Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Microsphere Characterisation
3.2. Ophthalmological Analysis

3.3. Computational Analysis
3.3.1. VIT/RPE Intensity

3.3.2. Correlation Analysis
3.3.3. In Vivo Analysis of Vitreous Immunity

Percentage of Opacities/Cells by Size


Average Eccentricity of the Opacities/Cells

Mean Intensity of Opacities/Cells

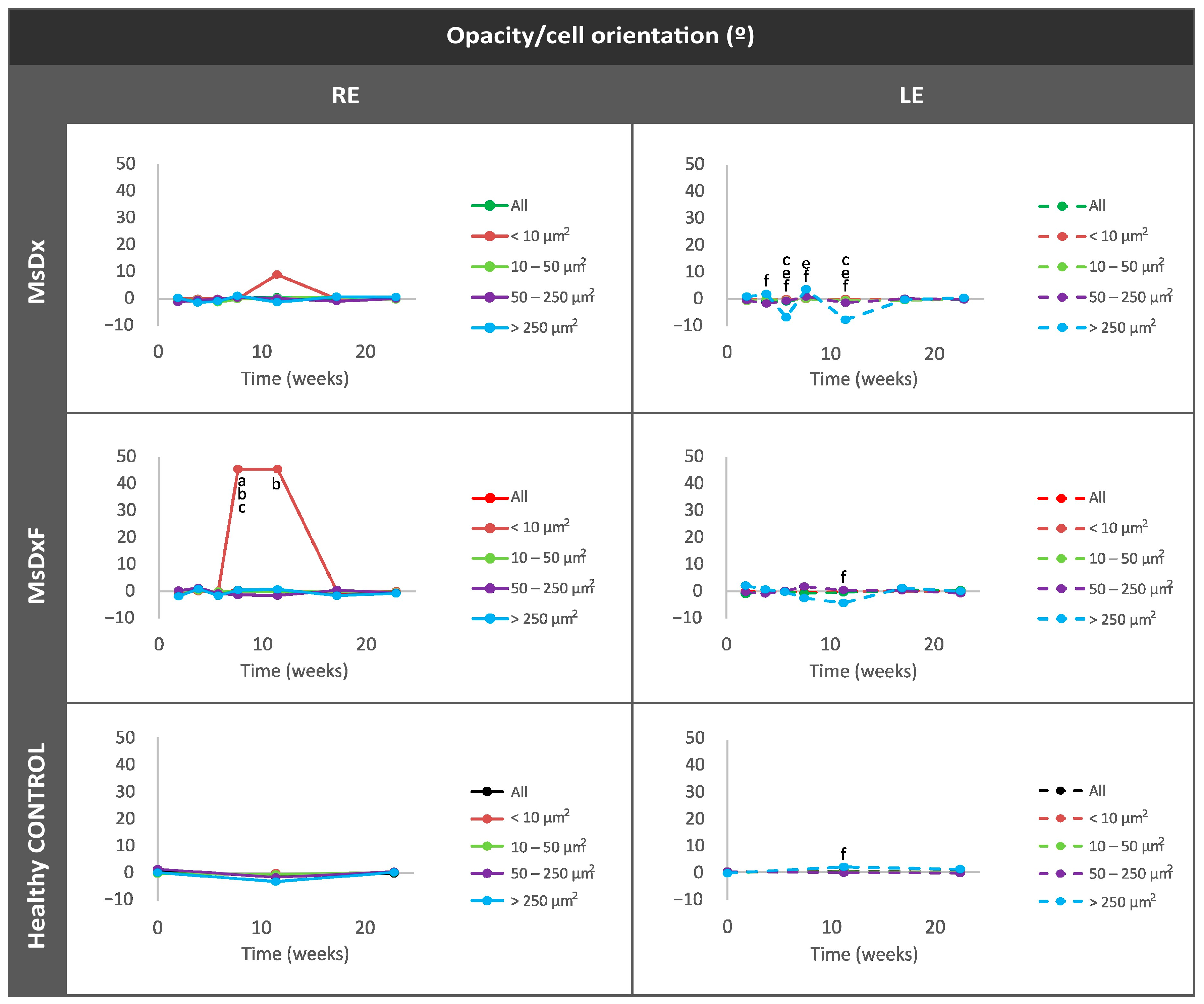
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Q.; Göktaş, E.; Gopal, K.; Al-Aswad, L.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Tezel, G. T-Lymphocyte Subset Distribution and Activity in Patients With Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Kametani, M.; Chen, D.F. Adaptive Immunity: New Aspects of Pathogenesis Underlying Neurodegeneration in Glaucoma and Optic Neuropath. Front. Immunol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Lau, L.I.; Liu, C.J.L.; Chou, J.C.K.; Hsu, W.M.; Liu, J.H. Patterns of visual field defects in chronic angle-closure glaucoma with different disease severity. Ophthalmology 2003, 110, 1890–1894. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, C.; Weimer, R.M. An Automated Method to Quantify Microglia Morphology and Application to Monitor Activation State Longitudinally In Vivo. PLoS ONE 2012, 7, e31814. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cho, K.-S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A.; Microglia, P.O. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Ambati, B.K.; Vetter, M.L. In Vivo Dynamics of Retinal Microglial Activation During Neurodegeneration: Confocal Ophthalmoscopic Imaging and Cell Morphometry in Mouse Glaucoma. J. Vis. Exp. 2015, 2015, e52731. [Google Scholar] [CrossRef]
- Kezic, J.M.; Chrysostomou, V.; Trounce, I.A.; McMenamin, P.G.; Crowston, J.G. Effect of anterior chamber cannulation and acute IOP elevation on retinal macrophages in the adult mouse. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3028–3036. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Boehm, M.R.R.; Oellers, P.; Thanos, S. Inflammation and immunology of the vitreoretinal compartment. Inflamm. Allergy Drug Targets 2011, 10, 283–309. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina 2011, 31, 222–228. [Google Scholar] [CrossRef]
- Vagaja, N.N.; Chinnery, H.R.; Binz, N.; Kezic, J.M.; Rakoczy, E.P.; McMenamin, P.G. Changes in murine hyalocytes are valuable early indicators of ocular disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1445–1451. [Google Scholar] [CrossRef]
- Keane, P.A.; Karampelas, M.; Sim, D.A.; Sadda, S.R.; Tufail, A.; Sen, H.N.; Nussenblatt, R.B.; Dick, A.D.; Lee, R.W.; Murray, P.I.; et al. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014, 121, 1706–1714. [Google Scholar] [CrossRef]
- Uji, A.; Yoshimura, N. Microarchitecture of the Vitreous Body: A High-Resolution Optical Coherence Tomography Study. Am. J. Ophthalmol. 2016, 168, 24–30. [Google Scholar] [CrossRef]
- Sreekantam, S.; Macdonald, T.; Keane, P.A.; Sim, D.A.; Murray, P.I.; Denniston, A.K. Quantitative analysis of vitreous inflammation using optical coherence tomography in patients receiving sub-Tenon’s triamcinolone acetonide for uveitic cystoid macular oedema. Br. J. Ophthalmol. 2017, 101, 175–179. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Subías, M.; Montolío, A.; Méndez-Martínez, S.; Martínez-Rincón, T.; Arias, L.; García-Herranz, D.; Bravo-Osuna, I.; Garcia-Feijoo, J.; Pablo, L.; et al. Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging. Biomedicines 2021, 9, 1792. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Garcia-Herranz, D.; Aragón-Navas, A.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Cardiel, M.J.; García-Feijoo, J.; Ruberte, J.; Herrero-Vanrell, R.; et al. Long-term corticosteroid-induced chronic glaucoma model produced by intracameral injection of dexamethasone-loaded PLGA microspheres. Drug Deliv. 2021, 28, 2427–2446. [Google Scholar] [CrossRef]
- Aragón-Navas, A.; Rodrigo, M.J.; Garcia-Herranz, D.; Martinez, T.; Subias, M.; Mendez, S.; Ruberte, J.; Pampalona, J.; Bravo-Osuna, I.; Garcia-Feijoo, J.; et al. Mimicking chronic glaucoma over 6 months with a single intracameral injection of dexamethasone/ fibronectin-loaded PLGA microspheres Mimicking chronic glaucoma over 6 months with a single intracameral injection of dexamethasone/fibronectin-loaded PLGA microspheres. Drug Deliv. 2022, 2022, 2357–2374. [Google Scholar] [CrossRef]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.X.; Sousa, N.; Sotiropoulos, I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Katz, L.J. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012, 47, 66–80. [Google Scholar] [CrossRef]
- Garcia-Herranz, D.; Rodrigo, M.J.; Subias, M.; Martinez-Rincon, T.; Mendez-Martinez, S.; Bravo-Osuna, I.; Bonet, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.; et al. Novel Use of PLGA Microspheres to Create an Animal Model of Glaucoma with Progressive Neuroretinal Degeneration. Pharmaceutics 2021, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.-H.; Dai, C.; Camesa, A.; Zhang, H.F.; Jiao, S. Effect of Contact Lens on Optical Coherence Tomography Imaging of Rodent Retina. Curr. Eye Res. 2013, 38, 1235. [Google Scholar] [CrossRef] [PubMed]
- Korot, E.; Comer, G.; Steffens, T.; Antonetti, D.A. Algorithm for the Measure of Vitreous Hyperreflective Foci in Optical Coherence Tomographic Scans of Patients With Diabetic Macular Edema. JAMA Ophthalmol. 2016, 134, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; del Palomar, A.P.; Montolío, A.; Mendez-Martinez, S.; Subias, M.; Cardiel, M.J.; Martinez-Rincon, T.; Cegoñino, J.; Fraile, J.M.; Vispe, E.; et al. Monitoring New Long-Lasting Intravitreal Formulation for Glaucoma with Vitreous Images Using Optical Coherence Tomography. Pharmaceutics 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Herrmann, P.; Carvalho, L.S.; Liyanage, S.E.; Bainbridge, J.W.B.; Ali, R.R.; Dick, A.D.; Luhmann, U.F.O. Assessment and In Vivo Scoring of Murine Experimental Autoimmune Uveoretinitis Using Optical Coherence Tomography. PLoS ONE 2013, 8, e63002. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K. Scanning electron microscopic study of hyalocytes in the guinea pig eye. Arch. Histol. Cytol. 2002, 65, 263–268. [Google Scholar] [CrossRef]
- Liba, O.; Lew, M.D.; Sorelle, E.D.; Dutta, R.; Sen, D.; Moshfeghi, D.M.; Chu, S.; De La Zerda, A. Speckle-modulating optical coherence tomography in living mice and humans. Nat. Commun. 2017, 8, 15845. [Google Scholar] [CrossRef]
- London, A.; Itskovich, E.; Benhar, I.; Kalchenko, V.; Mack, M.; Jung, S.; Schwartz, M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 2011, 208, 23–39. [Google Scholar] [CrossRef]
- Jacobs, A.H.; Tavitian, B. Noninvasive Molecular Imaging of Neuroinflammation. J. Cereb. Blood Flow. Metab. 2012, 32, 1393–1415. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; McNagny, K.M.; Rossi, F.M.V. Infiltrating monocytes trigger EAE progression. but do not contribute to the resident microglia pool. Nat. Neurosci. 2011, 14, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 2020, 6, eaba3239. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Béchade, C.; D’Andrea, I.; St-Pierre, M.K.; Henry, M.S.; Roumier, A.; Tremblay, M.E. Microglia gone rogue: Impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 2018, 10, 421. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Bravo-Osuna, I.; Subias, M.; Montolío, A.; Cegoñino, J.; Martinez-Rincón, T.; Mendez-Martinez, S.; Aragón-Navas, A.; Garcia-Herranz, D.; Pablo, L.E.; et al. Tunable degrees of neurodegeneration in rats based on microsphere-induced models of chronic glaucoma. Sci. Rep. 2022, 12, 20622. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Luna, C.; Pablo, L.E.; Polo, V.; Garcia-Martin, E. Effect of age and sex on neurodevelopment and neurodegeneration in the healthy eye: Longitudinal functional and structural study in the Long–Evans rat. Exp. Eye Res. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Garcia-Herranz, D.; Garcia-Feijoo, J.; Herrero-Vanrell, R.; Pablo, L.; Bravo-Osuna, I.; Munuera, I.; et al. Influence of sex on chronic steroid-induced glaucoma: 24-Weeks follow-up study in rats. Exp. Eye Res. 2023, 238, 109736. [Google Scholar] [CrossRef]
- Forrester, J.V.; Xu, H. Good news–bad news: The Yin and Yang of immune privilege in the eye. Front. Immunol. 2012, 3, 338. [Google Scholar] [CrossRef]
- Medawar, P.B. Immunity to homologous grafted skin; the fate of skin homografts. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Provis, J.M.; Penfold, P.L. The human hyaloid system: Cellular phenotypes and inter-relationships. Exp. Eye Res. 1999, 68, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Hata, Y.; Hisatomi, T.; Nakamura, Y.; Hirayama, K.; Miura, M.; Nakao, S.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Functional properties of hyalocytes under PDGF-rich conditions. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Hisatomi, T.; Sonoda, K.H.; Kura, S.; Sassa, Y.; Kinoshita, S.; Nakamura, T.; Sakamoto, T.; Ishibashi, T. The characterisation of hyalocytes: The origin. phenotype, and turnover. Br. J. Ophthalmol. 2005, 89, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Castanos, M.V.; Zhou, D.B.; Linderman, R.E.; Allison, R.; Milman, T.; Carroll, J.; Migacz, J.; Rosen, R.B.; Chui, T.Y.P. Imaging of Macrophage-Like Cells in Living Human Retina Using Clinical OCT. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef] [PubMed]
- Geyer, O.; Levo, Y. Glaucoma is an autoimmune disease. Autoimmun. Rev. 2020, 19, 102535. [Google Scholar] [CrossRef]
- Ramírez, A.I.; Fernández-Albarral, J.A.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Rojas, P.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Microglial changes in the early aging stage in a healthy retina and an experimental glaucoma model. In Progress in Brain Research; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 125–149. [Google Scholar] [CrossRef]
- Tezel, G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef]
- Sapienza, A.; Raveu, A.-L.; Reboussin, E.; Roubeix, C.; Boucher, C.; Dégardin, J.; Godefroy, D.; Rostène, W.; Goazigo, A.R.-L.; Baudouin, C.; et al. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J. Neuroinflamm. 2016, 13, 44. [Google Scholar] [CrossRef]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- Tsai, T.; Reinehr, S.; Maliha, A.M.; Joachim, S.C. Immune Mediated Degeneration and Possible Protection in Glaucoma. Front. Neurosci. 2019, 13, 931. [Google Scholar] [CrossRef]
- Todd, L.; Palazzo, I.; Suarez, L.; Liu, X.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 2019, 16, 118. [Google Scholar] [CrossRef]
- Roberti, G.; Oddone, F.; Agnifili, L.; Katsanos, A.; Michelessi, M.; Mastropasqua, L.; Quaranta, L.; Riva, I.; Tanga, L.; Manni, G. Steroid-induced glaucoma: Epidemiology; pathophysiology; clinical management. Surv. Ophthalmol. 2020, 65, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Couret, C.; Poinas, A.; Volteau, C.; Riche, V.P.; Le Lez, M.L.; Errera, M.H.; Creuzot-Garcher, C.; Baillif, S.; Kodjikian, L.; Ivan, C.L.M.; et al. Comparison of two techniques used in routine care for the treatment of inflammatory macular oedema, subconjunctival triamcinolone injection and intravitreal dexamethasone implant: Medical and economic importance of this randomized controlled trial. Trials 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W.; Ng, T.F. Negative regulators that mediate ocular immune privilege. J. Leukoc. Biol. 2018, 103, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hill, D.; Guo, L.; Nicholas, R.; Papadopoulos, D.; Cordeiro, M.F. Automated characterisation of microglia in ageing mice using image processing and supervised machine learning algorithms. Sci. Rep. 2022, 12, 1806. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef]
- Choudhry, N.; Duker, J.S.; Freund, K.B.; Kiss, S.; Querques, G.; Rosen, R.; Sarraf, D.; Souied, E.H.; Stanga, P.E.; Staurenghi, G.; et al. Classification and Guidelines for Widefield Imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol. Retin. 2019, 3, 843–849. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Weinreb, R.N.; Xu, G.; Lindsey, J.D.; Ye, C.; Yung, W.; Pang, C.-P.; Lam, D.S.C.; Leung, C.K. Tracking Retinal Microgliosis in Models of Retinal Ganglion Cell Damage. Investig. Opthalmol. Vis. Sci. 2012, 53, 6254. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.; Droho, S.; Lavine, J.A. Macrophages in close proximity to the vitreoretinal interface are potential biomarkers of inflammation during retinal vascular disease. J. Neuroinflamm. 2022, 19, 203. [Google Scholar] [CrossRef]
- Keane, P.A.; Balaskas, K.; Sim, D.A.; Aman, K.; Denniston, A.K.; Aslam, T.; Aslam, T. Automated analysis of vitreous inflammation using spectral-domain optical coherence tomography. Transl. Vis. Sci. Technol. 2015, 4, 4. [Google Scholar] [CrossRef]
- Zarranz-Ventura, J.; Keane, P.A.; Sim, D.A.; Llorens, V.; Tufail, A.; Sadda, S.R.; Dick, A.D.; Lee, R.W.; Pavesio, C.; Denniston, A.K.; et al. Evaluation of Objective Vitritis Grading Method Using Optical Coherence Tomography: Influence of Phakic Status and Previous Vitrectomy. Am. J. Ophthalmol. 2016, 161, 172–180.e4. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A.; Estrada, F.M. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Streilein, J.W. Immune Privilege Extended to Allogeneic Tumor Cells in the Vitreous Cavity. Investig. Ophthalmol. Vis. Sci. 1991, 32, 224–228. [Google Scholar]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef]
- O’Koren, E.G.; Mathew, R.; Saban, D.R. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep. 2016, 6, 20636. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Guo, L.; Luong, V.; Harding, G.; Wang, W.; Jones, H.E.; Moss, S.E.; Sillito, A.M.; Fitzke, F.W. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13352–13356. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Normando, E.M.; Cardoso, M.J.; Miodragovic, S.; Jeylani, S.; Davis, B.M.; Guo, L.; Ourselin, S.; A’Hern, R.; Bloom, P.A. Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain 2017, 140, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Coric, D.; Ometto, G.; Montesano, G.; Keane, P.A.; Balk, L.J.; Uitdehaag, B.M.J.; Petzold, A.; Crabb, D.P.; Denniston, A.K. Objective quantification of vitreous haze on optical coherence tomography scans: No evidence for relationship between uveitis and inflammation in multiple sclerosis. Eur. J. Neurol. 2020, 27, 144-e3. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.R.; St-Pierre, M.K.; Wendeln, A.C.; Makoni, N.J.; Gouwens, L.K.; Garrad, E.C.; Sohrabi, M.; Neher, J.J.; Tremblay, M.E.; Combs, C.K. Inflammatory Mechanisms in Neurodegeneration. J. Neurochem. 2019, 149, 562. [Google Scholar] [CrossRef] [PubMed]

| Right Eye | Left Eye | |||||
|---|---|---|---|---|---|---|
| MsDx | MsDxF | HC | MsDx | MsDxF | HC | |
| IOP/IOP | 4 w/6 w(m) | 6 w/12 w (m) | 4 w/8 w (r = 0.934, p = 0.020) | 2 w/18 w (im) | 2 w/4 w (m) 4 w/6-8-24 w (m) | |
| IOP/OCT | 2 w/24 w (r = 0.988, p = 0.002) 4 w/24 w (r = 0.896, p = 0.040) | 18 w/24 w (r = 0.854, p = 0.031) | 4 w/18 w (r = 0.889, p = 0.043) | 0 w/8 w (r = 0.882, p = 0.020) 0 w/12 w (r = −0.851, p = 0.032) 6 w/8 w (r = 0.813, p = 0.049) | 24 w/24 w (im) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, M.J.; Subías, M.; Montolío, A.; Martínez-Rincón, T.; Aragón-Navas, A.; Bravo-Osuna, I.; Pablo, L.E.; Cegoñino, J.; Herrero-Vanrell, R.; Garcia-Martin, E.; et al. Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma. Biomedicines 2024, 12, 633. https://doi.org/10.3390/biomedicines12030633
Rodrigo MJ, Subías M, Montolío A, Martínez-Rincón T, Aragón-Navas A, Bravo-Osuna I, Pablo LE, Cegoñino J, Herrero-Vanrell R, Garcia-Martin E, et al. Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma. Biomedicines. 2024; 12(3):633. https://doi.org/10.3390/biomedicines12030633
Chicago/Turabian StyleRodrigo, Maria J., Manuel Subías, Alberto Montolío, Teresa Martínez-Rincón, Alba Aragón-Navas, Irene Bravo-Osuna, Luis E. Pablo, Jose Cegoñino, Rocío Herrero-Vanrell, Elena Garcia-Martin, and et al. 2024. "Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma" Biomedicines 12, no. 3: 633. https://doi.org/10.3390/biomedicines12030633
APA StyleRodrigo, M. J., Subías, M., Montolío, A., Martínez-Rincón, T., Aragón-Navas, A., Bravo-Osuna, I., Pablo, L. E., Cegoñino, J., Herrero-Vanrell, R., Garcia-Martin, E., & Pérez del Palomar, A. (2024). Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma. Biomedicines, 12(3), 633. https://doi.org/10.3390/biomedicines12030633







