Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Formulations
2.2. Cell Culture
2.3. Effect of Ursolic Acid and Formulation on Cytokine Release of HaCaT upon Stimulation with M5 Cytokine Mix
2.4. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
2.5. Proliferation of HaCaT Cell Line upon Stimulation with M5 Cytokin Mix in Presence of Ursolic Acid Formulations
2.6. ELISA
2.7. Human Neutrophil Isolation
2.8. Stimulation of Human Neutrophils with Ursolic Acid and M5
2.9. Annexin V and Propidium Iodide Staining
2.10. Reactive Oxygen Species Production
2.11. Statistical Analysis
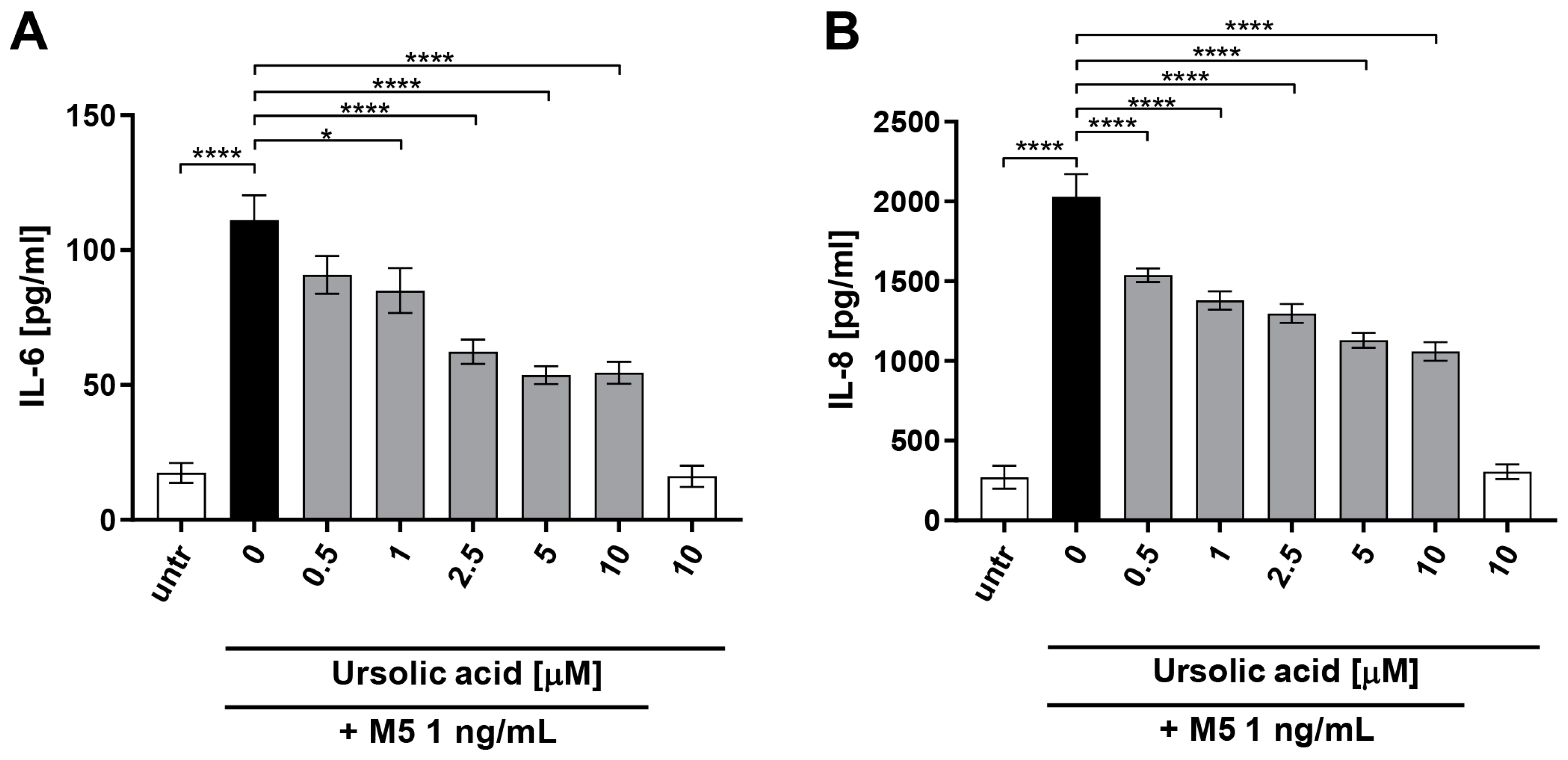
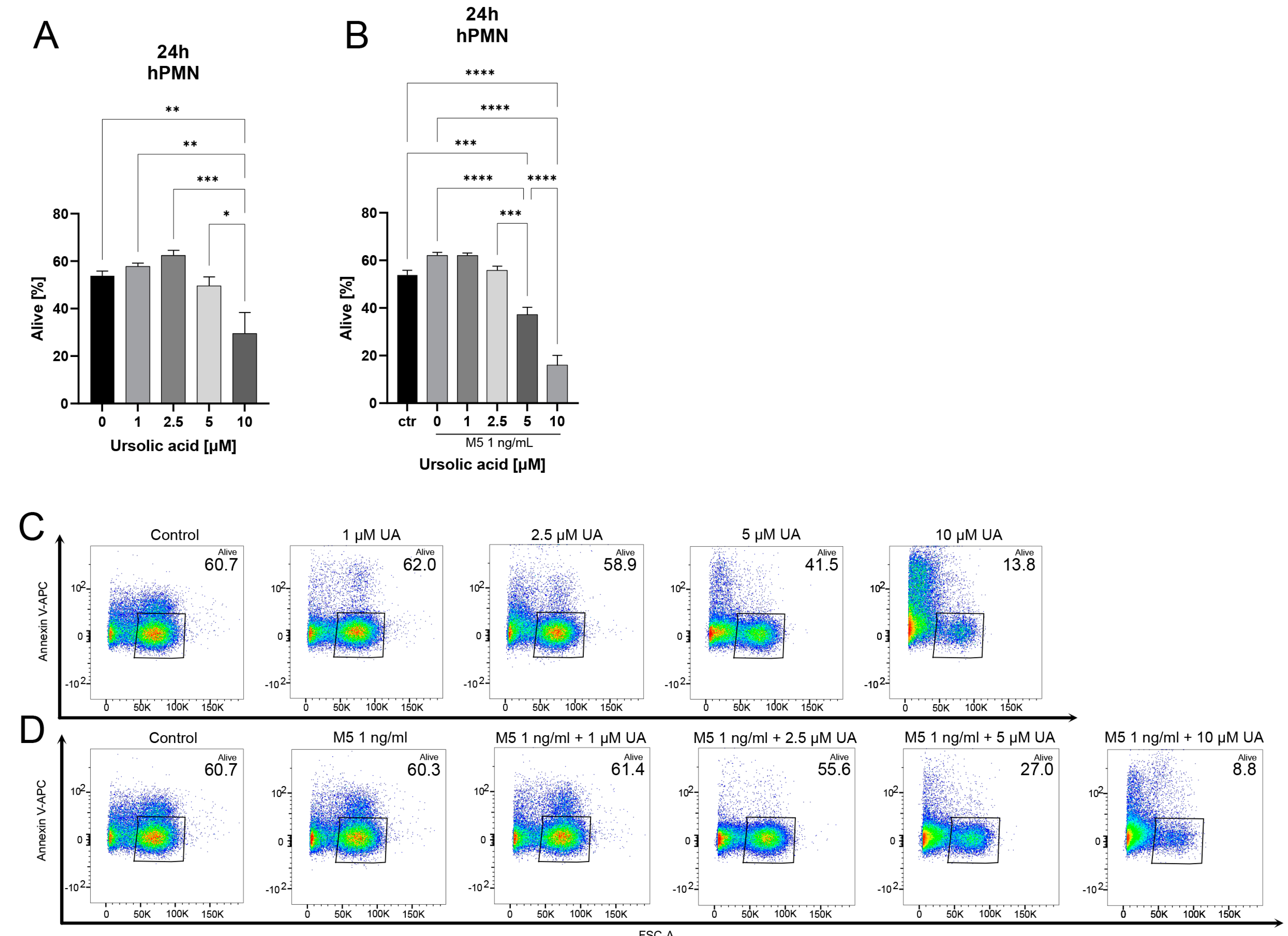
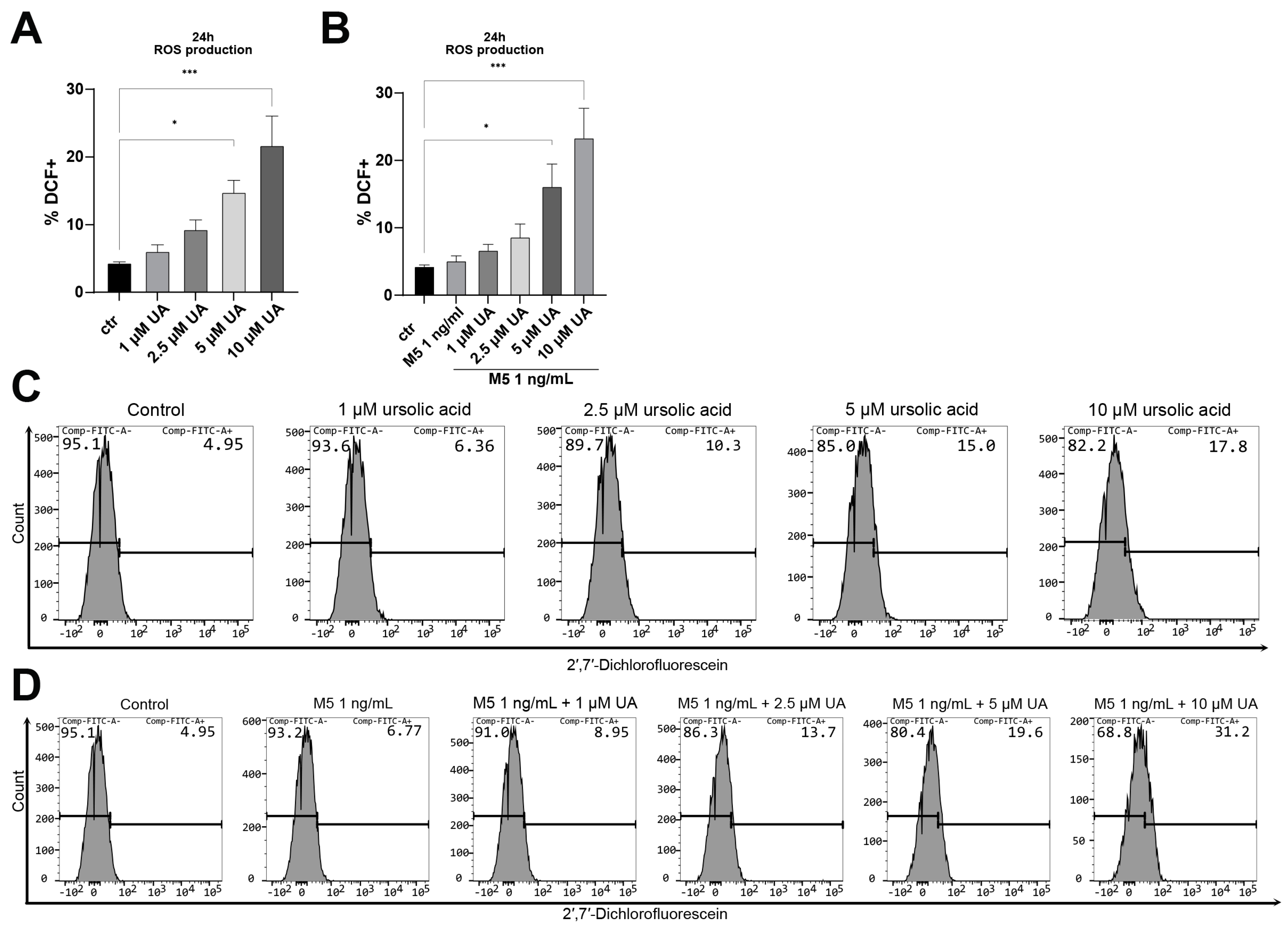
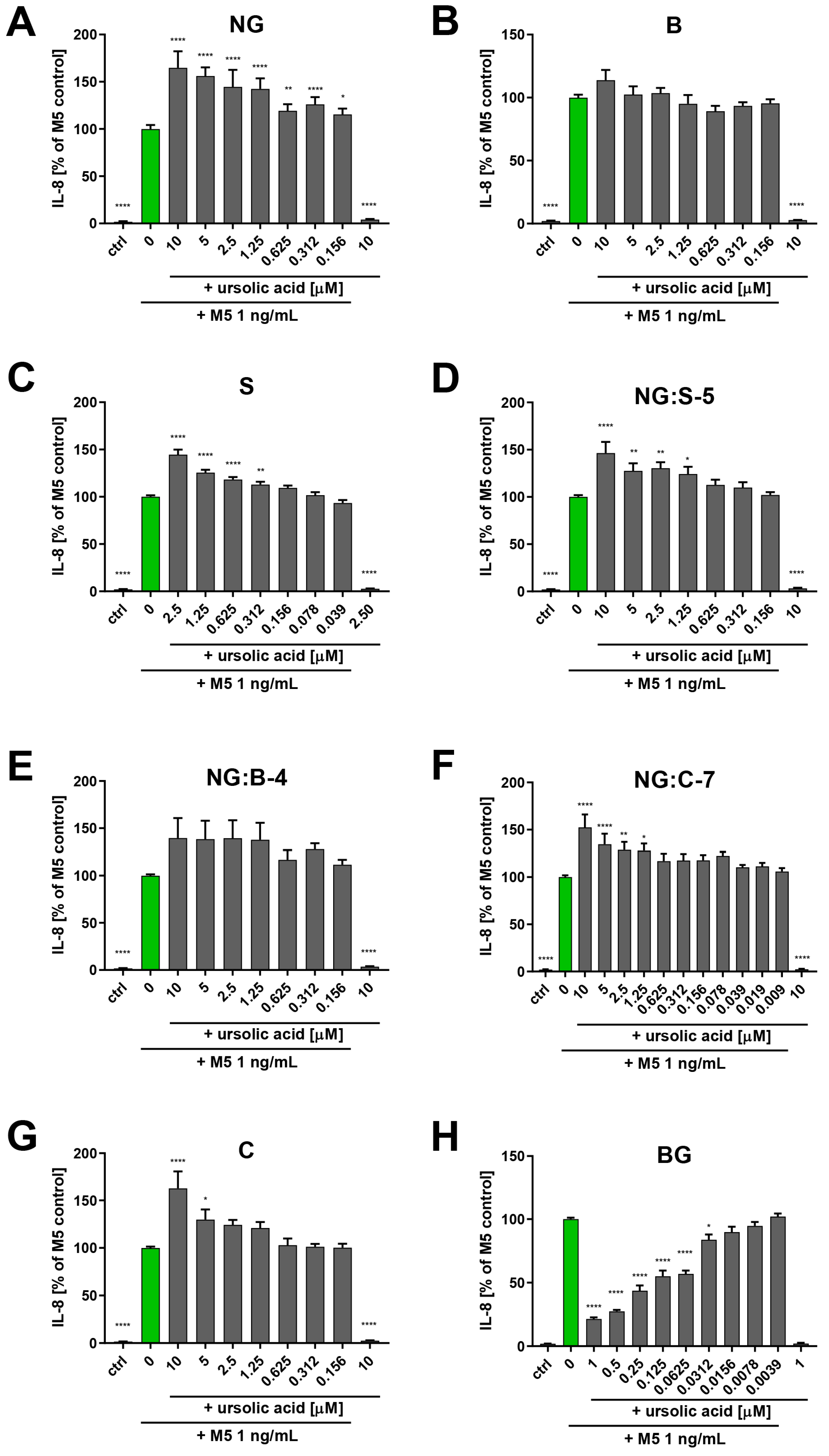
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B | balm |
| C | cream |
| DCF | 2′,7′-dichlorofluorescein diacetate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| h | hour |
| ME | macroemulsion |
| NE | nano-emulsion |
| NG | nano-emulgel |
| O | oleogel |
| PBS | phosphate-buffered saline |
| pDC | plasmacytoid dendritic cells |
| PMN | polymorphonuclear neutrophils |
| ROS | reactive oxygen species |
| S | serum |
| UA | ursolic acid |
References
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.P. Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders. Front. Immunol. 2019, 10, 1764. [Google Scholar] [CrossRef]
- Zheng, H.; Gu, L.; Wang, Z.; Zhou, H.; Zhang, C.; Teng, X.; Hu, Z.; Wei, X.; Liu, X.; Zeng, F.; et al. Establishing Transcription Profile of Psoriasiform Cutaneous In Vitro using HaCaT Cells Stimulated with Combination of Cytokines. J. Vis. Exp. 2021, 169, e61537. [Google Scholar] [CrossRef]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.F.; Guillet, G.; Huguier, V.; Lecron, J.C.; et al. Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1alpha, TNFalpha and oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef]
- Gilliet, M.; Conrad, C.; Geiges, M.; Cozzio, A.; Thurlimann, W.; Burg, G.; Nestle, F.O.; Dummer, R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 2004, 140, 1490–1495. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-alpha/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Chen, C.J.; Shih, Y.L.; Yeh, M.Y.; Liao, N.C.; Chung, H.Y.; Liu, K.L.; Lee, M.H.; Chou, P.Y.; Hou, H.Y.; Chou, J.S.; et al. Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release Through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro. Vivo 2019, 33, 383–391. [Google Scholar] [CrossRef]
- Wang, S.; Chang, X.; Zhang, J.; Li, J.; Wang, N.; Yang, B.; Pan, B.; Zheng, Y.; Wang, X.; Ou, H.; et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Front. Oncol. 2021, 11, 745584. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 16. [Google Scholar] [CrossRef]
- Dimitris, D.; Ekaterina-Michaela, T.; Christina, K.; Ioannis, S.; Ioanna, S.K.; Aggeliki, L.; Sophia, H.; Michael, R.; Helen, S. Melissa officinalis ssp. altissima extracts: A therapeutic approach targeting psoriasis in mice. J. Ethnopharmacol. 2020, 246, 112208. [Google Scholar] [CrossRef] [PubMed]
- NA, J.C.F.; Pirson, L.; Edelberg, H.; L, M.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef]
- Geerlofs, L.; He, Z.; Xiao, S.; Xiao, Z.C. Repeated dose (90 days) oral toxicity study of ursolic acid in Han-Wistar rats. Toxicol. Rep. 2020, 7, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Araujo, G.A.; Ferreira, P.S.; Victorelli, F.D.; Pironi, A.M.; Araujo, V.H.S.; Carvalho, S.G.; Chorilli, M. Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid. Pharmaceutics 2023, 15, 366. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pioro, A.; Lason, E.; Sliwa, K.; Malinowska, M.A.; Sikora, E.; Kantyka, T.; Bielecka, E.; Maksylewicz, A.; Klimaszewska, E.; et al. Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment. Pharmaceutics 2023, 15, 2559. [Google Scholar] [CrossRef]
- Bryzek, D.; Ciaston, I.; Dobosz, E.; Gasiorek, A.; Makarska, A.; Sarna, M.; Eick, S.; Puklo, M.; Lech, M.; Potempa, B.; et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019, 15, e1007773. [Google Scholar] [CrossRef]
- Sochalska, M.; Stanczyk, M.B.; Uzarowska, M.; Zubrzycka, N.; Kirschnek, S.; Grabiec, A.M.; Kantyka, T.; Potempa, J. Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil-LPS Interaction to Periodontal Disease. Pathogens 2020, 9, 530. [Google Scholar] [CrossRef]
- Bochenska, K.; Moskot, M.; Gabig-Ciminska, M. Use of Cytokine Mix-, Imiquimod-, and Serum-Induced Monoculture and Lipopolysaccharide- and Interferon Gamma-Treated Co-Culture to Establish In Vitro Psoriasis-like Inflammation Models. Cells 2021, 10, 2985. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert. Opin. Ther. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, W. Ursolic acid: Pharmacokinetics process in vitro and in vivo, a mini review. Arch. Pharm. 2019, 352, e1800222. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Zhong, B.; Nurieva, R.I.; Ding, S.; Dong, C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J. Biol. Chem. 2011, 286, 22707–22710. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, A.; Minutilli, E.; Mussi, A.; Bordignon, V.; Trento, E.; D’Agosto, G.; Cordiali-Fei, P.; Berardesca, E. Cytokine profiles during infliximab monotherapy in psoriatic arthritis. Br. J. Dermatol. 2005, 153, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.M.; Krueger, J.; Yourish, D.; Granelli-Piperno, A.; Murphy, D.P.; May, L.T.; Kupper, T.S.; Sehgal, P.B.; Gottlieb, A.B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 6367–6371. [Google Scholar] [CrossRef] [PubMed]
- Neuner, P.; Urbanski, A.; Trautinger, F.; Moller, A.; Kirnbauer, R.; Kapp, A.; Schopf, E.; Schwarz, T.; Luger, T.A. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J. Investig. Dermatol. 1991, 97, 27–33. [Google Scholar] [CrossRef]
- Ravipati, A.; Nolan, S.; Alphonse, M.; Dikeman, D.; Youn, C.; Wang, Y.; Orlando, N.; Patrick, G.; Lee, S.; Ortines, R.V.; et al. IL-6R/Signal Transducer and Activator of Transcription 3 Signaling in Keratinocytes rather than in T Cells Induces Psoriasis-Like Dermatitis in Mice. J. Investig. Dermatol. 2022, 142, 1126–1135.e4. [Google Scholar] [CrossRef]
- Goodman, W.A.; Levine, A.D.; Massari, J.V.; Sugiyama, H.; McCormick, T.S.; Cooper, K.D. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009, 183, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Anttila, H.S.; Reitamo, S.; Erkko, P.; Ceska, M.; Moser, B.; Baggiolini, M. Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J. Investig. Dermatol. 1992, 98, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.M.; Gregory, H.; Young, J.; Christophers, E. Neutrophil-activating proteins in psoriasis. J. Investig. Dermatol. 1992, 98, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Arenberger, P.; Kemeny, L.; Suss, R.; Michel, G.; Peter, R.U.; Ruzicka, T. Interleukin-8 receptors in normal and psoriatic polymorphonuclear leukocytes. Acta Derm. Venereol. 1992, 72, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, S.; Jiang, W.; Tang, S.; Shi, Y. Discovering Novel Biomarkers Associated with the Pathogenesis of Psoriasis: Evidence from Bioinformatic Analysis. Int. J. Gen. Med. 2022, 15, 2817–2833. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tsai, T.F. Anti-interleukin and interleukin therapies for psoriasis: Current evidence and clinical usefulness. Ther. Adv. Musculoskelet. Dis. 2017, 9, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, E.; Pilmane, M.; Kisis, J. Local antimicrobial, protease and cytokine defense systems in psoriatic skin. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 284–291. [Google Scholar] [CrossRef]
- Li, T.; Qi, Z.; Kong, F.; Li, Y.; Wang, R.; Zhang, W.; Shang, Y.; Huang, L.; He, D.; Xiao, X. S100A7 acts as a dual regulator in promoting proliferation and suppressing squamous differentiation through GATA-3/caspase-14 pathway in A431 cells. Exp. Dermatol. 2015, 24, 342–348. [Google Scholar] [CrossRef]
- Son, E.D.; Kim, H.J.; Kim, K.H.; Bin, B.H.; Bae, I.H.; Lim, K.M.; Yu, S.J.; Cho, E.G.; Lee, T.R. S100A7 (psoriasin) inhibits human epidermal differentiation by enhanced IL-6 secretion through IkappaB/NF-kappaB signalling. Exp. Dermatol. 2016, 25, 636–641. [Google Scholar] [CrossRef]
- Mellor, L.F.; Gago-Lopez, N.; Bakiri, L.; Schmidt, F.N.; Busse, B.; Rauber, S.; Jimenez, M.; Megias, D.; Oterino-Soto, S.; Sanchez-Prieto, R.; et al. Keratinocyte-derived S100A9 modulates neutrophil infiltration and affects psoriasis-like skin and joint disease. Ann. Rheum. Dis. 2022, 81, 1400–1408. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Hashimoto, Y.; Ishiko, A. Stratum corneum levels of calprotectin proteins S100A8/A9 correlate with disease activity in psoriasis patients. J. Dermatol. 2021, 48, 1518–1525. [Google Scholar] [CrossRef]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun. Rev. 2021, 20, 102846. [Google Scholar] [CrossRef]
- Pohin, M.; Guesdon, W.; Mekouo, A.A.; Rabeony, H.; Paris, I.; Atanassov, H.; Favot, L.; McHeik, J.; Bernard, F.X.; Richards, C.D.; et al. Oncostatin M overexpression induces skin inflammation but is not required in the mouse model of imiquimod-induced psoriasis-like inflammation. Eur. J. Immunol. 2016, 46, 1737–1751. [Google Scholar] [CrossRef]
- Soini, Y.; Kamel, D.; Paakko, P.; Lehto, V.P.; Oikarinen, A.; Vahakangas, K.V. Aberrant accumulation of p53 associates with Ki67 and mitotic count in benign skin lesions. Br. J. Dermatol. 1994, 131, 514–520. [Google Scholar] [CrossRef]
- Krueger, J.G.; Wolfe, J.T.; Nabeya, R.T.; Vallat, V.P.; Gilleaudeau, P.; Heftler, N.S.; Austin, L.M.; Gottlieb, A.B. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J. Exp. Med. 1995, 182, 2057–2068. [Google Scholar] [CrossRef]
- Castelijns, F.A.; Gerritsen, M.J.; van Vlijmen-Willems, I.M.; van Erp, P.J.; van de Kerkhof, P.C. Proliferation is the main epidermal target in the treatment of psoriatic plaques with once daily application of tacalcitol ointment. Acta Derm. Venereol. 1999, 79, 111–114. [Google Scholar]
- Laporte, M.; Galand, P.; Fokan, D.; de Graef, C.; Heenen, M. Apoptosis in established and healing psoriasis. Dermatology 2000, 200, 314–316. [Google Scholar] [CrossRef]
- Chen, S.H.; Arany, I.; Apisarnthanarax, N.; Rajaraman, S.; Tyring, S.K.; Horikoshi, T.; Brysk, H.; Brysk, M.M. Response of keratinocytes from normal and psoriatic epidermis to interferon-gamma differs in the expression of zinc-alpha(2)-glycoprotein and cathepsin D. FASEB J. 2000, 14, 565–571. [Google Scholar] [CrossRef]
- Oztas, P.; Lortlar, N.; Oztas, M.O.; Omeroglu, S.; Gurer, M.A.; Alli, N. Caspase 9 is decreased in psoriatic epidermis. Acta Histochem. 2006, 108, 497–499. [Google Scholar] [CrossRef]
- Takahashi, H.; Manabe, A.; Ishida-Yamamoto, A.; Hashimoto, Y.; Iizuka, H. Aberrant expression of apoptosis-related molecules in psoriatic epidermis. J. Dermatol. Sci. 2002, 28, 187–197. [Google Scholar] [CrossRef]
- McGill, A.; Frank, A.; Emmett, N.; Turnbull, D.M.; Birch-Machin, M.A.; Reynolds, N.J. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005, 19, 1012–1014. [Google Scholar] [CrossRef]
- El-Domyati, M.; Barakat, M.; Abdel-Razek, R.; El-Din Anbar, T. Apoptosis, P53 and Bcl-2 expression in response to topical calcipotriol therapy for psoriasis. Int. J. Dermatol. 2007, 46, 468–474. [Google Scholar] [CrossRef]
- Tse, W.P.; Cheng, C.H.; Che, C.T.; Lin, Z.X. Arsenic trioxide, arsenic pentoxide, and arsenic iodide inhibit human keratinocyte proliferation through the induction of apoptosis. J. Pharmacol. Exp. Ther. 2008, 326, 388–394. [Google Scholar] [CrossRef]
- Kruger-Krasagakis, S.; Galanopoulos, V.K.; Giannikaki, L.; Stefanidou, M.; Tosca, A.D. Programmed cell death of keratinocytes in infliximab-treated plaque-type psoriasis. Br. J. Dermatol. 2006, 154, 460–466. [Google Scholar] [CrossRef]
- Markham, T.; Mathews, C.; Rogers, S.; Mullan, R.; Bresnihan, B.; Fitzgerald, O.; Veale, D.J.; Fearon, U. Downregulation of the inhibitor of apoptosis protein survivin in keratinocytes and endothelial cells in psoriasis skin following infliximab therapy. Br. J. Dermatol. 2006, 155, 1191–1196. [Google Scholar] [CrossRef]
- Chowaniec, O.; Jablonska, S.; Beutner, E.H.; Proniewska, M.; Jarzabek-Chorzelska, M.; Rzesa, G. Earliest clinical and histological changes in psoriasis. Dermatologica 1981, 163, 42–51. [Google Scholar] [CrossRef]
- Breathnach, S.M.; Carrington, P.; Black, M.M. Neutrophil leukocyte migration in psoriasis vulgaris. J. Investig. Dermatol. 1981, 76, 271–274. [Google Scholar] [CrossRef]
- Kaneko, F.; Itoh, N.; Yoshida, H.; Suzuki, M.; Ono, I. The cell-components and cytokines in the subcorneal microabscess of psoriasis. Fukushima J. Med. Sci. 1991, 37, 103–112. [Google Scholar]
- Colotta, F.; Re, F.; Polentarutti, N.; Sozzani, S.; Mantovani, A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992, 80, 2012–2020. [Google Scholar] [CrossRef]
- Cao, T.; Yuan, X.; Fang, H.; Chen, J.; Xue, K.; Li, Z.; Dang, E.; Wang, G.; Shao, S. Neutrophil extracellular traps promote keratinocyte inflammation via AIM2 inflammasome and AIM2-XIAP in psoriasis. Exp. Dermatol. 2023, 32, 368–378. [Google Scholar] [CrossRef]
- Hu, S.C.; Yu, H.S.; Yen, F.L.; Lin, C.L.; Chen, G.S.; Lan, C.C. Neutrophil extracellular trap formation is increased in psoriasis and induces human beta-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef]
- Skrzeczynska-Moncznik, J.; Wlodarczyk, A.; Zabieglo, K.; Kapinska-Mrowiecka, M.; Marewicz, E.; Dubin, A.; Potempa, J.; Cichy, J. Secretory leukocyte proteinase inhibitor-competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: Implication for psoriasis. J. Immunol. 2012, 189, 1611–1617. [Google Scholar] [CrossRef]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhofer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Loffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, A.; Choi, E.J.; Hong, M.P.; Kie, J.H.; Lim, W.; Lee, H.K.; Moon, B.I.; Seoh, J.Y. Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLoS ONE 2014, 9, e91146. [Google Scholar] [CrossRef]
- Khmaladze, I.; Kelkka, T.; Guerard, S.; Wing, K.; Pizzolla, A.; Saxena, A.; Lundqvist, K.; Holmdahl, M.; Nandakumar, K.S.; Holmdahl, R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3669–E3678. [Google Scholar] [CrossRef]
- Cross, A.L.; Hawkes, J.; Frankland, H.; Mediana, A.; Wright, H.L.; Goodson, N.J.; Edwards, S.W.; Moots, R.J. Neutrophil function following treatment of psoriatic arthritis patients with secukinumab: Altered cytokine signalling but no impairment of host defence. Rheumatolology 2023, 62, 3025–3034. [Google Scholar] [CrossRef]
- Jankowski, A.; Dyja, R.; Sarecka-Hujar, B. Dermal and transdermal delivery of active substances from semisolid bases. Indian J. Pharm. Sci. 2017, 79, 488–500. [Google Scholar] [CrossRef]
- Sikora, E.; Miastkowska, M.; Lasoń, E. Selected Skin Delivery Systems; Cracow University of Techonology: Cracow, Poland, 2020. [Google Scholar]
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of Ursolic Acid: A Modern Natural Anticancer Molecule. Front. Pharmacol. 2021, 12, 706121. [Google Scholar] [CrossRef]
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H.; Ying, G. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2013, 8, 129–136. [Google Scholar]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef]
- Pribowo, A.; Girish, J.; Gustiananda, M.; Nandhira, R.G.; Hartrianti, P. Potential of Tamanu (Calophyllum inophyllum) Oil for Atopic Dermatitis Treatment. Evid. Based Complement. Altern. Med. 2021, 2021, 6332867. [Google Scholar] [CrossRef]
- Raharivelomanana, P.; Ansel, J.-L.; Lupo, E.; Mijouin, L.; Guillot, S.; Butaud, J.-F.; Ho, R.; Lecellier, G.; Pichon, C. Tamanu oil and skin active properties: From traditional to modern cosmetic uses. OCL 2018, 25, D504. [Google Scholar] [CrossRef]
- Franchesca Marie, D.; Ilagan, E.L.L.; Bernardita, O. Policarpio, Efficacy and Safety of Sunflower Oil for Mild to Moderate Plaque-type Psoriasis: A Double-blind, Randomized Controlled Trial. J. Med. Univ. St. Tomas Manila 2021, 2, 755–773. [Google Scholar]
- Rivier, M.; Safonova, I.; Lebrun, P.; Griffiths, C.E.; Ailhaud, G.; Michel, S. Differential expression of peroxisome proliferator-activated receptor subtypes during the differentiation of human keratinocytes. J. Investig. Dermatol. 1998, 111, 1116–1121. [Google Scholar] [CrossRef]
- Sheu, M.Y.; Fowler, A.J.; Kao, J.; Schmuth, M.; Schoonjans, K.; Auwerx, J.; Fluhr, J.W.; Man, M.Q.; Elias, P.M.; Feingold, K.R. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J. Investig. Dermatol. 2002, 118, 94–101. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, L.; Liu, S.; Dai, C.; Cui, T.; Lei, Y.; You, X.; Wang, X.; Wu, J.; Zheng, Z. Natural ursolic acid based self-therapeutic polymer as nanocarrier to deliver natural resveratrol for natural therapy of acute kidney injury. J. Nanobiotechnology 2023, 21, 484. [Google Scholar] [CrossRef]







| Nanoemulgel (NG) | Formulation (F) | |||
|---|---|---|---|---|
| Serum (S) | Cream (C) | Balm (B) | Oleogel (O) | |
| Ratios of NG:F (w/w) | 50:50 | 70:30 | 40:60 | 5:95 |
| Hybrid systems | NG:S-5 | NG:C-7 | NG:B-4 | BG-1 |
| GENE | FORWARD | REVERSE |
|---|---|---|
| S100A9 | CGGCTTTGACAGAGTGCAAG | CAGGTTAGCCTCGCCATCAG |
| S100A7 | ACACCAGACGTGATGACAAGA | AAGACATCGGCGAGGTAATTTGT |
| S100A8 | ATGTTGACCGAGCTGGAGAAAG | TTCAGGTCATCCCTGTAGACGG |
| HCCL20 | TGCTGTACCAAGAGTTTGCTC | CGCACACAGACAACTTTTTCTTT |
| CXCL1 | AACCGAAGTCATAGCCACAC | GTTGGATTTGTCACTGTTCAGC |
| EEF2 | GACATCACCAAGGGTGTGCAG | TTCAGCACACTGGCATAGAGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecka, E.; Zubrzycka, N.; Marzec, K.; Maksylewicz, A.; Sochalska, M.; Kulawik-Pióro, A.; Lasoń, E.; Śliwa, K.; Malinowska, M.; Sikora, E.; et al. Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro. Biomedicines 2024, 12, 732. https://doi.org/10.3390/biomedicines12040732
Bielecka E, Zubrzycka N, Marzec K, Maksylewicz A, Sochalska M, Kulawik-Pióro A, Lasoń E, Śliwa K, Malinowska M, Sikora E, et al. Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro. Biomedicines. 2024; 12(4):732. https://doi.org/10.3390/biomedicines12040732
Chicago/Turabian StyleBielecka, Ewa, Natalia Zubrzycka, Karolina Marzec, Anna Maksylewicz, Maja Sochalska, Agnieszka Kulawik-Pióro, Elwira Lasoń, Karolina Śliwa, Magdalena Malinowska, Elżbieta Sikora, and et al. 2024. "Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro" Biomedicines 12, no. 4: 732. https://doi.org/10.3390/biomedicines12040732
APA StyleBielecka, E., Zubrzycka, N., Marzec, K., Maksylewicz, A., Sochalska, M., Kulawik-Pióro, A., Lasoń, E., Śliwa, K., Malinowska, M., Sikora, E., Nowak, K., Miastkowska, M., & Kantyka, T. (2024). Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro. Biomedicines, 12(4), 732. https://doi.org/10.3390/biomedicines12040732










