ind. Pharm. 2021, 47, 153–162. [Google Scholar] [CrossRef] [PubMed]
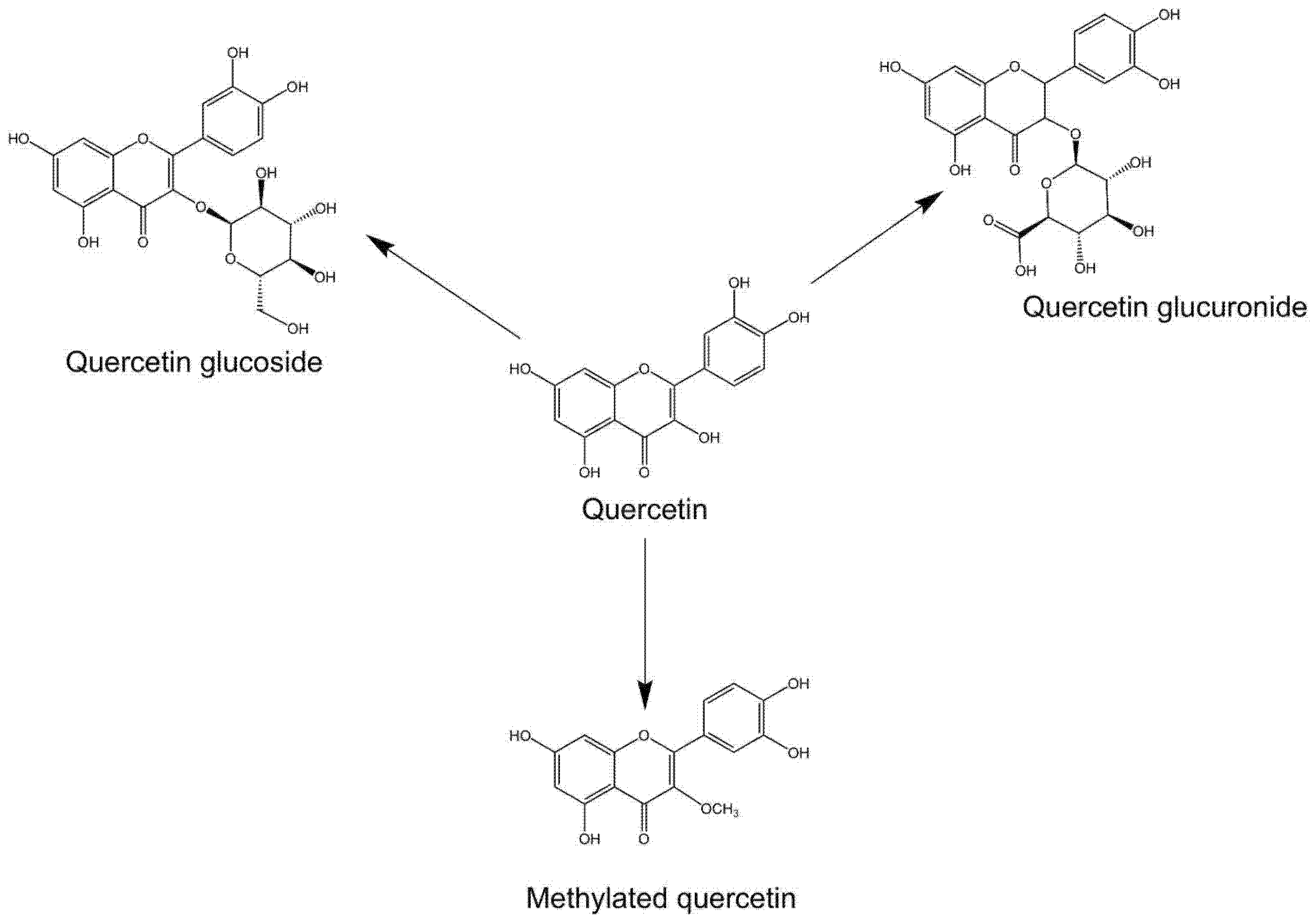
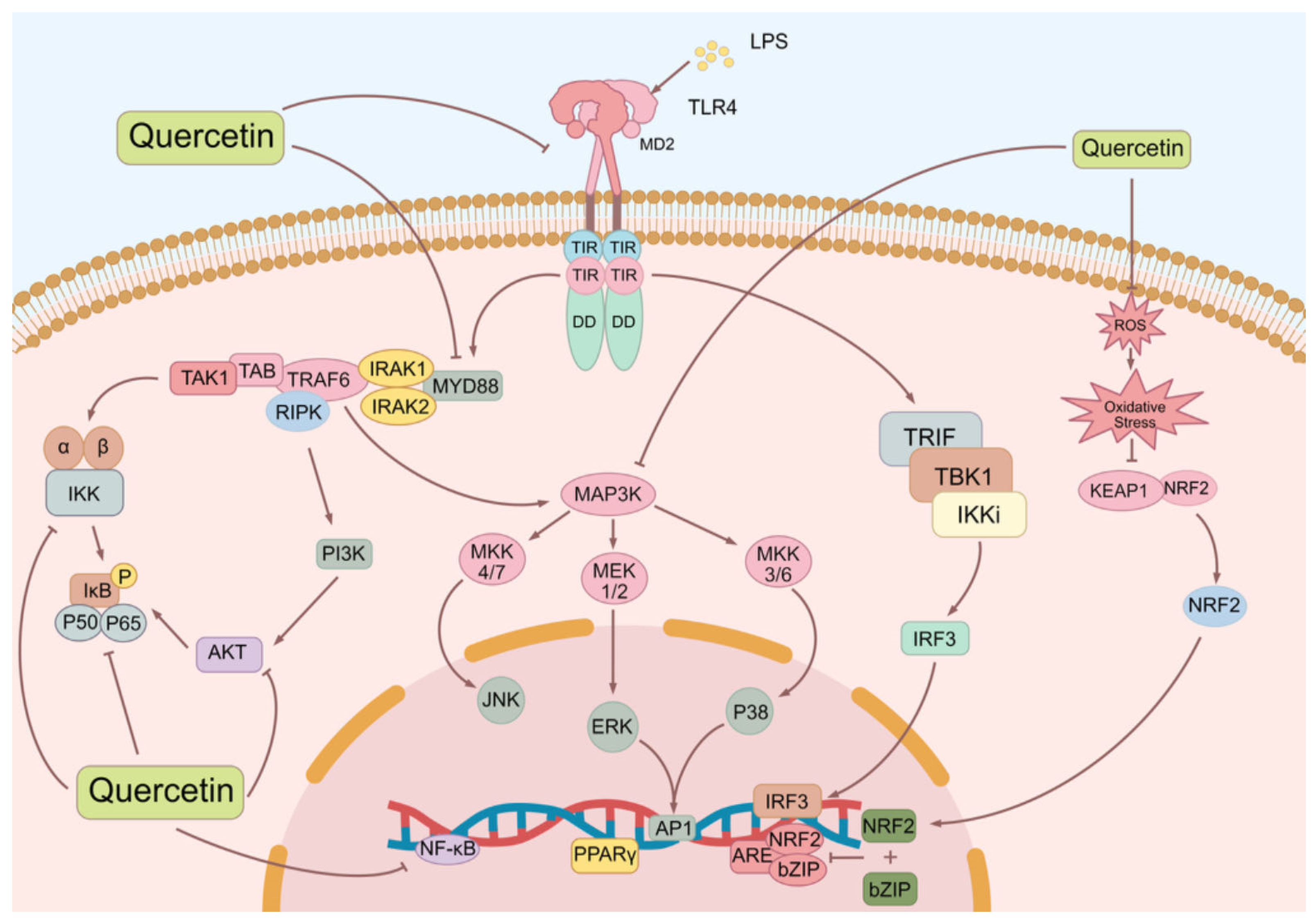
| Symptoms/Phenomena Associated with Sepsis | Stimulants | Main Study Subjects | Work Concentration | Ref. |
|---|---|---|---|---|
| ALI | LPS (10 μg/mL) | A549 cells | 10 μM | [38] |
| Macrophage pyroptosis | LPS (10 μg/mL), ATP (5 mM) | THP-1 cells | 50 μM | [39] |
| Immunodeficiency and hyperinflammation | In vivo: LPS (10 ng/kg) In vitro: LPS (1 μg/mL) | Human adherent monocyte; mouse BMDMs | In vivo: 50 mg/kg In vitro: 50 μM | [40] |
| CI/RI | the MCAO/R model | Male SD rats | 50 mg/kg | [44] |
| Irritable bowel syndrome and IBD | Diquat (100 μM) | IPEC-1 cells | 5 μM | [47] |
| Asthma | 1% aerosolized OVA | 8-week-old male Wistar rats | 50 mg/kg | [48] |
| Chemical Structure, Name, and Formula | Objectives | Subjects | Signaling Pathways/Axes | Main Outcome (Increase/Decrease) | Ref. |
|---|---|---|---|---|---|
| Miquelianin (C21H18O1) | Antioxidant effect | In vivo: male SD rats (275–300 g) | JNK and MAK-ERK | Decreased: ROS, cAMP and RAS, p-ERK1/2, HO-1, MMP-2, and MMP-9 | [51] |
| Neurodegenerative diseases | In vivo: 24-week-old male C57BL/6 mice; In vitro: HT22 and SH-SY5Y cells | TrKR and PI3K/AKT | Increased: TrKR, PI3K/AKT, neurite outgrowth levels | [52] | |
| Allergic diseases | In vitro: Th2 and CD4+ T cells In vivo: 6-week-old female BALB/c mice | C-Raf-ERK1/2-Nrf2 | Increased: HO-1 Decreased: IL-2 | [54] | |
| Reynoutrin (C20H18O11) | IHF | In vivo: male SD rats (180–220 g) | NF-κB | Decreased: S100A1, MMP-2, MMP-9, p-P65, TGF-β1 | [55] |
| Rutin (C27H30O16) | Nervous system inflammation and cognitive dysfunction | In vivo: 6-month-old male tau-P301S mice | NF-κB | Increased: PP2A Decreased: tau oligomers | [56] |
| Colitis | In vivo: 8-week-old female C57BL/6 mice | NF-κB | Increased: TJ protein Decreased: FITC-dextran and endotoxin, Romboutsia ilealis and Eubacterium fissicatena group | [57] | |
| Isoquercetin (C21H20O12) | ALD | In vitro: HepG2 cells | Nrf2/ARE, NF-κB | Decreased: NO, ROS and MDA levels, TNF-α | [58] |
| Birth defects in diabetic pregnancies | In vivo: female C57BL/6 mice | NF-κB, ER-stress | Increased: SOD1 Decreased: P65, IKK, NOS2 | [59] | |
| SD-induced neuronal injury | In vivo: C57BL/6 mice | Not mentioned | Decreased: NLRP3, caspase-1, ASC, IL-1β, IL-18, and GSDMD. | [60] | |
| NAFLD | In vivo: 6-week-old male C57BL/6 mice | Intestinal FXR-Fgf15 signaling | Decreased: cholesterol and triglyceride levels | [61] | |
| DOX-induced cardiomyocyte apoptosis | In vitro: H9c2 and HMC cells | AKT/Bcl2 | Increased: LDH, ΔΨm Decreased: oxidative stress levels, AKT, Bcl-2, Bax, CytC, and pro-caspase-3 | [62] | |
| Quercetin-3-O-sambubioside (C26H28O16) | Hepatoprotective effect of HDWE | In vivo: transgenic zebrafish with a liver-specific fluorescent probe (L-FABP: EGFP) | Not mentioned | Increased: SOD, GSH Decreased: ALT, AST | [63] |
| Quercitrin (C21H20O11) | OA | In vitro: chondrocytes, SW1353 cells In vivo: male SD rats (200 ± 20 g) | P110α/AKT/mTOR | Increased: collagen II Decreased: MMP-13 | [64] |
| APAP-induced liver injury | In vitro: L-02 cells In vivo: BALB/c mice | Not mentioned | Increased: SOD, GSH, GSH-Px, CAT Decreased: ALT, AST, LDH, IL-6, TNF-α, ROS, MDA | [65] | |
| Neuroinflammation and depression | In vivo: 6–8-week-old male ICR mice (22–24 g) | PI3K/AKT/NF-κB, MEK/ERK, pCREB/BDNF/PSD95/Synapsin1 | Increased: ERK signaling Decreased: IL-10, IL-1β, and TNF-α | [66] | |
| Spiraeoside (C21H20O12) | Antioxidative and anti-cancer effects | In vitro: HeLa cells | Bcl-2/Bid/caspase-9/-3 pathway, cell cycle-related CDK2-cyclin E pathway | Increased: caspase-9, caspase-3 Decreased: Bcl-2 and Bid, CDK2-cyclin E, MUDENG, aromatase, monoamine oxidase A/B, and angiotensin-converting enzyme | [67] |
| Rhamnetin (C16H12O7) | Sepsis caused by CRAB and E. coli | In vitro: RAW 264.7 cells and HEK cells In vivo: 6-week-old female ICR mice | Not mentioned | Decreased: IL-6 and NO | [68] |
| Tamarixetin (C16H12O7) | Cardiac hypertrophy | In vitro: H9c2 cells In vivo: 8–10-week-old male C57BL/6 mice | NFAT and PI3K/AKT | Decreased: VW/BW, LW/BW, echocardiographic parameters, hypertrophic markers, ROS | [69] |
| Bacterial sepsis | In vitro: BMDCs In vivo: 6-week-old female C57BL/6 mice | MAPK/JNK | Increased: IL-10 Decreased: p-JNK1, p-p38 and p-AKT, COX-2 | [70] | |
| S. aureus infection | In vivo: 6-week-old female C57BL/6J mice | Not mentioned | Increased: urease Decreased: HLa and PVL proteins, ClpP activity, hla, agr, RNAIII, pvl, PSM-α and spa genes | [71] | |
| Nepetin (C16H12O7) | MRSA infection | In vivo: 7-week-old female C57BL/6J mice | Not mentioned | Decreased: thermal stability of ClpP | [72] |
| RPE | In vitro: ARPE-19 cells | NF-κB and MAPKs | Decreased: IL-6, IL-8, and MCP-1 | [73] | |
| Inflammation and allergy | In vitro: BMMCs In vivo: male BALB/c mice | AKT/NF-κB/COX-2, and IGE/Ag | Decreased: COX-2, PLCγ1, cPLA2, LTC4, PGD2, Ca2+ | [74] | |
| Inflammatory osteolysis | In vitro: osteoclasts In vivo: 6-8-week-old male BALB/c mice | NF-κB and MAPK | Decreased: TRAF6- dependent ubiquitination of Beclin-1 | [75] | |
| CFD-induced pneumonia and asthma | In vitro: MH-S cells In vivo: 6-week-old C57BL/6 mice | NF-κB and MAPK, the localization of IRAK-1 | Decreased: NO, INOS, COX-2, IL-1β, IL-6, and TNF-α, ADMA, SDMA | [76] | |
| Isorhamnetin (C16H12O7) | DED | In vitro: FRT cells, T84 cells, HEK-293T cells, CHO cells, human CorE and CorjE cells In vivo: 6-week-old ICR mice (30 g) | PI3K/AKT and NF-κB | Increased: CFTR activity and tear secretion Decreased: IL-1β, IL-8, and TNF-α | [77] |
| Diabetes-associated cerebral I/R injury | In vitro: HT22 cells | AKT/SIRT1/Nrf2/HO-1 | Decreased: SIRT1, Nrf2, HO-1, p-AKT | [78] | |
| Liver fibrosis | In vivo: 8-week-old male C57 mice (22–24 g) | TGF-β1-mediated SMAD3 and p38-MAPK | Increased: PPAR-γ, MMP-2 Decreased: TGF-β1, SMAD3, ALT, AST, Collagen I and III, α-SMA, Beclin-1, LC3, TIMP-1 | [79] | |
| Antiplatelet and antithrombotic effects | In vitro: human peripheral blood | Not mentioned | Increased: Ca2+ Decreased: platelet ATP levels, ΔΨm | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Lv, L.; Feng, H.; Gu, W. Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis. Biomedicines 2024, 12, 444. https://doi.org/10.3390/biomedicines12020444
Wang T, Lv L, Feng H, Gu W. Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis. Biomedicines. 2024; 12(2):444. https://doi.org/10.3390/biomedicines12020444
Chicago/Turabian StyleWang, Tian, Linxi Lv, Hui Feng, and Wei Gu. 2024. "Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis" Biomedicines 12, no. 2: 444. https://doi.org/10.3390/biomedicines12020444
APA StyleWang, T., Lv, L., Feng, H., & Gu, W. (2024). Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis. Biomedicines, 12(2), 444. https://doi.org/10.3390/biomedicines12020444






