Mixture of Doxycycline, ML-7 and L-NAME Restores the Pro- and Antioxidant Balance during Myocardial Infarction—In Vivo Pig Model Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Experimental Treatment Protocol and Sample Size
- (1)
- The myocardial infarction group with an intracoronary infusion of DOXY (1.0 µM) + ML-7 (0.5 µM) + L-NAME (2 µM) diluted in saline by an infusion catheter distal to the angioplasty site constituted the MI-MIX (n = 6).
- (2)
- The myocardial infarction group with an intracoronary infusion of the same volume of 0.9% NaCl constituted the sham-MI control group (n = 6).
2.3. Premedication and Monitoring
2.4. Angiography, STEMI Induction, and Reperfusion
2.5. Postoperative Treatment and Maintaining
2.6. ECG
2.7. Collection and Analysis of Blood Samples
2.8. Heart Tissue Homogenization
2.9. ELISA Tests
2.10. Total Antioxidant Capacity
2.11. Total Oxidative Status
2.12. Oxidative Stress Index
2.13. Malondialdehyde Concentration
2.14. Glutathione Peroxidase Activity
2.15. Glutathione Reductase Activity
2.16. Glutathione S-Transferase Activity
2.17. Lipofuscin Concentration
2.18. Statistical Analysis
3. Results
3.1. Total Oxidative Status in Heart Tissue
3.2. Total Antioxidative Status in Heart Tissue
3.3. An Influence of MIX Treatment on Oxidative and Antioxidative Status in Heart Tissue
3.4. Oxidative and Antioxidative Parameters in Serum
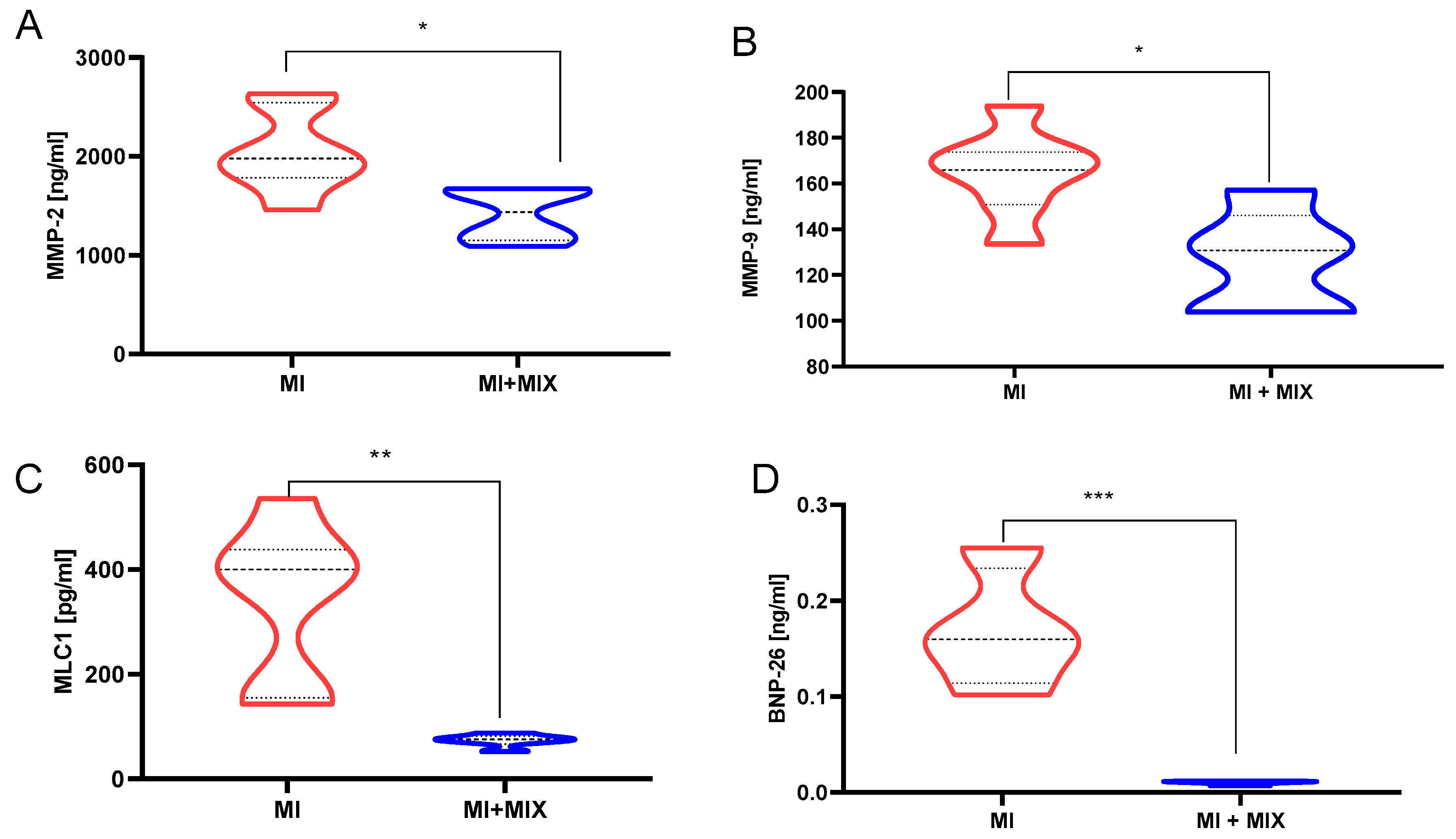
3.5. An Influence of Doxy, ML-7, and L-NAME Mixture on Proteins Release into Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamm, C.W.; Bassand, J.-P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevatio. Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef] [PubMed]
- Lønborg, J.; Vejlstrup, N.; Kelbæk, H.; Bøtker, H.E.; Kim, W.Y.; Mathiasen, A.B.; Jørgensen, E.; Helqvist, S.; Saunamäki, K.; Clemmensen, P.; et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 2012, 33, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Klotho protein contributes to cardioprotection during ischaemia/reperfusion injury. J. Cell. Mol. Med. 2020, 24, 6448–6458. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Gersh, B.J.; Stone, G.W. Pharmacological Facilitation of Coronary Intervention in ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2010, 3, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, L.; Liu, Y. Short-term Effect of Verapamil on Coronary No-Reflow Associated with Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Cardiol. 2013, 36, E11–E16. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S.; Deisher, A.J.; Suzuki, A.; Konishi, H.; Rettmann, M.E.; Merrell, K.W.; Kruse, J.J.; Newman, L.K.; Parker, K.D.; Monahan, K.H.; et al. Left ventricular function after noninvasive cardiac ablation using proton beam therapy in a porcine model. Heart Rhythm. 2019, 16, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Jiao, X.; Gao, E.; Koch, W.J.; Sharifi-Azad, S.; Grunwald, Z.; Ma, X.L.; Sun, J.-Z. Chronic β-Adrenergic Receptor Stimulation Induces Cardiac Apoptosis and Aggravates Myocardial Ischemia/Reperfusion Injury by Provoking Inducible Nitric-Oxide Synthase-Mediated Nitrative Stress. J. Pharmacol. Exp. Ther. 2006, 318, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Nitric Oxide: From Good to Bad. Ann. Vasc. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef]
- Yasmin, W.; Strynadka, K.D.; Schulz, R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc. Res. 1997, 33, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Polewicz, D.; Sawicka, J.; Richardson, J.S.; Cheung, P.-Y.; Sawicki, G. Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res. Cardiol. 2009, 104, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc. Res. 2002, 53, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, G.; Leon, H.; Sawicka, J.; Sariahmetoglu, M.; Schulze, C.J.; Scott, P.G.; Szczesna-Cordary, D.; Schulz, R. Degradation of Myosin Light Chain in Isolated Rat Hearts Subjected to Ischemia-Reperfusion Injury. Circulation 2005, 112, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Cadete, V.J.J.; Arcand, S.A.; Lin, H.-B.; Sawicki, G. Synergistic protection of MLC 1 against cardiac ischemia/reperfusion-induced degradation: A novel therapeutic concept for the future. Future Med. Chem. 2013, 5, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Cadete, V.J.J.; Sawicka, J.; Jaswal, J.S.; Lopaschuk, G.D.; Schulz, R.; Szczesna-Cordary, D.; Sawicki, G. Ischemia/reperfusion-induced myosin light chain 1 phosphorylation increases its degradation by matrix metalloproteinase 2. FEBS J. 2012, 279, 2444–2454. [Google Scholar] [CrossRef]
- Krzywonos-Zawadzka, A.; Franczak, A.; Sawicki, G.; Bil-Lula, I. Mixture of MMP-2, MLC, and NOS Inhibitors Affects NO Metabolism and Protects Heart from Cardiac I/R Injury. Cardiol. Res. Pract. 2020, 2020, 1561478. [Google Scholar] [CrossRef]
- Bil-Lula, I.; Krzywonos-Zawadzka, A.; Sawicka, J.; Bialy, D.; Wawrzynska, M.; Wozniak, M.; Sawicki, G. L-NAME improves doxycycline and ML-7 cardioprotection from oxidative stress. Front. Biosci. Landmark Ed. 2018, 23, 298–309. [Google Scholar] [CrossRef]
- Krzywonos-Zawadzka, A.; Wozniak, M.; Sawicki, G.; Bil-Lula, I. A drug cocktail for protecting against ischemia-reperfusion injury. Front. Biosci.-Landmark 2020, 25, 722–735. [Google Scholar] [CrossRef]
- Bil-Lula, I.; Lin, H.-B.; Biały, D.; Wawrzyńska, M.; Diebel, L.; Sawicka, J.; Woźniak, M.; Sawicki, G. Subthreshold nitric oxide synthase inhibition improves synergistic effects of subthreshold MMP-2/MLCK-mediated cardiomyocyte protection from hypoxic injury. J. Cell. Mol. Med. 2016, 20, 1086–1094. [Google Scholar] [CrossRef]
- Kawicka, M.; Lewicki, M.; Frydrychowski, P.; Michałek, M.; Noszczyk-Nowak, A. Comparative analysis of ECG records depending on body position in domestic swine (Sus scrofa domestica). Porc. Health Manag. 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Cbl Distribution Limited. Electrocardiography of the Dog and Cat: Diagnosis of Arrhythmias, 2nd ed.; Cbl Distribution Limited: Birmingham, UK, 2018. [Google Scholar]
- Pierson, R.N. A major advance toward clinical cardiac xenotransplantation. J. Thorac. Cardiovasc. Surg. 2020, 159, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. Glutathione peroxidase. Methods Enzymol. 1985, 113, 490–495. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Tsuchida, M.; Miura, T.; Mizutani, K.; Aibara, K. Fluorescent substances in mouse and human sera as a parameter of in vivo lipid peroxidation. Biochim. Biophys. Acta 1985, 834, 196–204. [Google Scholar]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. PROTEOMICS–Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef]
- Komaru, T.; Kanatsuka, H.; Shirato, K. Coronary microcirculation. Pharmacol. Ther. 2000, 86, 217–261. [Google Scholar] [CrossRef] [PubMed]
- Papaharalambus, C.A.; Griendling, K.K. Basic Mechanisms of Oxidative Stress and Reactive Oxygen Species in Cardiovascular Injury. Trends Cardiovasc. Med. 2007, 17, 48–54. [Google Scholar] [CrossRef]
- Wildhirt, S. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: Evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc. Res. 1999, 43, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.-Y.; Sawicki, G.; Wozniak, M.; Wang, W.; Radomski, M.W.; Schulz, R. Matrix Metalloproteinase-2 Contributes to Ischemia-Reperfusion Injury in the Heart. Circulation 2000, 101, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Polewicz, D.; Cadete, V.J.J.; Doroszko, A.; Hunter, B.E.; Sawicka, J.; Szczesna-Cordary, D.; Light, P.E.; Sawicki, G. Ischemia induced peroxynitrite dependent modifications of cardiomyocyte MLC1 increases its degradation by MMP-2 leading to contractile dysfunction. J. Cell. Mol. Med. 2011, 15, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C.; Xi, L. eNOS phosphorylation: A pivotal molecular switch in vasodilation and cardioprotection? J. Mol. Cell. Cardiol. 2007, 42, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Di Filippo, C.; Esposito, K.; Nappo, F.; Piegari, E.; Cuzzocrea, S.; Berrino, L.; Rossi, F.; Giugliano, D.; D’Amico, M. Absence of Inducible Nitric Oxide Synthase Reduces Myocardial Damage During Ischemia Reperfusion in Streptozotocin-Induced Hyperglycemic Mice. Diabetes 2004, 53, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.-A.A.; Pershad, S.; Chen, Q.; McGraw, S.; Adams, J.S.; Zambrano, C.; Krass, S.; Emrich, J.; Bell, B.; Iyamu, M.; et al. The effects of modulating eNOS activity and coupling in ischemia/reperfusion (I/R). Naunyn. Schmiedebergs Arch. Pharmacol. 2012, 385, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Xie, L.; Portbury, A.L.; Kumar, S.; Lockyer, P.; Li, X.; Patterson, C. NADPH Oxidase–Generated Reactive Oxygen Species Are Required for Stromal Cell–Derived Factor-1α–Stimulated Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2023–2032. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr. Opin. Pharmacol. 2006, 6, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH Oxidases in Vascular Pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Chavan, V.; Karnik, N.D. Antioxidant status in patients with acute myocardial infarction. Indian J. Clin. Biochem. 2007, 22, 45–51. [Google Scholar] [CrossRef]
- Dwivedi, V.K.; Chandra, M.; Misra, P.C.; Misra, A.; Misra, M.K. Status of some free radical scavenging enzymes in the blood of myocardial infarction patients. J. Enzym. Inhib. Med. Chem. 2006, 21, 43–46. [Google Scholar] [CrossRef]
- Lubrano, V. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F.; Parinandi, N.; Maulik, N. Thioredoxins in cardiovascular disease. Can. J. Physiol. Pharmacol. 2015, 93, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Shioji, K.; Nakamura, H.; Masutani, H.; Yodoi, J. Redox regulation by thioredoxin in cardiovascular diseases. Antioxid. Redox Signal. 2003, 5, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Park, J.-G.; Oh, G.T. Peroxiredoxins as Potential Targets for Cardiovascular Disease. Antioxidants 2021, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yin, G.; Huang, C.; Wang, H.; Gao, J.; Luo, J.; Zhang, Z.; Wang, J.; Hong, J.; Chai, X. Peroxiredoxin-1 ameliorates pressure overload-induced cardiac hypertrophy and fibrosis. Biomed. Pharmacother. 2020, 129, 110357. [Google Scholar] [CrossRef] [PubMed]
- Bernt, E.; Bergmeyer, H.U. Glutathione; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Zuzak, E.; Horecka, A.; Kiełczykowska, M.; Dudek, A.; Musik, I.; Kurzepa, J.; Kurzepa, J. Glutathione level and glutathione reductase activity in serum of coronary heart disease patients. J. Pre-Clin. Clin. Res. 2017, 11, 103–105. [Google Scholar] [CrossRef]
- Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar] [PubMed]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 5028181. [Google Scholar] [CrossRef] [PubMed]
- Kariž, S.; Nikolajević Starčević, J.; Petrovič, D. Association of manganese superoxide dismutase and glutathione S-transferases genotypes with myocardial infarction in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 98, 144–150. [Google Scholar] [CrossRef]
- Conklin, D.J.; Guo, Y.; Jagatheesan, G.; Kilfoil, P.J.; Haberzettl, P.; Hill, B.G.; Baba, S.P.; Guo, L.; Wetzelberger, K.; Obal, D.; et al. Genetic Deficiency of Glutathione S -Transferase P Increases Myocardial Sensitivity to Ischemia–Reperfusion Injury. Circ. Res. 2015, 117, 437–449. [Google Scholar] [CrossRef]
- Okamoto, T.; Akaike, T.; Nagano, T.; Miyajima, S.; Suga, M.; Ando, M.; Ichimori, K.; Maeda, H. Activation of Human Neutrophil Procollagenase by Nitrogen Dioxide and Peroxynitrite: A Novel Mechanism for Procollagenase Activation Involving Nitric Oxide. Arch. Biochem. Biophys. 1997, 342, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van der Vliet, A.; Maeda, H. Activation of Matrix Metalloproteinases by Peroxynitrite-induced Protein S-Glutathiolation via Disulfide S-Oxide Formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-Nitrosylation of Matrix Metalloproteinases: Signaling Pathway to Neuronal Cell Death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; León, H.; van Mulligen, T.; Schulz, R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Schulz, R. Activation of intracellular matrix metalloproteinase-2 by reactive oxygen–nitrogen species: Consequences and therapeutic strategies in the heart. Arch. Biochem. Biophys. 2013, 540, 82–93. [Google Scholar] [CrossRef]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.B.; Sawicki, G.; Schulz, R. Intracellular Action of Matrix Metalloproteinase-2 Accounts for Acute Myocardial Ischemia and Reperfusion Injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.; Cho, W.J.; Hudson, B.; Kassiri, Z.; Granzier, H.; Schulz, R. Titin is a Target of Matrix Metalloproteinase-2. Circulation 2010, 122, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Polewicz, D.; Cadete, V.J.J.; Sawicka, J.; Jones, M.; Szczesna-Cordary, D.; Cheung, P.-Y.; Sawicki, G. Neonatal Asphyxia Induces the Nitration of Cardiac Myosin Light Chain 2 That is Associated with Cardiac Systolic Dysfunction. Shock 2010, 34, 592–600. [Google Scholar] [CrossRef]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin. Ann. N. Y. Acad. Sci. 2007, 1119, 97–111. [Google Scholar] [CrossRef]
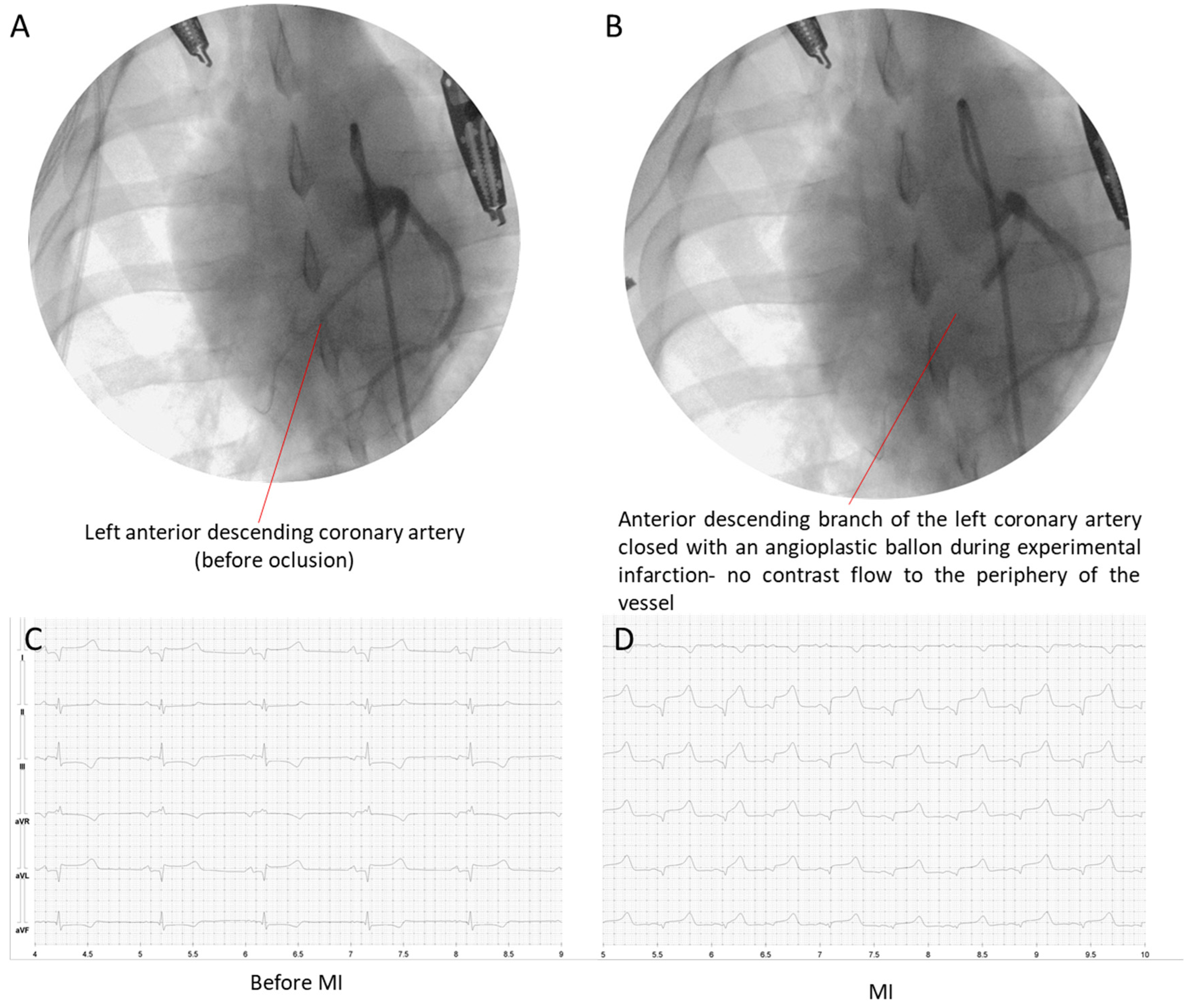
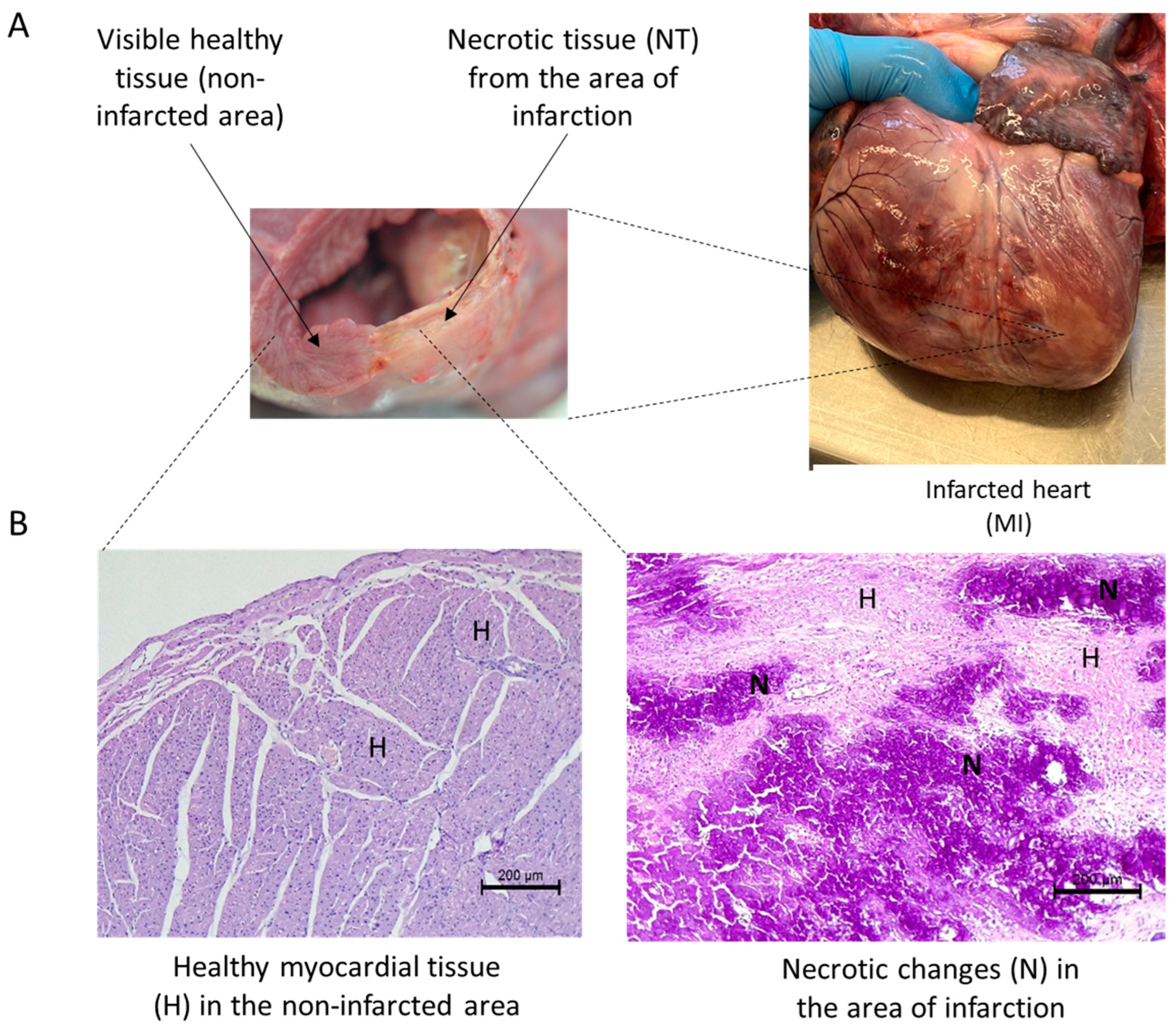


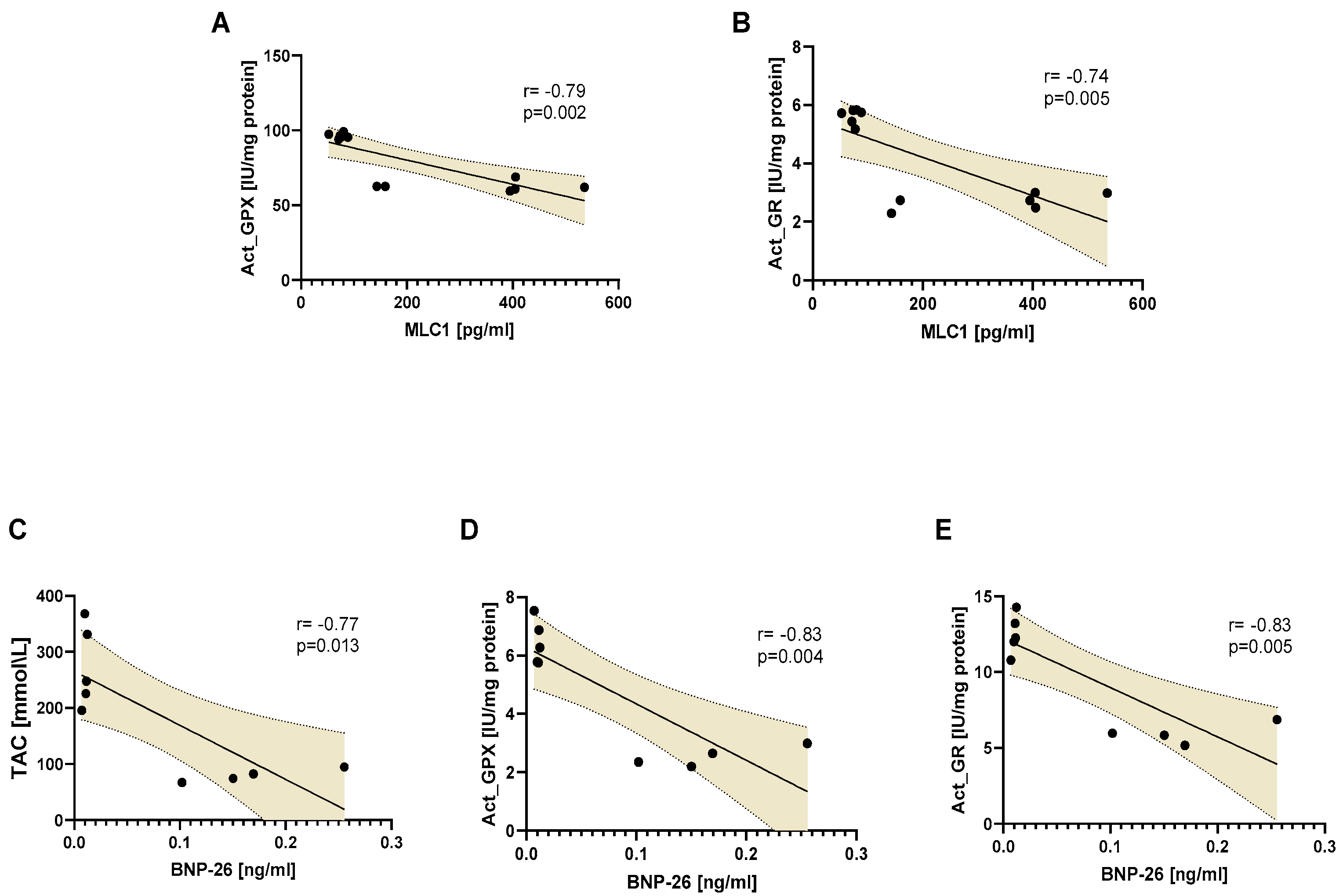
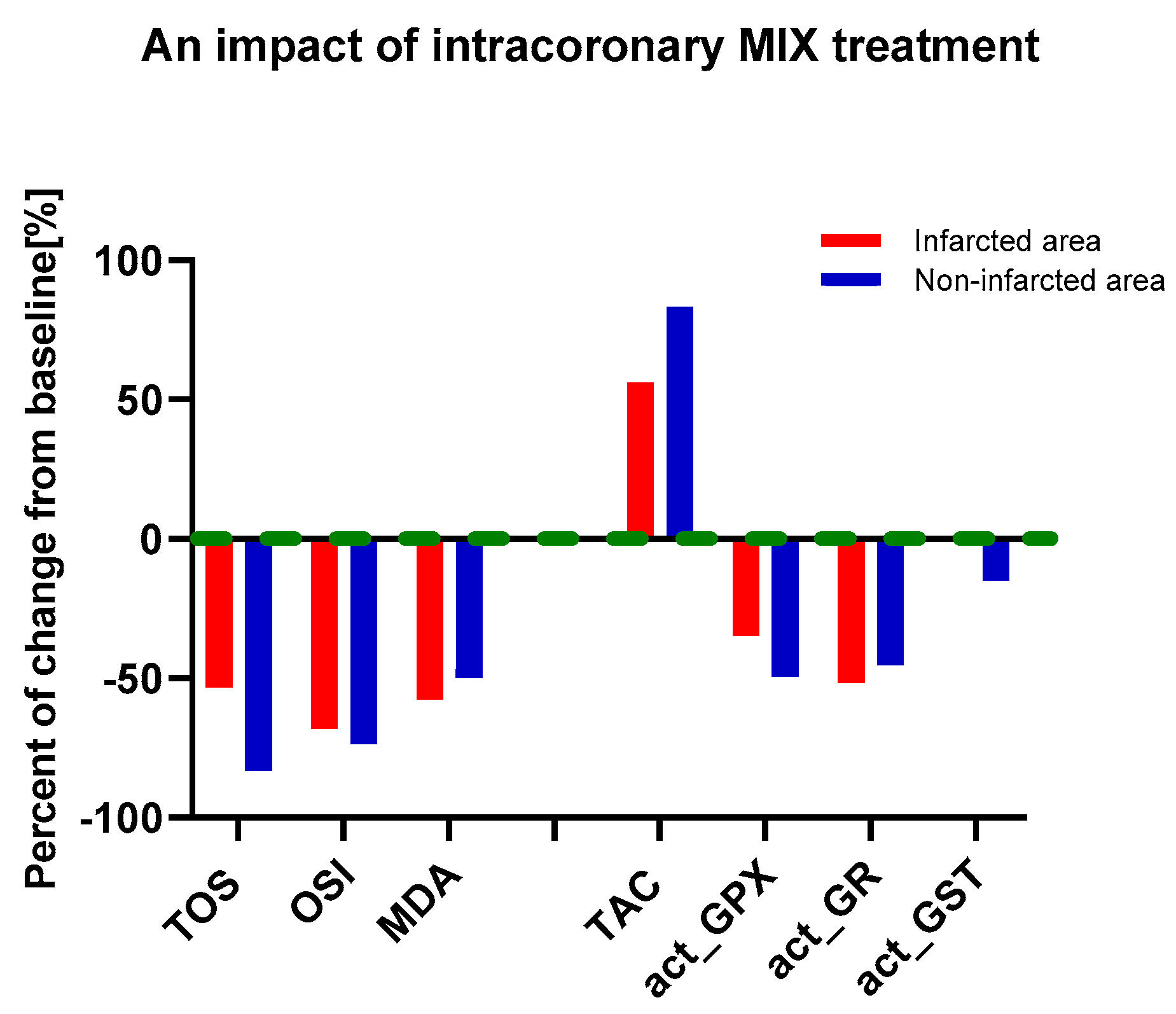

| Oxidative Marker | Heart Tissue Area | MI | p Value a | MI-MIX | p Value a |
|---|---|---|---|---|---|
| TOS [μmol/L] | LVIA | 12.2 ± 1.4 | <0.001 | 5.7 ± 0.8 | <0.001 |
| LVCA | 6.4 ± 0.9 | 2.8 ± 1.0 | |||
| OSI [index] | LVIA | 236.7 (220.7–331.6) | <0.001 | 75.6 (67.1–82.4) | <0.001 |
| LVCA | 21.7 (19.6–24.5) | 5.7 (3.8–6.7) | |||
| MDA [μmol/L] | LVIA | 6.4 ± 0.7 | <0.001 | 2.7 ± 0.3 | <0.01 |
| LVCA | 1.2 ± 0.2 | 0.6 ± 0.1 |
| Antioxidant | Heart Tissue Area | MI | p Value a | MI-MIX | p Value a |
|---|---|---|---|---|---|
| TAC [mmol/L] | LVIA | 4.8 ± 0.9 | <0.001 | 7.5 ± 0.9 | <0.001 |
| LVCA | 29.4 ± 2.2 | 53.9 ± 2.0 | |||
| act_GPX [IU/mg protein] | LVIA | 96.5 ± 1.7 | <0.001 | 62.7 ± 3.2 | <0.001 |
| LVCA | 40.1 ± 3.0 | 20.3 ± 2.7 | |||
| act_GR [IU/mg protein] | LVIA | 5.6 ± 0.3 | <0.001 | 2.7 ± 0.3 | <0.001 |
| LVCA | 3.3 ± 0.7 | 1.8 ± 0.1 | |||
| act_GST [IU/mg protein] | LVIA | 18.9 ± 1.0 | <0.001 | 18.7 ± 0.4 | <0.001 |
| LVCA | 14.7 ± 0.4 | 12.5 ± 0.3 |
| Parameter | MI | MI-MIX | p Value | MI | MI-MIX | p Value |
|---|---|---|---|---|---|---|
| Infarcted Area | Non-Infarcted Area | |||||
| TAC [mmol/L] | 4.8 ± 0.9 | 7.5 ± 0.9 | <0.001 | 29.4 ± 2.2 | 53.9 ± 2.0 | <0.01 |
| TOS [μmol/L] | 12.2 ± 1.4 | 5.7 ± 0.8 | <0.001 | 6.4 ± 0.9 | 2.8 ± 1.0 | <0.001 |
| OSI [index] | 236.7 | 75.6 | <0.001 | 21.7 | 5.7 | <0.001 |
| (220.7–331.6) | (67.1–82.4) | (19.6–24.5) | (3.8–6.7) | |||
| MDA [μmol/L] | 6.4 ± 0.7 | 2.7 ± 0.3 | <0.001 | 1.2 ± 0.2 | 0.6 ± 0.1 | <0.001 |
| act_GPX [IU/mg protein] | 96.5 ± 1.7 | 62.7 ± 3.2 | <0.001 | 40.1 ± 3.0 | 20.3 ± 2.7 | <0.001 |
| act_GR [IU/mg protein] | 5.6 ± 0.3 | 2.7 ± 0.3 | <0.001 | 3.3 ± 0.7 | 1.8 ± 0.1 | <0.001 |
| act_GST [IU/mg protein] | 18.9 ± 1.0 | 18.7 ± 0.4 | >0.05 | 14.7 ± 0.4 | 12.5 ± 0.3 | <0.01 |
| Oxidative/Antioxidative Marker | Heart Tissue Area | p Value | ||
|---|---|---|---|---|
| MI vs. MI-MIX | MI | MI-MIX | ||
| TAC [mmol/L] | LVIA | <0.01 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
| TOS [μmol/L] | LVIA | <0.001 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
| OSI index | LVIA | <0.001 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
| MDA [μmol/L] | LVIA | <0.001 | <0.001 | <0.001 |
| LVCA | <0.01 | |||
| act_GPX [IU/mg protein] | LVIA | <0.001 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
| act_GR [IU/mg protein] | LVIA | <0.001 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
| act_GST [IU/mg protein] | LVIA | 0.722 | <0.001 | <0.001 |
| LVCA | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bil-Lula, I.; Kuliczkowski, W.; Krzywonos-Zawadzka, A.; Frydrychowski, P.; Stygar, D.; Hałucha, K.; Noszczyk-Nowak, A. Mixture of Doxycycline, ML-7 and L-NAME Restores the Pro- and Antioxidant Balance during Myocardial Infarction—In Vivo Pig Model Study. Biomedicines 2024, 12, 784. https://doi.org/10.3390/biomedicines12040784
Bil-Lula I, Kuliczkowski W, Krzywonos-Zawadzka A, Frydrychowski P, Stygar D, Hałucha K, Noszczyk-Nowak A. Mixture of Doxycycline, ML-7 and L-NAME Restores the Pro- and Antioxidant Balance during Myocardial Infarction—In Vivo Pig Model Study. Biomedicines. 2024; 12(4):784. https://doi.org/10.3390/biomedicines12040784
Chicago/Turabian StyleBil-Lula, Iwona, Wiktor Kuliczkowski, Anna Krzywonos-Zawadzka, Piotr Frydrychowski, Dominika Stygar, Kornela Hałucha, and Agnieszka Noszczyk-Nowak. 2024. "Mixture of Doxycycline, ML-7 and L-NAME Restores the Pro- and Antioxidant Balance during Myocardial Infarction—In Vivo Pig Model Study" Biomedicines 12, no. 4: 784. https://doi.org/10.3390/biomedicines12040784
APA StyleBil-Lula, I., Kuliczkowski, W., Krzywonos-Zawadzka, A., Frydrychowski, P., Stygar, D., Hałucha, K., & Noszczyk-Nowak, A. (2024). Mixture of Doxycycline, ML-7 and L-NAME Restores the Pro- and Antioxidant Balance during Myocardial Infarction—In Vivo Pig Model Study. Biomedicines, 12(4), 784. https://doi.org/10.3390/biomedicines12040784





