Mechanisms of Myocardial Edema Development in CVD Pathophysiology
Abstract
1. Introduction
2. The Fluid Flow in Myocardial Tissue under Normal Conditions
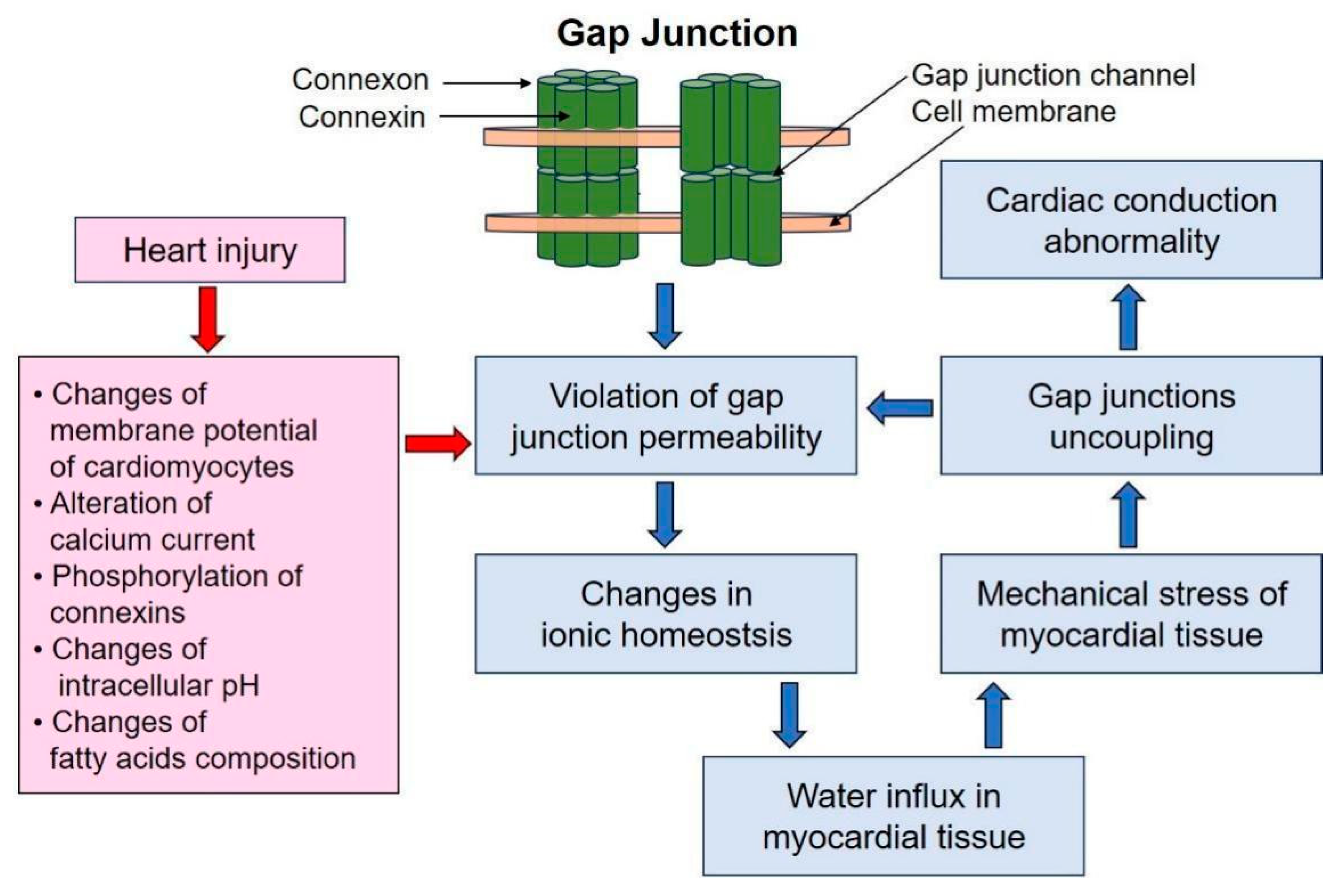
3. The Role of Gap Junctions in Myocardial Edema
4. Cellular Models of Myocardial Edema
5. Diagnostic Methods of Myocardial Edema and Potential Treatment
5.1. Diagnostic Methods
5.2. Myocardial Edema Treatment
5.2.1. Therapy Aimed at Stimulating Lymphangiogenesis
VEGF Induction
Adrenomedullin
Apelin
Reelin
5.2.2. Other Therapy Approaches
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehlhorn, U.; Geissler, H.J.; Laine, G.A.; Allen, S.J. Myocardial Fluid Balance. Eur. J. Cardiothorac. Surg. 2001, 20, 1220–1230. [Google Scholar] [CrossRef]
- Rame, J.E.; Müller, J. Myocardial Edema Revisited in a New Paradigm of Cardiac Electrical Microcurrent Application in Heart Failure. Bioelectricity 2021, 3, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Friedrich, M.G. T2-Weighted Cardiovascular Magnetic Resonance in Acute Cardiac Disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Aengevaeren, V.L. Exercise-Induced Myocardial Damage Is Associated with Cardiac Edema and Dysfunction: Adding Another Piece to the Troponin Puzzle. Eur. J. Appl. Physiol. 2023, 123, 2103–2105. [Google Scholar] [CrossRef]
- Huang, L.H.; Lavine, K.J.; Randolph, G.J. Cardiac Lymphatic Vessels, Transport, and Healing of the Infarcted Heart. JACC Basic. Transl. Sci. 2017, 2, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, C.; Tian, D.; Bai, J.; Li, Y.; Yu, X.; Yang, J.; Wang, X.; Dong, Y.; Yang, M.; et al. Application of 7.0 T Ultra-High-Field MRI in Evaluating the Structure and Function of the Right Ventricle of the Heart in Rats under a Chronic Hypoxic Environment at High Altitude. Ann. Transl. Med. 2021, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Zagrosek, A.; Wassmuth, R.; Abdel-Aty, H.; Rudolph, A.; Dietz, R.; Schulz-Menger, J. Relation between Myocardial Edema and Myocardial Mass during the Acute and Convalescent Phase of Myocarditis—A CMR Study. J. Cardiovasc. Magn. Reson. 2008, 10, 19. [Google Scholar] [CrossRef]
- Nensa, F.; Mahabadi, A.A.; Erbel, R.; Schlosser, T.W. Myocardial Edema during Acute Myocardial Infarction Visualized by Diffusion-Weighted MRI. Herz 2013, 38, 509–510. [Google Scholar] [CrossRef]
- Joudar, I.; Aichouni, N.; Nasri, S.; Kamaoui, I.; Skiker, I. Diagnostic Criteria for Myocarditis on Cardiac Magnetic Resonance Imaging: An Educational Review. Ann. Med. Surg. 2023, 85, 3960–3964. [Google Scholar] [CrossRef]
- World Heart Report 2023: Full Report—World Heart Federation. Available online: https://world-heart-federation.org/resource/world-heart-report-2023/ (accessed on 16 January 2024).
- Dongaonkar, R.M.; Stewart, R.H.; Geissler, H.J.; Laine, G.A. Myocardial Microvascular Permeability, Interstitial Oedema, and Compromised Cardiac Function. Cardiovasc. Res. 2010, 87, 331–339. [Google Scholar] [CrossRef]
- Egan, J.R.; Butler, T.L.; Au, C.G.; Tan, Y.M.; North, K.N.; Winlaw, D.S. Myocardial Water Handling and the Role of Aquaporins. Biochim. Biophys. Acta 2006, 1758, 1043–1052. [Google Scholar] [CrossRef]
- Nielsen, N.R.; Rangarajan, K.V.; Mao, L.; Rockman, H.A.; Caron, K.M. A Murine Model of Increased Coronary Sinus Pressure Induces Myocardial Edema with Cardiac Lymphatic Dilation and Fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H895–H907. [Google Scholar] [CrossRef]
- Bueno-Orovio, A.; Kay, D.; Grau, V.; Rodriguez, B.; Burrage, K. Fractional Diffusion Models of Cardiac Electrical Propagation: Role of Structural Heterogeneity in Dispersion of Repolarization. J. R. Soc. Interface 2014, 11, 20140352. [Google Scholar] [CrossRef]
- Starling, E.H. On the Absorption of Fluids from the Connective Tissue Spaces. J. Physiol. 1896, 19, 312–326. [Google Scholar] [CrossRef]
- Landis, E.M. Micro-Injection Studies of Capillary Permeability. Am. J. Physiol. 1927, 82, 217–238. [Google Scholar] [CrossRef]
- Klaourakis, K.; Vieira, J.M.; Riley, P.R. The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration. Nat. Rev. Cardiol. 2021, 18, 368–379. [Google Scholar] [CrossRef]
- Brakenhielm, E.; González, A.; Díez, J. Role of Cardiac Lymphatics in Myocardial Edema and Fibrosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Huxley, V.H.; Scallan, J. Lymphatic Fluid: Exchange Mechanisms and Regulation. J. Physiol. 2011, 589, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, Z.B.; Ahmed, N.S.; Horder, J.L.; Storr, S.J.; Benest, A.V. Transcription Factor Control of Lymphatic Quiescence and Maturation of Lymphatic Neovessels in Development and Physiology. Front. Physiol. 2021, 12, 672987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, C.; Hu, Y.; Xu, Q.; Hu, X. Lymphatics in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, E275–E283. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.P.; Morykwas, M.J.; Jordan, J.E.; Wang, R.; Argenta, L.C. Local Fluid Transfer Regulation in Heart Extracellular Matrix. J. Physiol. Biochem. 2016, 72, 255–268. [Google Scholar] [CrossRef]
- Moore, J.E.; Bertram, C.D. Lymphatic System Flows. Annu. Rev. Fluid. Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Lymphatic Vessels and Their Surroundings: How Local Physical Factors Affect Lymph Flow. Biology 2020, 9, 463. [Google Scholar] [CrossRef]
- Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Alvarenga, J.M.G.; Nobrega, J.; Cerqueira, R.J.; Mancio, J.; Leite-Moreira, A.F.; Roncon-Albuquerque, R. Myocardial Oedema: Pathophysiological Basis and Implications for the Failing Heart. ESC Heart Fail. 2022, 9, 958–976. [Google Scholar] [CrossRef]
- Bravo-Reyna, C.C.; Torres-Villalobos, G.; Aguilar-Blas, N.; Frías-Guillén, J.; Guerra-Mora, J.R. Comparative Study of Capillary Filtration Coefficient (Kfc) Determination by a Manual and Automatic Perfusion System. Step by Step Technique Review. Physiol. Res. 2019, 68, 901–907. [Google Scholar] [CrossRef]
- Curry, F.E.; Michel, C.C. The Colloid Osmotic Pressure Across the Glycocalyx: Role of Interstitial Fluid Sub-Compartments in Trans-Vascular Fluid Exchange in Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 729873. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ishihara, K.; Ehara, T.; Shioya, T. Cell-Volume Regulation by Swelling-Activated Chloride Current in Guinea-Pig Ventricular Myocytes. Jpn. J. Physiol. 2004, 54, 31–38. [Google Scholar] [CrossRef]
- Ogura, T.; Matsuda, H.; Imanishi, S.; Shibamoto, T. Sarcolemmal Hydraulic Conductivity of Guinea-Pig and Rat Ventricular Myocytes. Cardiovasc. Res. 2002, 54, 590–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Au, C.G.; Cooper, S.T.; Lo, H.P.; Compton, A.G.; Yang, N.; Wintour, E.M.; North, K.N.; Winlaw, D.S. Expression of Aquaporin 1 in Human Cardiac and Skeletal Muscle. J. Mol. Cell Cardiol. 2004, 36, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.T.; Shaw, R.M. Cardiac T-Tubule Microanatomy and Function. Physiol. Rev. 2017, 97, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Qi, Y.; Ye, Y.; Yue, P.; Zhang, D.; Li, Y. Mechanotranduction Pathways in the Regulation of Mitochondrial Homeostasis in Cardiomyocytes. Front. Cell Dev. Biol. 2021, 8, 625089. [Google Scholar] [CrossRef]
- Ibrahim, M.; Gorelik, J.; Yacoub, M.H.; Terracciano, C.M. The Structure and Function of Cardiac T-Tubules in Health and Disease. Proc. Biol. Sci. 2011, 278, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, R.; Li, R.; Peng, Y.; Zhu, P.; Zhou, H. Molecular Mechanisms of Mitochondrial Quality Control in Ischemic Cardiomyopathy. Int. J. Biol. Sci. 2023, 19, 426–448. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Soda, T.; De Francesco, E.M.; Fiorillo, M.; Moccia, F.; Viglietto, G.; Angelone, T.; Amodio, N. Mitochondrial Dysfunction at the Crossroad of Cardiovascular Diseases and Cancer. J. Transl. Med. 2023, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular Matrix Composition of Connective Tissues: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Latella, G.; Gaudio, E.; Sferra, R.; Vetuschi, A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front. Med. 2021, 8, 610189. [Google Scholar] [CrossRef]
- Lee, C.; Liang, L.W.; Hasegawa, K.; Maurer, M.S.; Tower-Rader, A.; Fifer, M.A.; Reilly, M.; Shimada, Y.J. Signaling Pathways Associated with Prior Cardiovascular Events in Hypertrophic Cardiomyopathy. J. Card. Fail. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef]
- Bueno-Orovio, A.; Teh, I.; Schneider, J.E.; Burrage, K.; Grau, V. Anomalous Diffusion in Cardiac Tissue as an Index of Myocardial Microstructure. IEEE Trans. Med. Imaging 2016, 35, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, H.J.; Wilders, R. Gap Junctions in Cardiovascular Disease. Circ. Res. 2000, 86, 1193–1197. [Google Scholar] [CrossRef]
- Johnson, R.D.; Camelliti, P. Role of Non-Myocyte Gap Junctions and Connexin Hemichannels in Cardiovascular Health and Disease: Novel Therapeutic Targets? Int. J. Mol. Sci. 2018, 19, 866. [Google Scholar] [CrossRef]
- Sáez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V.L. Cell Membrane Permeabilization via Connexin Hemichannels in Living and Dying Cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef]
- Lueck, S.; Delis, A.; Minor, T.; Preusse, C.J.; Schaefer, M. Age-Related Differences of Intraischemic Gap Junction Uncoupling in Hearts during Ischemia. J. Thorac. Cardiovasc. Surg. 2016, 152, 729–736. [Google Scholar] [CrossRef]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, e019338. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, R.; Salama, M.E.; Poelzing, S. Interstitial Volume Modulates the Conduction Velocity-Gap Junction Relationship. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H278–H286. [Google Scholar] [CrossRef]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 Hemichannels Mediate the Ca2+ Influx Induced by Extracellular Alkalinization. Am. J. Physiol. Cell Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, N.; Frinak, S.; Yee, J. Sodium-Based Osmotherapy for Hyponatremia in Acute Decompensated Heart Failure. Heart Fail. Rev. 2022, 27, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Marti, C.N.; Mentz, R.J.; Greene, S.J.; Ambrosy, A.P.; Subacius, H.P.; Fonarow, G.C.; Chioncel, O.; Bazari, H.; Maggioni, A.P.; et al. Serum Osmolality and Postdischarge Outcomes after Hospitalization for Heart Failure. Am. J. Cardiol. 2016, 117, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Meana, M.; García-Dorado, D.; González, M.A.; Barrabés, J.A.; Soler-Soler, J. Effect of Osmotic Stress on Sarcolemmal Integrity of Isolated Cardiomyocytes Following Transient Metabolic Inhibition. Cardiovasc. Res. 1995, 30, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.C.; Shivell, C.L.; Ganote, C.E. Sarcolemmal Blebs and Osmotic Fragility as Correlates of Irreversible Ischemic Injury in Preconditioned Isolated Rabbit Cardiomyocytes. J. Mol. Cell Cardiol. 2001, 33, 149–160. [Google Scholar] [CrossRef]
- Lyon, A.R.; MacLeod, K.T.; Zhang, Y.; Garcia, E.; Kanda, G.K.; Lab, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Loss of T-Tubules and Other Changes to Surface Topography in Ventricular Myocytes from Failing Human and Rat Heart. Proc. Natl. Acad. Sci. USA 2009, 106, 6854–6859. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Rees, S.A.; Wright, A.R.; Powell, T. Cell Swelling and Ion Transport Pathways in Cardiac Myocytes. Cardiovasc. Res. 1996, 32, 85–97. [Google Scholar] [CrossRef]
- Hermsmeyer, K.; Rulon, R.; Sperelakis, N. Loss of the Plateau of the Cardiac Action Potential in Hypertonic Solutions. J. Gen. Physiol. 1972, 59, 779–793. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kawano, S.; Hirano, Y.; Furukawa, T. Role of Cardiac Chloride Currents in Changes in Action Potential Characteristics and Arrhythmias. Cardiovasc. Res. 1998, 40, 23–33. [Google Scholar] [CrossRef]
- Kozeny, G.A.; Murdock, D.K.; Euler, D.E.; Hano, J.E.; Scanlon, P.J.; Bansal, V.K.; Vertuno, L.L. In Vivo Effects of Acute Changes in Osmolality and Sodium Concentration on Myocardial Contractility. Am. Heart J. 1985, 109, 290–296. [Google Scholar] [CrossRef]
- Kourouklis, A.P.; Wahlsten, A.; Stracuzzi, A.; Martyts, A.; Paganella, L.G.; Labouesse, C.; Al-Nuaimi, D.; Giampietro, C.; Ehret, A.E.; Tibbitt, M.W.; et al. Control of Hydrostatic Pressure and Osmotic Stress in 3D Cell Culture for Mechanobiological Studies. Biomater. Adv. 2023, 145, 213241. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, E.; Han, Y.L.; Guo, M.; Shenoy, V.B. Gap Junctions Amplify Spatial Variations in Cell Volume in Proliferating Tumor Spheroids. Nat. Commun. 2020, 11, 6148. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pegoraro, A.F.; Mao, A.; Zhou, E.H.; Arany, P.R.; Han, Y.; Burnette, D.T.; Jensen, M.H.; Kasza, K.E.; Moore, J.R.; et al. Cell Volume Change through Water Efflux Impacts Cell Stiffness and Stem Cell Fate. Proc. Natl. Acad. Sci. USA 2017, 114, E8618–E8627. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Mattesi, G.; Baldi, E.; Toniolo, M.; Guerra, F.; Cauti, F.M.; Cipriani, A.; De Lazzari, M.; Muser, D.; Stronati, G.; et al. Prognostic Role of Myocardial Edema as Evidenced by Early Cardiac Magnetic Resonance in Survivors of Out-of-Hospital Cardiac Arrest: A Multicenter Study. J. Am. Heart Assoc. 2021, 10, e021861. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Urzua Fresno, C.; Sanchez Tijmes, F.; Shaw, K.E.; Huang, F.; Thavendiranathan, P.; Khullar, S.; Seidman, M.A.; Hanneman, K. Cardiac Imaging in Myocarditis: Current Evidence and Future Directions. Can. Assoc. Radiol. J. 2023, 74, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Hughes, S.G. The Bright Side of Myocardial Edema. J. Am. Heart Assoc. 2021, 10, e023731. [Google Scholar] [CrossRef]
- Westbrook, C.; Talbot, J. Writer on magnetic resonance imaging. In MRI in Practice; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 416. [Google Scholar]
- Naenstein, K.; Nensa, F.; Schlosser, T.; Bruder, O.; Umutlu, L.; Lauenstein, T.; Maderwald, S.; Ladd, M.E. Cardiac MRI: T2-Mapping Versus T2-Weighted Dark-Blood TSE Imaging for Myocardial Edema Visualization in Acute Myocardial Infarction. Rofo 2014, 186, 166–172. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; Di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, E011492. [Google Scholar] [CrossRef]
- Kamp, N.J.; Chery, G.; Kosinski, A.S.; Desai, M.Y.; Wazni, O.; Schmidler, G.S.; Patel, M.; Lopes, R.D.; Morin, D.P.; Al-Khatib, S.M. Risk Stratification Using Late Gadolinium Enhancement on Cardiac Magnetic Resonance Imaging in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Prog. Cardiovasc. Dis. 2021, 66, 10–16. [Google Scholar] [CrossRef]
- Sinigiani, G.; De Michieli, L.; De Conti, G.; Ricci, F.; De Lazzari, M.; Migliore, F.; Perazzolo Marra, M.; Zorzi, A.; Corrado, D.; Cipriani, A. Cardiac Magnetic Resonance-Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2023, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Vermes, E.; Childs, H.; Faris, P.; Friedrich, M.G. Predictive Value of CMR Criteria for LV Functional Improvement in Patients with Acute Myocarditis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Capasso, R.; Imperato, M.C.; Serra, N.; Rodriguez, R.; Rivellini, M.; De Filippo, M.; Pinto, A. Infarct-like versus Non-Infarct-like Clinical Presentation of Acute Myocarditis: Comparison of Cardiac Magnetic Resonance (CMR) Findings. Diagnostics 2023, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, A.; Chareonthaitawee, P.; Raman, S.V.; Cooper, L.T. Imaging of Inflammation in Unexplained Cardiomyopathy. JACC Cardiovasc. Imaging 2016, 9, 603–617. [Google Scholar] [CrossRef]
- Filomena, D.; Dresselaers, T.; Bogaert, J. Role of Cardiovascular Magnetic Resonance to Assess Cardiovascular Inflammation. Front. Cardiovasc. Med. 2022, 9, 877364. [Google Scholar] [CrossRef]
- Montant, P.; Sigovan, M.; Revel, D.; Douek, P. MR Imaging Assessment of Myocardial Edema with T2 Mapping. Diagn. Interv. Imaging 2015, 96, 885–890. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Myocardial Edema and Prognosis in Amyloidosis. J. Am. Coll. Cardiol. 2018, 71, 2919–2931. [Google Scholar] [CrossRef]
- Chinali, M.; Franceschini, A.; Ciancarella, P.; Lisignoli, V.; Curione, D.; Ciliberti, P.; Esposito, C.; Del Pasqua, A.; Rinelli, G.; Secinaro, A. Echocardiographic Two-Dimensional Speckle Tracking Identifies Acute Regional Myocardial Edema and Sub-Acute Fibrosis in Pediatric Focal Myocarditis with Normal Ejection Fraction: Comparison with Cardiac Magnetic Resonance. Sci. Rep. 2020, 10, 11321. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.; Zareba, K.M.; Nagaraja, H.N.; Schaal, S.F.; Simonetti, O.P.; Ambrosio, G.; Raman, S.V. T-Wave Abnormality as Electrocardiographic Signature of Myocardial Edema in Non-ST-Elevation Acute Coronary Syndromes. J. Am. Heart Assoc. 2018, 7, e007118. [Google Scholar] [CrossRef]
- Andrés-Villarreal, M.; Barba, I.; Poncelas, M.; Inserte, J.; Rodriguez-Palomares, J.; Pineda, V.; Garcia-Dorado, D. Measuring Water Distribution in the Heart: Preventing Edema Reduces Ischemia-Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003843. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Andres-Villarreal, M.; Ruiz-Meana, M.; Inserte, J.; Barba, I. Myocardial Edema: A Translational View. J. Mol. Cell Cardiol. 2012, 52, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Michael Felker, G. Diuretic Management in Heart Failure. Congest. Heart Fail. 2010, 16, S68–S72. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. The Role of Lymphangiogenesis in Cardiovascular Diseases and Heart Transplantation. Heart Fail. Rev. 2022, 27, 1837–1856. [Google Scholar] [CrossRef]
- Liu, X.; Cui, K.; Wu, H.; Li, K.S.; Peng, Q.; Wang, D.; Cowan, D.B.; Dixon, J.B.; Sathish Srinivasan, R.; Bielenberg, D.R.; et al. Promoting Lymphangiogenesis and Lymphatic Growth and Remodeling to Treat Cardiovascular and Metabolic Diseases. Arterioscler. Thromb. Vasc. Biol. 2023, 43, E1–E10. [Google Scholar] [CrossRef]
- Wada, H.; Suzuki, M.; Matsuda, M.; Ajiro, Y.; Shinozaki, T.; Sakagami, S.; Yonezawa, K.; Shimizu, M.; Funada, J.; Takenaka, T.; et al. VEGF-C and Mortality in Patients with Suspected or Known Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e010355. [Google Scholar] [CrossRef]
- Han, X.; Liu, L.; Niu, J.; Yang, J.; Zhang, Z.; Zhang, Z. Serum VEGF Predicts Worse Clinical Outcome of Patients with Coronary Heart Disease after Percutaneous Coronary Intervention Therapy. Med. Sci. Monit. 2015, 21, 3247–3251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Z.; Zhao, X.; Wu, Z.; Qu, B.; Yuan, M.; Xing, Y.; Song, Y.; Wang, Z. Lymphatic Vessel: Origin, Heterogeneity, Biological Functions, and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 9. [Google Scholar] [CrossRef]
- Tan, K.W.; Chong, S.Z.; Wong, F.H.S.; Evrard, M.; Tan, S.M.L.; Keeble, J.; Kemeny, D.M.; Ng, L.G.; Abastado, J.P.; Angeli, V. Neutrophils Contribute to Inflammatory Lymphangiogenesis by Increasing VEGF-A Bioavailability and Secreting VEGF-D. Blood 2013, 122, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Wuest, T.R.; Carr, D.J.J. VEGF-A Expression by HSV-1-Infected Cells Drives Corneal Lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.I.; Jiang, Y.J.; Chang, A.C.; Huang, C.L.; Fong, Y.C.; Guo, J.H.; Liu, C.L.; Wang, S.W.; Liu, J.F.; Chang, S.L.Y.; et al. ANGPTL2 Promotes VEGF-A Synthesis in Human Lung Cancer and Facilitates Lymphangiogenesis. Aging 2023, 15, 1652. [Google Scholar] [CrossRef] [PubMed]
- Houssari, M.; Dumesnil, A.; Tardif, V.; Kivelä, R.; Pizzinat, N.; Boukhalfa, I.; Godefroy, D.; Schapman, D.; Hemanthakumar, K.A.; Bizou, M.; et al. Lymphatic and Immune Cell Cross-Talk Regulates Cardiac Recovery after Experimental Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1722–1737. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dubé, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac Lymphatics Are Heterogeneous in Origin and Respond to Injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.C.S.; Lim, L.; Shewale, S.V.; McDaid, K.; Martí-Pàmies, Í.; Tang, A.T.; Wittig, C.; Guerrero, A.A.; Sterling, S.; Adrian Leu, N.; et al. Genetic Blockade of Lymphangiogenesis Does Not Impair Cardiac Function after Myocardial Infarction. J. Clin. Investig. 2021, 131, e147070. [Google Scholar] [CrossRef]
- Alitalo, A.K.; Proulx, S.T.; Karaman, S.; Aebischer, D.; Martino, S.; Jost, M.; Schneider, N.; Bry, M.; Detmar, M. VEGF-C and VEGF-D Blockade Inhibits Inflammatory Skin Carcinogenesis. Cancer Res. 2013, 73, 4212–4221. [Google Scholar] [CrossRef]
- Trincot, C.E.; Xu, W.; Zhang, H.; Kulikauskas, M.R.; Caranasos, T.G.; Jensen, B.C.; Sabine, A.; Petrova, T.V.; Caron, K.M. Adrenomedullin Induces Cardiac Lymphangiogenesis after Myocardial Infarction and Regulates Cardiac Edema Via Connexin 43. Circ. Res. 2019, 124, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.; Tan, C.; Kume, T. Cardiac and Intestinal Tissue Conduct Developmental and Reparative Processes in Response to Lymphangiocrine Signaling. Front. Cell Dev. Biol. 2023, 11, 1329770. [Google Scholar] [CrossRef]
- Hinrichs, S.; Scherschel, K.; Krüger, S.; Neumann, J.T.; Schwarzl, M.; Yan, I.; Warnke, S.; Ojeda, F.M.; Zeller, T.; Karakas, M.; et al. Precursor Proadrenomedullin Influences Cardiomyocyte Survival and Local Inflammation Related to Myocardial Infarction. Proc. Natl. Acad. Sci. USA 2018, 115, E8727–E8736. [Google Scholar] [CrossRef] [PubMed]
- Kuwasako, K.; Kitamura, K.; Nagata, S.; Hikosaka, T.; Takei, Y.; Kato, J. Shared and Separate Functions of the RAMP-Based Adrenomedullin Receptors. Peptides 2011, 32, 1540–1550. [Google Scholar] [CrossRef]
- Fritz-Six, K.L.; Dunworth, W.P.; Li, M.; Caron, K.M. Adrenomedullin Signaling Is Necessary for Murine Lymphatic Vascular Development. J. Clin. Investig. 2008, 118, 40. [Google Scholar] [CrossRef]
- Tatin, F.; Renaud-Gabardos, E.; Godet, A.C.; Hantelys, F.; Pujol, F.; Morfoisse, F.; Calise, D.; Viars, F.; Valet, P.; Masri, B.; et al. Apelin Modulates Pathological Remodeling of Lymphatic Endothelium after Myocardial Infarction. JCI Insight 2017, 2, e93887. [Google Scholar] [CrossRef] [PubMed]
- Berta, J.; Hoda, M.A.; Laszlo, V.; Rozsas, A.; Garay, T.; Torok, S.; Grusch, M.; Berger, W.; Paku, S.; Renyi-Vamos, F.; et al. Apelin Promotes Lymphangiogenesis and Lymph Node Metastasis. Oncotarget 2014, 5, 4426–4437. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Jiang, G.; Hu, B.; Zheng, H.; Hong, Y.; Cui, Z.; Shi, L.; Li, X.; Lin, F.; et al. Apelin Rejuvenates Aged Human Mesenchymal Stem Cells by Regulating Autophagy and Improves Cardiac Protection after Infarction. Front. Cell Dev. Biol. 2021, 9, 628463. [Google Scholar] [CrossRef]
- Lutter, S.; Xie, S.; Tatin, F.; Makinen, T. Smooth Muscle–Endothelial Cell Communication Activates Reelin Signaling and Regulates Lymphatic Vessel Formation. J. Cell Biol. 2012, 197, 837. [Google Scholar] [CrossRef]
- Alexander, A.; Herz, J.; Calvier, L. Reelin Through the Years: From Brain Development to Inflammation. Cell Rep. 2023, 42, 112669. [Google Scholar] [CrossRef]
- Liu, X.; De la Cruz, E.; Gu, X.; Balint, L.; Oxendine-Burns, M.; Terrones, T.; Ma, W.; Kuo, H.H.; Lantz, C.; Bansal, T.; et al. Lymphoangiocrine Signals Promote Cardiac Growth and Repair. Nature 2020, 588, 705. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, G.; Ben-Aicha, S.; Gutiérrez, M.; Casani, L.; Aržanauskaitė, M.; Carreras, F.; Sabate, M.; Badimon, L.; Vilahur, G. Intravenous Statin Administration During Myocardial Infarction Compared with Oral Post-Infarct Administration. J. Am. Coll. Cardiol. 2020, 75, 1386–1402. [Google Scholar] [CrossRef]
- Kolwelter, J.; Kannenkeril, D.; Linz, P.; Jung, S.; Nagel, A.M.; Bosch, A.; Ott, C.; Bramlage, P.; Nöh, L.; Schiffer, M.; et al. The SGLT2 Inhibitor Empagliflozin Reduces Tissue Sodium Content in Patients with Chronic Heart Failure: Results from a Placebo-Controlled Randomised Trial. Clin. Res. Cardiol. 2023, 112, 134–144. [Google Scholar] [CrossRef]
- Eltobshy, S.A.G.; Messiha, R.; Metias, E.; Sarhan, M.; El-Gamal, R.; El-Shaieb, A.; Ghalwash, M. Effect of SGLT2 Inhibitor on Cardiomyopathy in a Rat Model of T2DM: Possible Involvement of Cardiac Aquaporins. Tissue Cell 2023, 85, 102200. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Bianchi, C.; Araujo, E.; Voisine, P.; Xu, S.H.; Feng, J.; Li, J.; Sellke, F.W. Aprotinin Preserves Cellular Junctions and Reduces Myocardial Edema after Regional Ischemia and Cardioplegic Arrest. Circulation 2005, 112, I196–I201. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseleva, D.G.; Kirichenko, T.V.; Markina, Y.V.; Cherednichenko, V.R.; Gugueva, E.A.; Markin, A.M. Mechanisms of Myocardial Edema Development in CVD Pathophysiology. Biomedicines 2024, 12, 465. https://doi.org/10.3390/biomedicines12020465
Kiseleva DG, Kirichenko TV, Markina YV, Cherednichenko VR, Gugueva EA, Markin AM. Mechanisms of Myocardial Edema Development in CVD Pathophysiology. Biomedicines. 2024; 12(2):465. https://doi.org/10.3390/biomedicines12020465
Chicago/Turabian StyleKiseleva, Diana G., Tatiana V. Kirichenko, Yuliya V. Markina, Vadim R. Cherednichenko, Ekaterina A. Gugueva, and Alexander M. Markin. 2024. "Mechanisms of Myocardial Edema Development in CVD Pathophysiology" Biomedicines 12, no. 2: 465. https://doi.org/10.3390/biomedicines12020465
APA StyleKiseleva, D. G., Kirichenko, T. V., Markina, Y. V., Cherednichenko, V. R., Gugueva, E. A., & Markin, A. M. (2024). Mechanisms of Myocardial Edema Development in CVD Pathophysiology. Biomedicines, 12(2), 465. https://doi.org/10.3390/biomedicines12020465





