Artificial Intelligence Applied to Electrical and Non-Invasive Hemodynamic Markers in Elderly Decompensated Chronic Heart Failure Patients
Abstract
1. Introduction
2. Methods
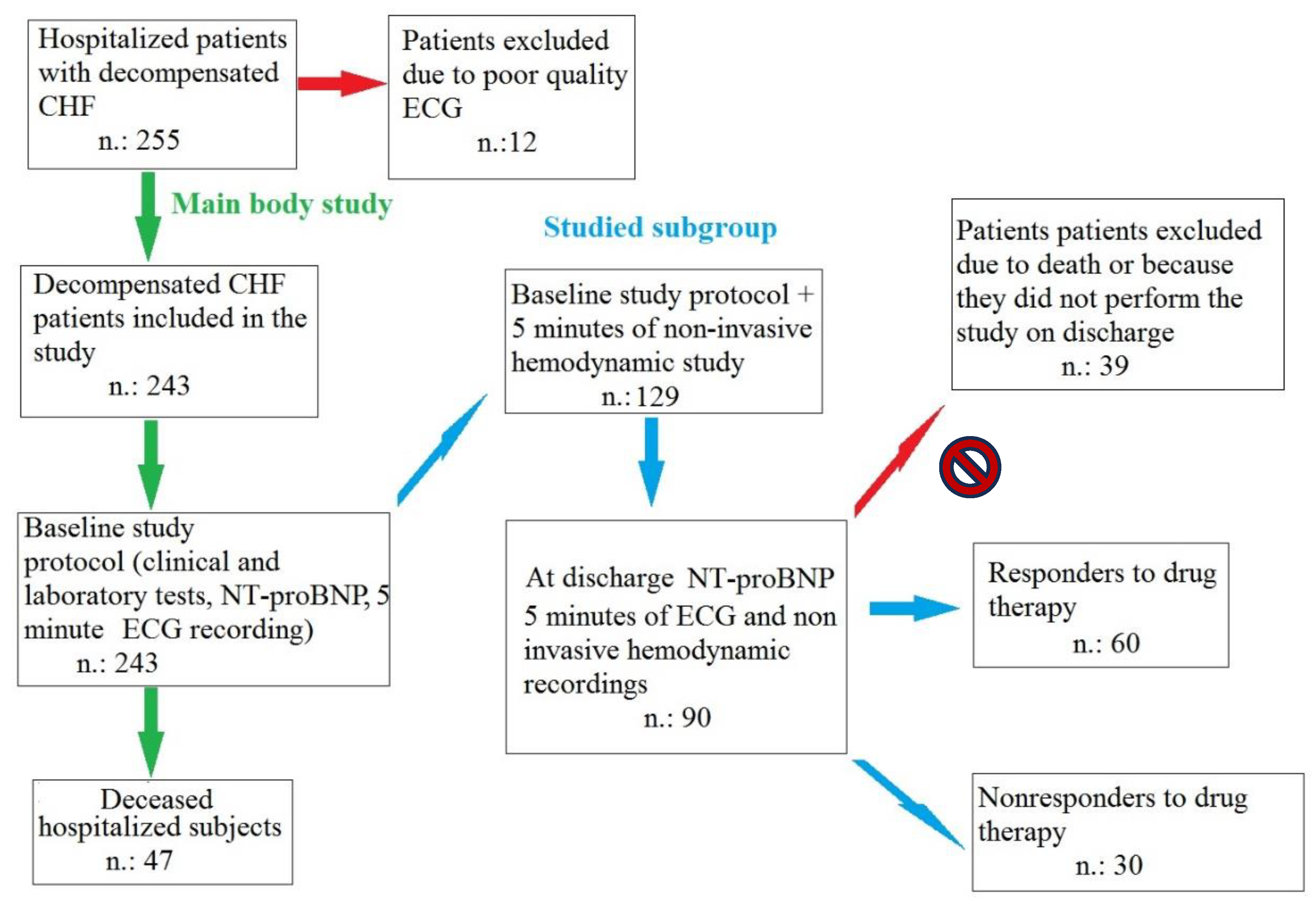
2.1. Study Subjects and ECG Analysis
2.2. Statistical Analysis
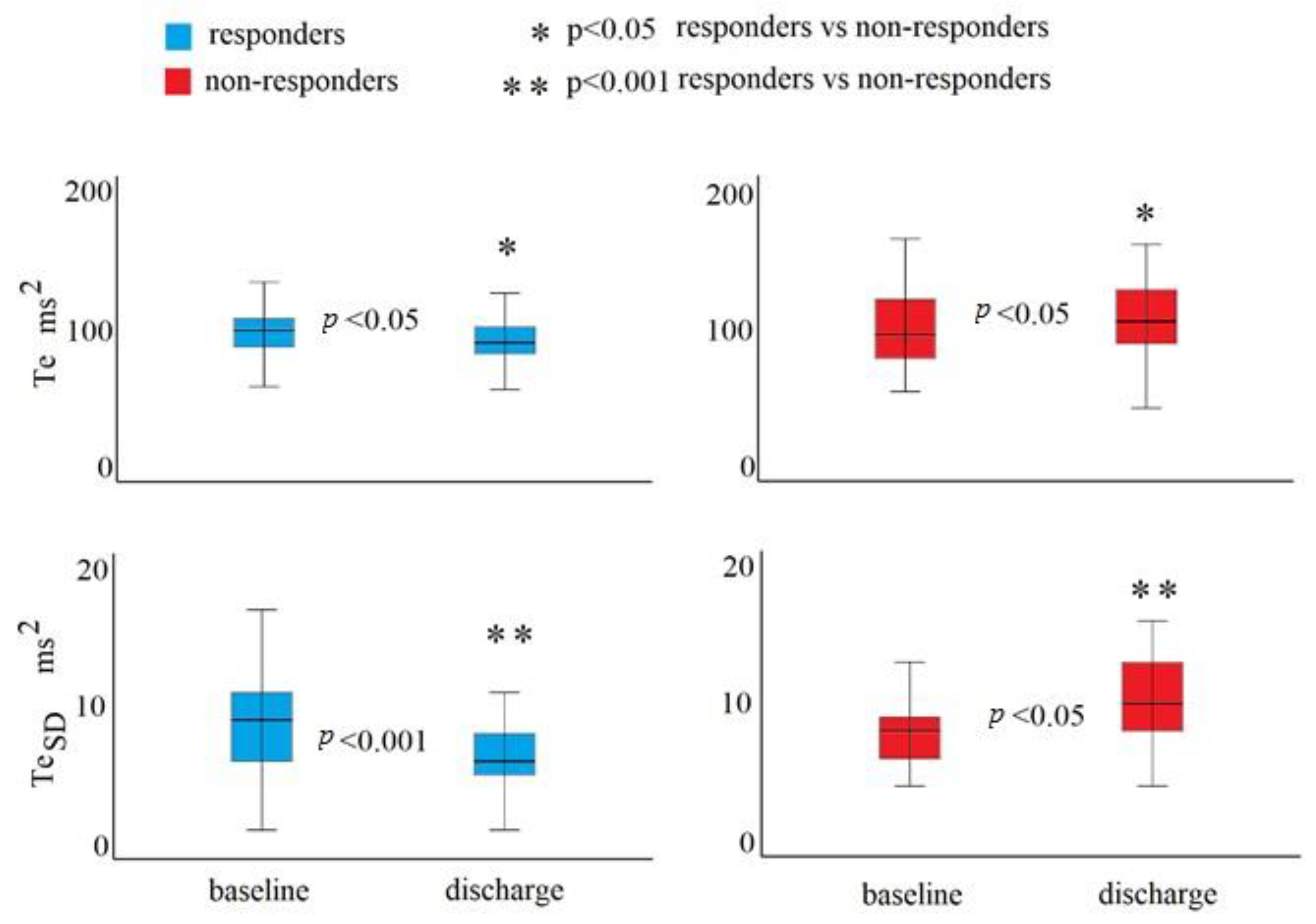
3. Results
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S. Comparison of International Guidelines for Managing Chronic Heart Failure with Reduced Ejection Fraction. Curr. Probl. Cardiol. 2023, 48, 101867. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Seferovic, P.; Savarese, G.; Spoletini, I.; Lopatin, Y.; Gustafsson, F.; Bayes-Genis, A.; Jaarsma, T.; Abdelhamid, M.; Miqueo, A.G.; et al. Impact analysis of heart failure across European countries: An ESC-HFA position paper. ESC Heart Fail. 2022, 9, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Ploux, S.; Strik, M.; Ramirez, F.D.; Buliard, S.; Chauvel, R.; Dos Santos, P.; Haïssaguerre, M.; Jobbé-Duval, A.; Picard, F.; Riocreux, C.; et al. Remote management of worsening heart failure to avoid hospitalization in a real-world setting. ESC Heart Fail. 2023, 10, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; von Haehling, S.; Papp, Z.; Piepoli, M.F. A year in heart failure: Updates of clinical and preclinical findings. ESC Heart Fail. 2023, 10, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.P.; Nikorowitsch, J.; Bei der Kellen, R.; Magnussen, C.; Bonin-Schnabel, R.; Westermann, D.; Twerenbold, R.; Kirchhof, P.; Blankenberg, S.; Schrage, B. Heart failure in the general population and impact of the 2021 European Society of Cardiology Heart Failure Guidelines. ESC Heart Fail. 2022, 9, 2157–2169. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Sciomer, S.; Magrì, D. Chronic Heart Failure Management: Monitoring Patients and Intercepting Exacerbations. Rev. Cardiovasc. Med. 2023, 24, 208. [Google Scholar] [CrossRef]
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014. [Google Scholar] [CrossRef]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef]
- Gheorghiade, M.; De Luca, L.; Fonarow, G.C.; Filippatos, G.; Metra, M.; Francis, G.S. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am. J. Cardiol. 2005, 96, 11G–17G. [Google Scholar] [CrossRef]
- Reynard, J.T.; Oshodi, O.M.; Lai, J.C.; Lai, R.W.; Bazoukis, G.; Fragakis, N.; Letsas, K.P.; Korantzopoulos, P.; Liu, F.Z.; Liu, T.; et al. Electrocardiographic conduction and repolarization markers associated with sudden cardiac death: Moving along the electrocardiography waveform. Minerva Cardioangiol. 2019, 67, 131–144. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Bachmann, T.N.; Skov, M.W.; Rasmussen, P.V.; Graff, C.; Pietersen, A.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Haunsø, S.; Køber, L.; et al. Electrocardiographic Tpeak-Tend interval and risk of cardiovascular morbidity and mortality: Results from the Copenhagen ECG study. Heart Rhythm. 2016, 13, 915–924. [Google Scholar] [CrossRef]
- Tse, G.; Gong, M.; Wong, W.T.; Georgopoulos, S.; Letsas, K.P.; Vassiliou, V.S.; Chan, Y.S.; Yan, B.P.; Wong, S.H.; Wu, W.K.; et al. The Tpeak-Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta-analysis. Heart Rhythm. 2017, 14, 1131–1137. [Google Scholar] [CrossRef]
- Braun, C.C.; Zink, M.D.; Gozdowsky, S.; Hoffmann, J.M.; Hochhausen, N.; Röhl, A.B.; Beckers, S.K.; Kork, F. A Longer Tpeak-Tend Interval Is Associated with a Higher Risk of Death: A Meta-Analysis. J. Clin. Med. 2023, 12, 992. [Google Scholar] [CrossRef]
- Moscucci, F.; Sciomer, S.; Maffei, S.; Meloni, A.; Lospinuso, I.; Carnovale, M.; Corrao, A.; Di Diego, I.; Caltabiano, C.; Mezzadri, M.; et al. Sex Differences in Repolarization Markers: Telemonitoring for Chronic Heart Failure Patients. J. Clin. Med. 2023, 12, 4714. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Mezzadri, M.; Caltabiano, C.; Di Diego, I.; Carnovale, M.; Corrao, A.; Stefano, S.; Scinicariello, C.; Giuffrè, M.; et al. Electrocardiographic and other Noninvasive Hemodynamic Markers in Decompensated CHF Patients. J. Cardiovasc. Dev. Dis. 2023, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Corrao, A.; Carnovale, M.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Rossi, P.; Magrì, D. Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments. Biomedicines 2022, 10, 2407. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Carnovale, M.; Corrao, A.; Di Diego, I.; Lospinuso, I.; Sciomer, S.; Rossi, P.; Magrì, D. Glucose dysregulation and repolarization variability markers are short-term mortality predictors in decompensated heart failure. Cardiovasc. Endocrinol. Metab. 2022, 11, e0264. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 2017, 19, 712–721. [Google Scholar] [CrossRef]
- Cullington, D.; Goode, K.M.; Zhang, J.; Cleland, J.G.; Clark, A.L. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014, 2, 213–220. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Mariani, M.V.; Di Iorio, C.; Fabietti, M.; Mastropietri, F.; Crapanzano, D.; Bertani, G.; Sabatino, T.; Zaccagnini, G.; et al. Hospital mortality in decompensated heart failure. A pilot study. J. Electrocardiol. 2020, 61, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Rodríguez, A.; Pello, A.M.; López-Castillo, M.; Taibo Urquía, M.; Balaguer-Germán, J.; Munté, A.; González-Martín, G.; Carriazo-Julio, S.M.; Martínez-Milla, J.; Kallmeyer, A.; et al. The role of bioimpedance analysis in overweight and obese patients with acute heart failure: A pilot study. ESC Heart Fail. 2023, 10, 2418–2426. [Google Scholar] [CrossRef]
- Senarath, S.; Fernie, G.; Roshan Fekr, A. Influential Factors in Remote Monitoring of Heart Failure Patients: A Review of the Literature and Direction for Future Research. Sensors 2021, 21, 3575. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.; Fouhey, D.; Shepard, S.; Valley, T.S.; Kazerooni, E.A.; Banovic, N.; Wiens, J.; Sjoding, M.W. Measuring the Impact of AI in the Diagnosis of Hospitalized Patients: A Randomized Clinical Vignette Survey Study. JAMA 2023, 330, 2275–2284. [Google Scholar] [CrossRef]
- Leto, L.; Testa, M.; Feola, M. The predictive value of plasma biomarkers in discharged heart failure patients: Role of plasma NT-proBNP. Minerva Cardioangiol. 2016, 64, 157–164. [Google Scholar]
- Orini, M.; Al-Amodi, F.; Koelsch, S.; Bailón, R. The Effect of Emotional Valence on Ventricular Repolarization Dynamics Is Mediated by Heart Rate Variability: A Study of QT Variability and Music-Induced Emotions. Front. Physiol. 2019, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Saal, D.P.; Thijs, R.D.; van Zwet, E.W.; Bootsma, M.; Brignole, M.; Benditt, D.G.; van Dijk, J.G. Temporal Relationship of Asystole to Onset of Transient Loss of Consciousness in Tilt-Induced Reflex Syncope. JACC Clin. Electrophysiol. 2017, 3, 1592–1598. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yoshitomi, H.; Ito, S.; Adachi, T.; Sato, H.; Watanabe, N.; Kodani, N.; Sugamori, T.; Endo, A.; Takahashi, N.; et al. Left atrial remodeling and recurrence of congestive heart failure in patients initially diagnosed with heart failure. Echocardiography 2014, 31, 936–940. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; D’Alessandro, G.; Fiorucci, C.; Moscucci, F.; Di Iorio, C.; Mastropietri, F.; Parrotta, I.; Ogawa, M.; Lin, S.F.; et al. Oscillatory behavior of P wave duration and PR interval in experimental congestive heart failure: A preliminary study. Physiol. Meas. 2018, 39, 035010. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Magrì, D. Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity. Atmosphere 2022, 13, 123. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Di Diego, I.; Mezzadri, M.; Caltabiano, C.; Carnovale, M.; Corrao, A.; Lospinuso, I.; Stefano, S.; Scinicariello, C.; et al. Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects. Biology 2023, 12, 960. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Corrao, A.; Carnovale, M.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Magrì, D. Time and Frequency Repolarization Domains in Elderly Candidates to Transcatheter Aortic Valve Replacement. La Clin. Ter. 2023, 174, 139–145. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Pofi, R.; D’Alessandro, G.; Minnetti, M.; Isidori, A.M.; Francomano, D.; Lenzi, A.; Puddu, P.E.; Alexandre, J.; et al. Changes in left ventricular repolarization after short-term testosterone replacement therapy in hypogonadal males. J. Endocrinol. Investig. 2019, 42, 1051–1065. [Google Scholar] [CrossRef]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.R.; Barnholt, K.E.; Grundmann, N.K.; Lin, J.H.; McCallum, S.W.; Friedlander, A.L. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J. Appl. Physiol. 2006, 100, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, P.M.; Koralsztein, J.P.; Billat, V.L. Effect of exercise intensity on relationship between VO2max and cardiac output. Med. Sci. Sports Exerc. 2004, 36, 1357–1363. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Alnuaimat, H.; Li, N.; Carrie, R.; Mubarak, K.K. Value of impedance cardiography in patients studied for pulmonary hypertension. Lung 2011, 189, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.; Abbiss, C.R.; Maiorana, A.J.; Marston, K.J.; Peiffer, J.J. Intrarater reliability and agreement of the physioflow bioimpedance cardiography device during rest, moderate and high-intensity exercise. Kinesiology 2018, 50 (Suppl. 1), 140–149. [Google Scholar]
- Leão, R.N.; Da Silva, P.M. Impedance Cardiography in the Evaluation of Patients with Arterial Hypertension. Int. J. Cardiovasc. Sci. 2019, 32, 61–69. [Google Scholar] [CrossRef]
- Anand, G.; Yu, Y.; Lowe, A.; Kalra, A. Bioimpedance analysis as a tool for hemodynamic monitoring: Overview, methods and challenges. Physiol. Meas. 2021, 42, 03TR01. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Carnovale, M.; Corrao, A.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Rossi, P.; Magrì, D. Short-Period Temporal Dispersion Repolarization Markers in Elderly Patients with Decompensated Heart Failure. La Clin. Ter. 2022, 173, 356–361. [Google Scholar]
- Alhakak, A.S.; Teerlink, J.R.; Lindenfeld, J.; Böhm, M.; Rosano, G.M.C.; Biering-Sørensen, T. The significance of left ventricular ejection time in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C. Heterogeneity and cardiac arrhythmias: An overview. Heart Rhythm. 2007, 4, 964–972. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Di Diego, J.M. Tpeak-Tend interval as a marker of arrhythmic risk. Heart Rhythm. 2019, 16, 954–955. [Google Scholar] [CrossRef]
- Opthof, T.; Coronel, R.; Wilms-Schopman, F.J.; Plotnikov, A.N.; Shlapakova, I.N.; Danilo, P.; Rosen, M.R., Jr.; Janse, M.J. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007, 4, 341–348. [Google Scholar] [CrossRef]
- Malik, M.; Huikuri, H.; Lombardi, F.; Schmidt, G.; Zabel, M. e-Rhythm Study Group of EHRA Conundrum of the Tpeak-Tend interval. J. Cardiovasc. Electrophysiol. 2018, 29, 767–770. [Google Scholar] [CrossRef]
- Malik, M.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Verrier, R.L.; Zabel, M. e-Rhythm Group of EHRA Is the Tpeak-Tend interval as a measure of repolarization heterogeneity dead or just seriously woded? Heart Rhythm. 2019, 16, 952–953. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Iorio, C.D.; Fabietti, M.; Mastropietri, F.; Crapanzano, D.; Bertani, G.; Sabatino, T.; Zaccagnini, G.; Lospinuso, I.; et al. Time- and frequency-domain analysis of repolarization phase during recovery from exercise in healthy subjects. Pacing Clin. Electrophysiol. PACE 2020, 43, 1096–1103. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; D’Alessandro, G.; Pascucci, M.; Rossi, P.; Han, S.; Chen, L.S.; Lin, S.F.; Chen, P.S.; Magrì, D. Myocardial repolarization dispersion and autonomic nerve activity in a canine experimental acute myocardial infarction model. Heart Rhythm. 2014, 11, 110–118. [Google Scholar] [CrossRef]
- Yagishita, D.; Chui, R.W.; Yamakawa, K.; Rajendran, P.S.; Ajijola, O.A.; Nakamura, K.; So, E.L.; Mahajan, A.; Shivkumar, K.; Vaseghi, M. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circ. Arrhythmia Electrophysiol. 2015, 8, 174–185. [Google Scholar] [CrossRef]
- Aiba, T.; Tomaselli, G.F. Electrical remodeling in the failing heart. Curr. Opin. Cardiol. 2010, 25, 29–36. [Google Scholar] [CrossRef]
- Rahm, A.K.; Lugenbiel, P.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Role of ion channels in heart failure and channelopathies. Biophys. Rev. 2018, 10, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Bezzina, C.R. Sodium channel (dys)function and cardiac arrhythmias. Cardiovasc. Ther. 2010, 28, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered myocardial calcium cycling and energetics in heart failure—A rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational modeling of cardiac electrophysiology and arrhythmogenesis. Physiol. Rev. 2023. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.P.; Paixão, G.M.M.; Gomes, P.R.; Ribeiro, M.H.; Ribeiro, A.H.; Canazart, J.A.; Oliveira, D.M.; Ferreira, M.P.; Lima, E.M.; Moraes, J.L.; et al. Tele-electrocardiography and bigdata: The CODE (Clinical Outcomes in Digital Electrocardiography) study. J. Electrocardiol. 2019, 57S, S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, K.; Nishimori, M.; Kiuchi, K.; Shinohara, M.; Fukuzawa, K.; Takami, M.; El Hamriti, M.; Sommer, P.; Sakai, J.; Nakamura, T.; et al. Development of a Visualization Deep Learning Model for Classifying Origins of Ventricular Arrhythmias. Circ. J. Off. J. Jpn. Circ. Soc. 2022, 86, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Hughes, J.W.; Rogers, A.J.; Kang, G.; Narayan, S.M.; Ashley, E.A.; Perez, M.V. Race, Sex, and Age Disparities in the Performance of ECG Deep Learning Models Predicting Heart Failure. Circ. Heart Fail. 2024, 17, e010879. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.U.; Alsenani, Y.; Zafar, A.; Ullah, K.; Rabie, K.; Shongwe, T. Enhancing heart disease prediction using a self-attention-based transformer model. Sci. Rep. 2024, 14, 514. [Google Scholar] [CrossRef]
- Zhang, C.J.; Tang, F.Q.; Cai, H.P.; Qian, Y.F. Heart failure classification using deep learning to extract spatiotemporal features from ECG. BMC Med. Inform. Decis. Mak. 2024, 24, 17. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Biswas, D.; Ryan, M.; Bernstein, B.M.; Rizvi, M.; Fairhurst, N.; Kaye, G.; Baral, R.; Searle, T.; Melikian, N.; et al. Artificial intelligence methods for improved detection of undiagnosed heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2023. Advance online publication. [Google Scholar] [CrossRef]
- Ramírez, J.; Monasterio, V.; Mincholé, A.; Llamedo, M.; Lenis, G.; Cygankiewicz, I.; Bayés de Luna, A.; Malik, M.; Martínez, J.P.; Laguna, P.; et al. Automatic SVM classification of sudden cardiac death and pump failure death from autonomic and repolarization ECG markers. J. Electrocardiol. 2015, 48, 551–557. [Google Scholar] [CrossRef]
- Palacios, S.; Cygankiewicz, I.; Bayés de Luna, A.; Pueyo, E.; Martínez, J.P. Periodic repolarization dynamics as predictor of risk for sudden cardiac death in chronic heart failure patients. Sci. Rep. 2021, 11, 20546. [Google Scholar] [CrossRef]
- Barrett, M.; Boyne, J.; Brandts, J.; Brunner-La Rocca, H.P.; De Maesschalck, L.; De Wit, K.; Dixon, L.; Eurlings, C.; Fitzsimons, D.; Golubnitschaja, O.; et al. Artificial intelligence supported patient self-care in chronic heart failure: A paradigm shift from reactive to predictive, preventive and personalised care. EPMA J. 2019, 10, 445–464. [Google Scholar] [CrossRef]
- Pagallo, U.; O'Sullivan, S.; Nevejans, N.; Holzinger, A.; Friebe, M.; Jeanquartier, F.; Jean-Quartier, C.; Miernik, A. The underuse of AI in the health sector: Opportunity costs, success stories, risks and recommendations. Health Technol. 2024, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Croon, P.M.; Selder, J.L.; Allaart, C.P.; Bleijendaal, H.; Chamuleau, S.A.J.; Hofstra, L.; Išgum, I.; Ziesemer, K.A.; Winter, M.M. Current state of artificial intelligence-based algorithms for hospital admission prediction in patients with heart failure: A scoping review. Eur. Heart J. Digit. Health 2022, 3, 415–425. [Google Scholar] [CrossRef] [PubMed]
| Heart Failure with Reduced Ejection Fraction | Heart Failure with Mildly Reduced Ejection Fraction | Heart Failure with Preserved Ejection Fraction | ||
|---|---|---|---|---|
| N: 112 | N: 38 | N: 93 | p | |
| Gender, M/F | 65/47 | 16/22 | 41/52 | >0.5 |
| Age, years | 83 ± 10 | 85 ± 7 | 83 ± 10 | 0.497 |
| BMI, kg/m2 | 26 ± 5 | 25 ± 4 | 26 ± 5 | 0.536 |
| Heart Rate (radial pulse), beats/m | 74 ± 12 | 73 ± 15 | 76 ± 13 | 0.463 |
| Systolic Blood Pressure, mmHg | 120 ± 17 ** | 127 ± 17 | 130 ± 21 ** | <0.001 |
| Diastolic Blood Pressure, mmHg | 67 ± 9 ** | 67 ± 11 | 73 ± 11 ** | <0.001 |
| Left Ventricular Ejection Fraction, % | 32 ± 7 **## | 45 ± 1 ##§§ | 52 ± 3 **§§ | <0.001 |
| Left Ventricular Mass Index, g/m2 | 147 ± 34 **# | 129 ± 34 # | 123 ± 26 ** | <0.001 |
| Left Ventricular End-Diastolic Diameter, mm | 57 ± 8 **## | 51 ± 5 ## | 50 ± 5 ** | <0.001 |
| Posterior Wall Thickness, mm | 11 ± 2 | 11 ± 2 | 11 ± 2 | 0.691 |
| Interventricular Septum Thickness, mm | 12 ± 2 | 12 ± 1 | 12 ± 1 | 0.594 |
| Left Atrial Transversal Diameter, mm | 49 ± 8 *# | 45 ± 6 # | 45 ± 6 * | <0.001 |
| Tricuspid Annular Plane Systolic Excursion, mm | 19 ± 3 ** | 21 ± 5 | 22 ± 4 ** | <0.001 |
| Tricuspid Regurgitation Peak Gradient, mmHg | 47 ± 13 # | 40 ± 11 # | 43 ± 15 | 0.030 |
| Hemoglobin (g/dL) | 11.4 ± 2.1 | 11.1 ± 1.6 | 11.4 ± 2.1 | 0.689 |
| Arterial O2 Saturation, % | 97 ± 3 | 97 ± 2 | 97 ± 4 | 0.655 |
| Fraction of Inspired O2,% | 27 ± 9 | 26 ± 7 | 26 ± 8 | 0.828 |
| PaO2/FiO2 ratio | 334 ± 103 | 339 ± 126 | 329 ± 90 | 0.884 |
| A-ADO2, mmHg | 39 [51] | 35 [49] | 33 [41] | 0.671 |
| NT-proBNP, pg/mL | 6170 [9995] * | 2725 [4590] | 1660 [3085] * | <0.001 |
| C-ReactiveProtein (mg/dL) | 4.0 [9.4] | 3.6 [11.7] | 5.1 [8.8] | 0.926 |
| High-Sensitivity Cardiac Troponin/(pg/L) | 62 [98] **## | 36 [47] ## | 31 [29] ** | <0.001 |
| Serum Sodium (mmol/L) | 141 ± 6 | 141 ± 5 | 141 ± 5 | 0.957 |
| Serum Potassium (mmol/L) | 4.0 ± 0.6 | 4.1 ± 0.6 | 4.1 ± 0.6 | 0.532 |
| Serum Calcium (mmol/L) | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.054 |
| Creatinine Clearance (mL/m) | 38 [24] *# | 48 [30] #§ | 50 [38] *§ | 0.025 |
| Serum Urea (mmol/L) | 12.3 [8.3] | 10.2 [7.8] | 8.3 [5.6] | 0.003 |
| Albumin (g/dL) | 3.4 ± 0.6 | 3.4 ± 0.5 | 3.5 ± 0.6 | 0.736 |
| Fasting Glucose (mmol/L) | 6.8 ± 2.2 | 6.6 ± 2.7 | 6.2 ± 2.7 | 1.131 |
| HbA1c (%) | 6.2 ± 1.2 | 5.9 ± 1.3 | 5.8 ± 1.0 | 0.099 |
| Total Cholesterol (mmol/L) | 3.6 ± 0.9 | 3.8 ± 0.9 | 3.9 ± 1.0 | 0.444 |
| HDL-Cholesterol (mmol/L) | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.975 |
| LDL-Cholesterol (mmol/L) | 1.9 ± 0.8 | 2.1 ± 0.6 | 2.1 ± 0.9 | 0.608 |
| Triglycerides (mmol/L) | 1.9 ± 1.6 | 1.6 ± 0.7 | 1.5 ± 0.7 | 0.219 |
| Hypertension, n (%) | 80 (71) | 32 (84) | 77 (41) | 0.087 |
| Hypercholesterolemia, n (%) | 54 (48) | 14 (37) | 41 (44) | 0.468 |
| Diabetes, n (%) | 57 (51) | 12 (32) | 32 (34) | 0.087 |
| Renal Insufficiency, n (%) | 67 (60) * | 16 (42) | 37 (40) * | 0.010 |
| Known Myocardial Ischemia History, n (%) | 57 (51) **# | 8 (21) # | 18 (19) ** | <0.001 |
| Valve Diseases, n (%) | 26 (23) | 12 (32) | 24 (25) | 0.651 |
| Premature Supraventricular Complexes, n (%) | 12 (11) | 2 (5) | 9 (10) | 0.609 |
| Premature Ventricular Complexes, n (%) | 26 (23) | 11 (29) | 16 (17) | 0.298 |
| Permanent Atrial Fibrillation, n (%) | 46 (41) | 18 (47) | 30 (32) | 0.213 |
| Left Bundle Branch Block, n (%) | 34 (30) ** | 10 (26) §§ | 6 (7) **§§ | <0.001 |
| Right Bundle Branch Block, n (%) | 18 (16) | 4 (11) | 13 (14) | 0.694 |
| Pacemaker–ICD, n (%) | 37 (33) ** | 10 (26) §§ | 8 (9) **§§ | <0.001 |
| Deceased Hospitalized Patients, n (%) | 27 (24) | 7 (18) | 13 (14) | 0.186 |
| β-Blockers, n (%) | 82 (73) * | 30 (80) § | 52 (56) *§ | 0.008 |
| Furosemide, n (%) | 97 (87) ** | 31 (82) | 60 (65) ** | 0.001 |
| ACE/Sartans | 45 (40) | 10 (26) | 41 (44) | 0.165 |
| Aldosterone Antagonists, n (%) | 25 (22) * | 7 (18) | 8 (9) * | 0.029 |
| Potassium, n (%) | 7 (6) | 2 (5) | 8 (9) | 0.726 |
| Nitrates, n (%) | 14 (13) | 6 (16) | 8 (9) | 0.458 |
| Digoxin, n (%) | 6 (5) | 3 (8) | 3 (3) | 0.514 |
| Statins, n (%) | 36 (32) | 8 (21) | 26 (28) | 0.416 |
| Antiplatelet Drugs, n (%) | 47 (42) | 9 (24) | 35 (38) | 0.132 |
| Oral Anticoagulants, n (%) | 27 (24) | 13 (34) | 30 (32) | 0.319 |
| Diltiazem or Verapamil, n (%) | 1 (1) | 1 (3) | 6 (7) | 0.082 |
| Ivabradine, n | 2 (2) | 1 (3) | 2 (2) | 0.948 |
| Dihydropyridine Calcium Channel Blockers, n (%) | 8 (7) * | 6 (16) | 17 (18) * | 0.049 |
| Propafenone, n (%) | 0 (0) | 0 (0) | 2 (2) | 0.197 |
| Amiodarone, n (%) | 11 (10) | 1 (3) | 7 (8) | 0.358 |
| Valsartan/Sacubitril, n (%) | 4 (4) | 0 (0) | 0 (0) | 0.093 |
| SGLT-2i, n (%) | 1 (1) | 0 (0) | 0 (0) | 0.999 |
| Heart Failure with Reduced Ejection Fraction | Heart Failure with Mildly Reduced Ejection Fraction | Heart Failure with Preserved Ejection Fraction | ||
|---|---|---|---|---|
| Variables | N: 112 | N: 38 | N: 93 | p |
| RR, ms | 850 ± 160 | 864 ± 164 | 871 ± 174 | 0.671 |
| QTe, ms | 490 ± 87 ** | 457 ± 94 | 427 ± 65 ** | <0.001 |
| QTeSD, ms | 10 [5] * | 10 [5] | 8 [5] * | 0.043 |
| QTp, ms | 373 ± 85 * | 353 ± 83 | 332 ± 56 * | 0.001 |
| QTpSD, ms | 9 [5] | 9 [4] | 8 [3] | 0.216 |
| Te, ms | 110 ± 27 ** | 106 ± 31 | 94 ± 22 ** | <0.001 |
| TeSD, ms | 8 [6] ** | 9 [6] § | 6 [4] **§ | <0.001 |
| Heart Failure with Reduced Ejection Fraction | Heart Failure with Mildly Reduced Ejection Fraction | Heart Failure with Preserved Ejection Fraction | ||
|---|---|---|---|---|
| Variables | N: 63 | N: 20 | N: 46 | p |
| Heart Rate, b/m | 81 ± 23 * | 86 ± 30 § | 72 ± 13 | 0.031 |
| Stroke Volume, mL | 59 ± 17 * | 64 ± 23 | 71 ± 20 * | 0.008 |
| Stroke Volume Index, mL/m2 | 33 ± 10 * | 37 ± 14 | 40 ± 10 * | 0.004 |
| Cardiac Output, L/m | 4.59 ± 1.39 | 5.04 ± 1.60 | 4.97 ± 1.16 | 0.239 |
| Cardiac Index, L/m/m2 | 2.53 ± 0.75 * | 2.95 ± 0.87 | 2.81 ± 0.74 * | 0.047 |
| Systemic Vascular Resistance, Dyn.s/cm2 | 3390 ± 1440 | 2901 ± 739 | 2932 ± 877 | 0.090 |
| Systemic Vascular Resistance Index, Dyn.s/cm2.m2 | 1849 ± 692 | 1716 ± 458 | 1639 ± 491 | 0.188 |
| SBP, mmHg | 122 ± 17 | 124 ± 13 | 125 ± 13 | 0.503 |
| MBP, mmHg | 71 ± 10 | 73 ± 9 | 72 ± 10 | 0.691 |
| DBP, mmHg | 93 ± 12 | 95 ± 9 | 95 ± 10 | 0.563 |
| Left Ventricular Ejection Fraction, % | 34 ± 13 ** | 39 ± 14 | 46 ± 15 ** | <0.001 |
| Contractility Index | 61 ± 40 * | 78 ± 44 | 86 ± 53 * | 0.031 |
| Left Ventricular Ejection Time, ms | 267 ± 77 | 271 ± 74 | 291 ± 86 | 0.271 |
| Left Cardiac Work Index, kg.m/m2 | 3.04± | 3.63 ± 1.20 | 3.49 ± 1.04 | 0.063 |
| Left Ventricular End-Diastolic Volume, mL | 194 ± 90 | 195 ± 134 | 164 ± 49 | 0.162 |
| Early Diastolic Filling Ratio | 92 ± 35 * | 98 ± 57 | 77 ± 25 * | 0.045 |
| Responders | Non-Responders | |||||
|---|---|---|---|---|---|---|
| Baseline | Discharge | Baseline | Discharge | |||
| Variables | N: 60 | N: 60 | p | N: 30 | N: 30 | p |
| Heart Rate, b/m | 82 ± 26 | 74 ± 17 | 0.020 | 74 ± 17 | 77 ± 19 | 0.308 |
| Stroke Volume, mL | 64 ± 20 | 66 ± 24 | 0.490 | 65 ± 15 | 63 ± 15 | 0.576 |
| Stroke Volume Index, mL/m2 | 36 ± 12 | 38 ± 14 | 0.536 | 35 ± 9 | 35 ± 10 | 0.445 |
| Cardiac Output, L/m | 4.88 ± 1.48 | 4.72 ± 1.73 | 0.558 | 4.58 ± 1.09 | 4.60 ± 1.24 | 0.974 |
| Cardiac Index, L/m/m2 | 2.79 ± 0.87 | 2.69 ± 1.02 | 0.520 | 2.53 ± 0.63 | 2.52 ± 0.62 | 0.840 |
| Systemic Vascular Resistance, Dyn.s/cm2 | 3099 ± 1279 | 3049 ± 1113 | 0.868 | 3221 ± 1048 | 3307 ± 1166 | 0.747 |
| Systemic Vascular Resistance Index, Dyn.s/cm2.m2 | 1748 ± 600 | 1730 ± 619 | 0.795 | 1760 ± 506 | 1852 ± 783 | 0.532 |
| SBP, mmHg | 124 ± 14 | 119 ± 13 | 0.025 | 120 ± 15 | 123 ± 14 | 0.190 |
| MBP, mmHg | 94 ± 9 | 90 ± 10 | 0.006 | 92 ± 11 | 94 ± 14 | 0.297 |
| DBP, mmHg | 71 ± 10 | 68 ± 10 | 0.016 | 71 ± 10 | 72 ± 9 | 0.693 |
| Left Ventricular Ejection Fraction, % | 40 ± 16 | 41 ± 17 * | 0.688 | 36 ± 14 | 34 ± 14 * | 0.427 |
| Contractility Index | 78 ± 56 | 86 ± 56 * | 0.296 | 67 ± 36 | 58 ± 29 * | 0.108 |
| Left Ventricular Ejection Time, ms | 271 ± 98 | 299 ± 91 | 0.019 | 275 ± 60 | 264 ± 80 | 0.631 |
| Left Cardiac Work Index, kg.m/m2 | 3.39 ± 1.29 | 3.14 ± 1.44 | 0.300 | 3.05 ± 0.97 | 3.06 ± 0.89 | 0.459 |
| Left Ventricular End-Diastolic Volume, mL | 172 ± 77 | 171 ± 64 * | 0.907 | 196 ± 71 | 200 ± 73 * | 0.459 |
| Early Diastolic Filling Ratio | 91 ± 45 | 81 ± 25 | 0.520 | 88 ± 32 | 91 ± 47 | 0.471 |
| Variables | χ2 | B | Univariable Analysis Odds Ratio (95% CI) | p Values | χ2 | B | Multivariable Analysis Odds Ratio (95% CI) | p Values |
|---|---|---|---|---|---|---|---|---|
| 35.45 | ||||||||
| QTe | 0.009 | 0.00 | 1.00 (1.00–1.00) | 0.924 | −0.003 | 1.00 (0.98–1.01) | 0.429 | |
| QTeSD | 2.90 | 0.04 | 1.04 (0.99–1.09) | 0.096 | 0.057 | 1.06 (0.98–1.14) | 0.142 | |
| QTp | 2.82 | −0.01 | 1.00 (0.99–1.00) | 0.058 | −0.005 | 1.00 (0.99–1.00) | 0.206 | |
| QTpSD | 0.36 | 0.02 | 1.02 (0.95–1.11) | 0.542 | −0.086 | 0.92 (0.81–1.05) | 0.198 | |
| Te | 19.49 | 0.03 | 1.03 (1.01–1.04) | <0.001 | 0.032 | 1.03 (1.01–1.05) | 0.001 | |
| TeSD | 7.92 | 0.07 | 1.07 (1.01–1.14) | 0.027 | 0.047 | 1.05 (0.98–1.12) | 0.141 |
| Variables | χ2 | B | Univariable Analysis Odds Ratio (95% CI) | p Values | χ2 | B | Multivariable Analysis Odds Ratio (95% CI) | p Values |
|---|---|---|---|---|---|---|---|---|
| 32.58 | ||||||||
| QTe | 0.54 | 0.00 | 1.00 (1.00–1.01) | 0.454 | −0.01 | 1.00 (0.99–1.01) | 0.453 | |
| QTeSD | 1.97 | 0.05 | 1.05 (0.98–1.13) | 0.145 | 0.06 | 1.07 (0.94–1.21) | 0.317 | |
| QTp | 2.10 | −0.00 | 1.00 (0.99–1.00) | 0.153 | −0.01 | 1.00 (0.99–1.00) | 0.187 | |
| QTpSD | 0.67 | 0.04 | 1.04 (0.95–1.14) | 0.401 | −0.09 | 0.92 (0.77–1.09) | 0.317 | |
| Te | 20.83 | 0.03 | 1.03 (1.02–1.05) | <0.001 | 0.036 | 1.04 (1.02–1.06) | 0.001 | |
| TeSD | 8.77 | 0.08 | 1.08 (1.01–1.16) | 0.024 | 0.052 | 1.05 (1.98–1.03) | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Moscucci, F.; Mezzadri, M.; Caltabiano, C.; Cisaria, G.; Vizza, G.; De Santis, V.; Giuffrè, M.; Stefano, S.; Scinicariello, C.; et al. Artificial Intelligence Applied to Electrical and Non-Invasive Hemodynamic Markers in Elderly Decompensated Chronic Heart Failure Patients. Biomedicines 2024, 12, 716. https://doi.org/10.3390/biomedicines12040716
Piccirillo G, Moscucci F, Mezzadri M, Caltabiano C, Cisaria G, Vizza G, De Santis V, Giuffrè M, Stefano S, Scinicariello C, et al. Artificial Intelligence Applied to Electrical and Non-Invasive Hemodynamic Markers in Elderly Decompensated Chronic Heart Failure Patients. Biomedicines. 2024; 12(4):716. https://doi.org/10.3390/biomedicines12040716
Chicago/Turabian StylePiccirillo, Gianfranco, Federica Moscucci, Martina Mezzadri, Cristina Caltabiano, Giovanni Cisaria, Guendalina Vizza, Valerio De Santis, Marco Giuffrè, Sara Stefano, Claudia Scinicariello, and et al. 2024. "Artificial Intelligence Applied to Electrical and Non-Invasive Hemodynamic Markers in Elderly Decompensated Chronic Heart Failure Patients" Biomedicines 12, no. 4: 716. https://doi.org/10.3390/biomedicines12040716
APA StylePiccirillo, G., Moscucci, F., Mezzadri, M., Caltabiano, C., Cisaria, G., Vizza, G., De Santis, V., Giuffrè, M., Stefano, S., Scinicariello, C., Carnovale, M., Corrao, A., Lospinuso, I., Sciomer, S., & Rossi, P. (2024). Artificial Intelligence Applied to Electrical and Non-Invasive Hemodynamic Markers in Elderly Decompensated Chronic Heart Failure Patients. Biomedicines, 12(4), 716. https://doi.org/10.3390/biomedicines12040716







