Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
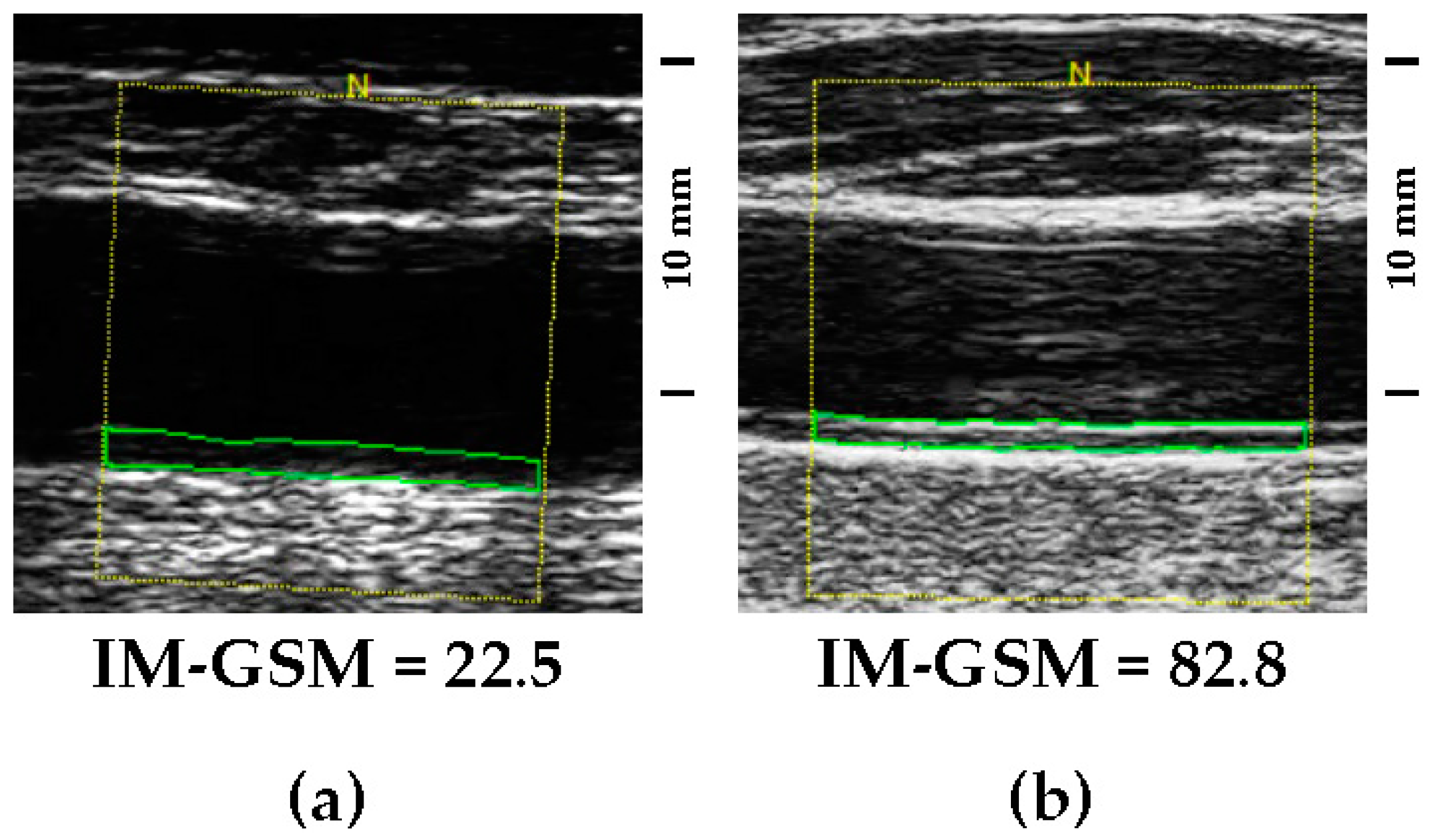
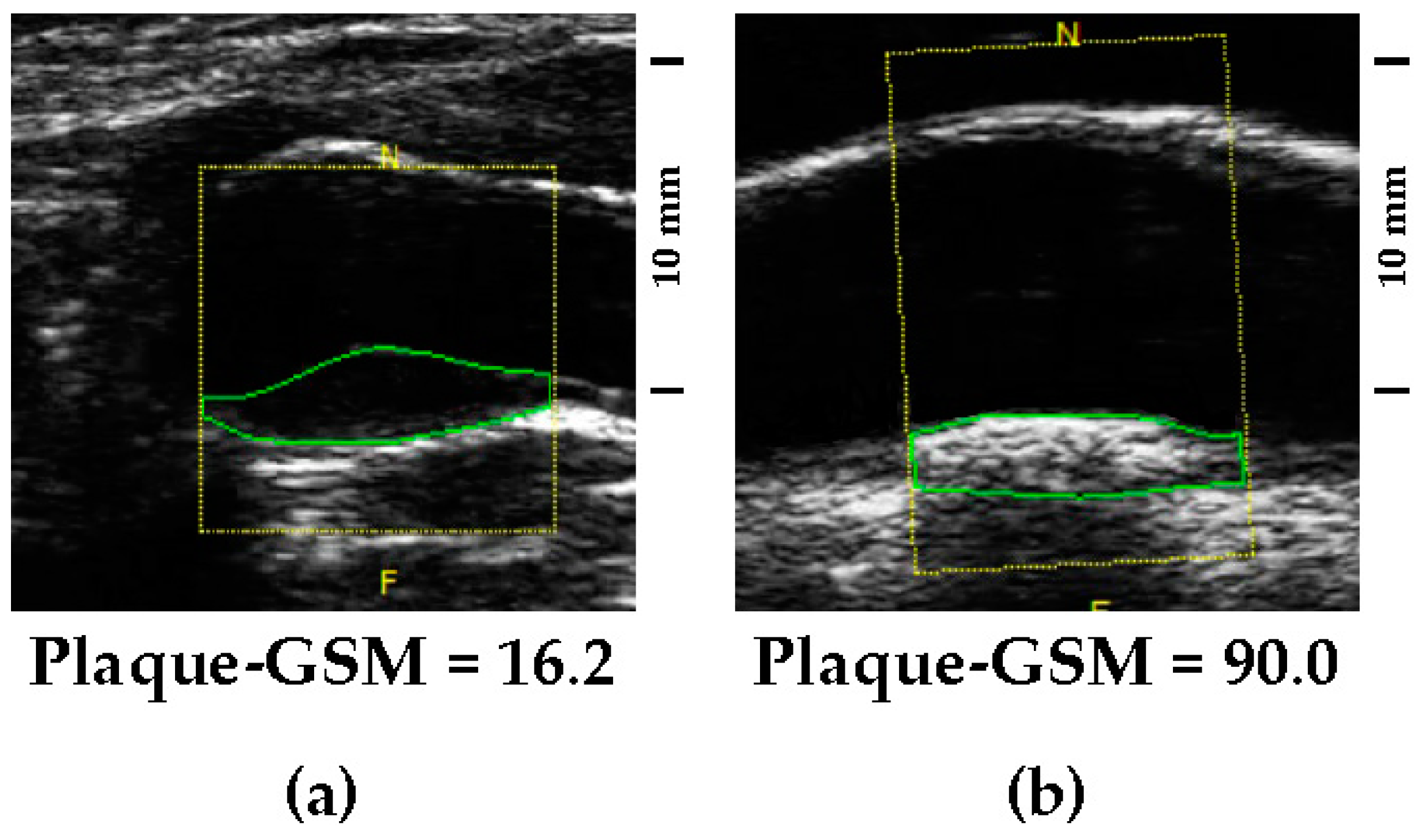
2.2. Quantitative Assessment of Carotid Artery Echolucency
2.3. Statistical Analysis
3. Results
3.1. Associations between Plaque-GSM, IM-GSM and Participants’ Characteristics
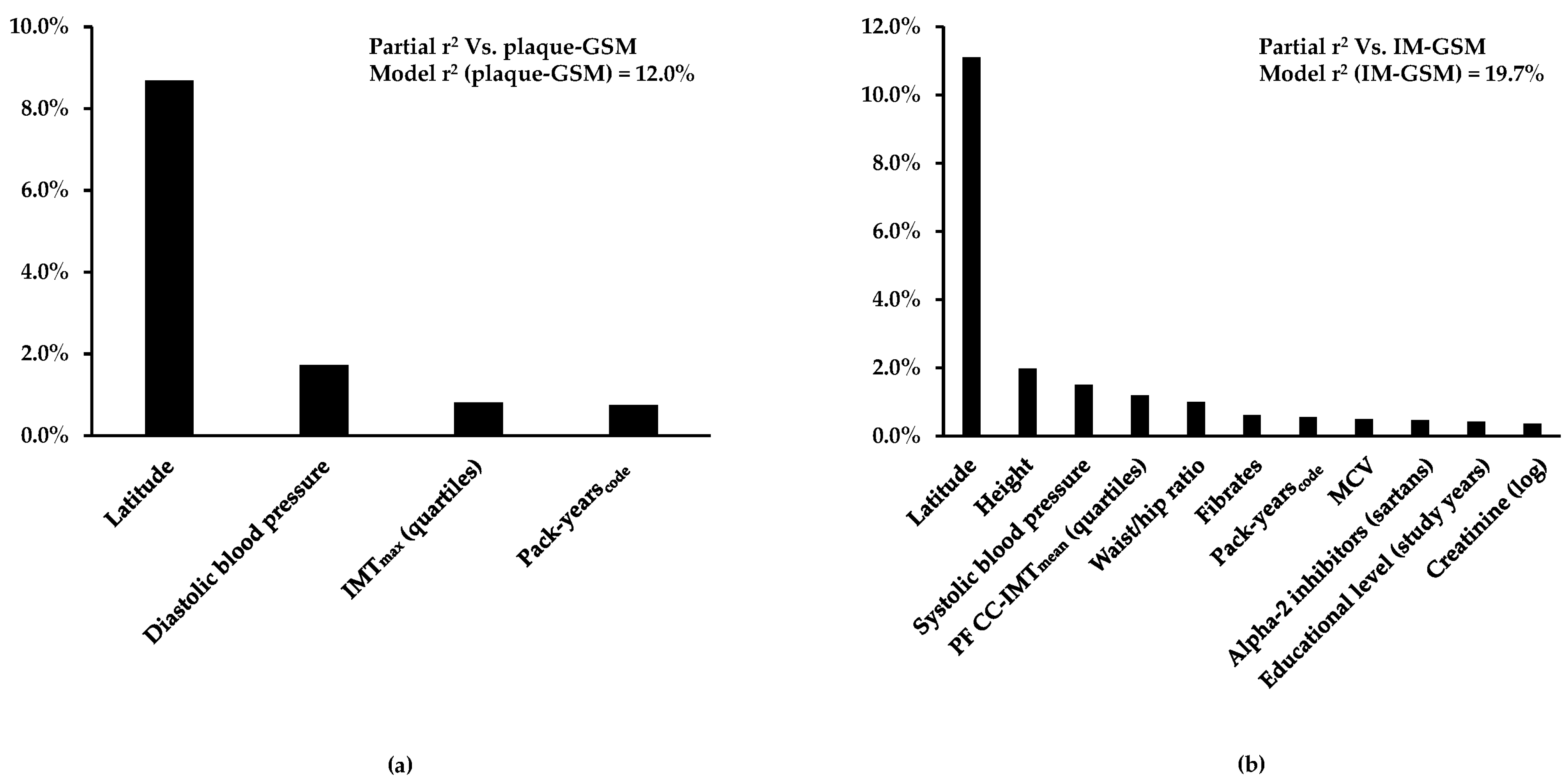
3.2. Independent Determinants of Carotid Plaque-GSM and IM-GSM
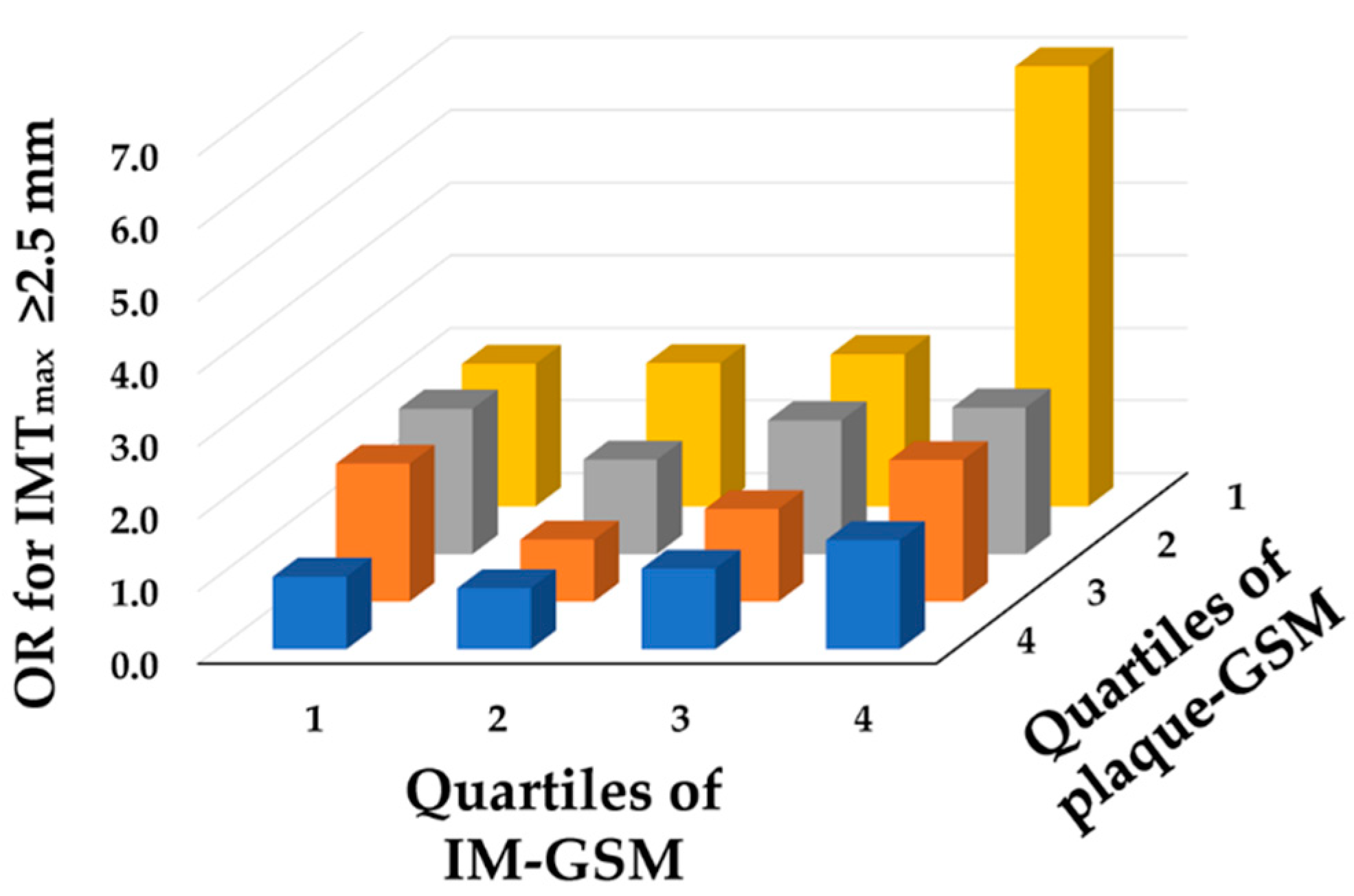
3.3. Relationship between Plaque-GSM, IM-GSM and Atherosclerotic Burden
4. Discussion
4.1. Positive and Negative Predictors of GSM
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doonan, R.J.; Gorgui, J.; Veinot, J.P.; Lai, C.; Kyriacou, E.; Corriveau, M.M.; Steinmetz, O.K.; Daskalopoulou, S.S. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J. Vasc. Surg. 2016, 64, 671–677.e678. [Google Scholar] [CrossRef] [PubMed]
- Sztajzel, R.; Momjian, S.; Momjian-Mayor, I.; Murith, N.; Djebaili, K.; Boissard, G.; Comelli, M.; Pizolatto, G. Stratified gray-scale median analysis and color mapping of the carotid plaque: Correlation with endarterectomy specimen histology of 28 patients. Stroke 2005, 36, 741–745. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Gronholdt, M.L.; Sillesen, H. Echolucent rupture-prone plaques. Curr. Opin. Lipidol. 2003, 14, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Charitanti, A.; Tegos, T.; Destanis, E.; Chryssogonidis, I. Contrast-enhanced ultrasound of the carotid system: A review of the current literature. J. Ultrasound 2017, 20, 97–109. [Google Scholar] [CrossRef]
- Gronholdt, M.L.; Nordestgaard, B.G.; Schroeder, T.V.; Vorstrup, S.; Sillesen, H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001, 104, 68–73. [Google Scholar] [CrossRef]
- Gronholdt, M.L. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2–13. [Google Scholar] [CrossRef]
- Gronholdt, M.L.; Nordestgaard, B.G.; Bentzon, J.; Wiebe, B.M.; Zhou, J.; Falk, E.; Sillesen, H. Macrophages are associated with lipid-rich carotid artery plaques, echolucency on B-mode imaging, and elevated plasma lipid levels. J. Vasc. Surg. 2002, 35, 137–145. [Google Scholar]
- Lind, L.; Peters, S.A.; den Ruijter, H.M.; Palmer, M.K.; Grobbee, D.E.; Crouse, J.R., 3rd; O’Leary, D.H.; Evans, G.W.; Raichlen, J.S.; Bots, M.L.; et al. Effect of rosuvastatin on the echolucency of the common carotid intima-media in low-risk individuals: The METEOR trial. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2012, 25, 1120–1127.e1121. [Google Scholar] [CrossRef]
- Lind, L.; Andersson, J.; Ronn, M.; Gustavsson, T.; Holdfelt, P.; Hulthe, J.; Elmgren, A.; Zilmer, K.; Zilmer, M. Brachial artery intima-media thickness and echogenicity in relation to lipids and markers of oxidative stress in elderly subjects: The prospective investigation of the vasculature in Uppsala Seniors (PIVUS) Study. Lipids 2008, 43, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, E.B.; Bonaa, K.H.; Joakimsen, O. Low levels of high-density lipoprotein cholesterol are associated with echolucent carotid artery plaques: The tromso study. Stroke 2001, 32, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wohlin, M.; Andren, B.; Sundstrom, J. The echogenicity of the intima-media complex in the common carotid artery is related to insulin resistance measured by the hyperinsulinemic clamp in elderly men. Clin. Physiol. Funct. Imaging 2013, 33, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Z.; Paul-Labrador, M.; Merz, C.N.; Iribarren, C.; Dwyer, J.H. Smoking status and common carotid artery intima-medial thickness among middle-aged men and women based on ultrasound measurement: A cohort study. BMC Cardiovasc. Disord. 2006, 6, 42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peters, S.A.; Lind, L.; Palmer, M.K.; Grobbee, D.E.; Crouse, J.R., 3rd; O’Leary, D.H.; Evans, G.W.; Raichlen, J.; Bots, M.L.; den Ruijter, H.M.; et al. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: The METEOR study. J. Intern. Med. 2012, 272, 257–266. [Google Scholar] [CrossRef] [PubMed]
- With Noto, A.T.; Bogeberg Mathiesen, E.; Amiral, J.; Vissac, A.M.; Hansen, J.B. Endothelial dysfunction and systemic inflammation in persons with echolucent carotid plaques. Thromb. Haemost. 2006, 96, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.H.; Mathiesen, E.B. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr. Cardiol. Rep. 2009, 11, 21–27. [Google Scholar] [CrossRef]
- Jashari, F.; Ibrahimi, P.; Bajraktari, G.; Gronlund, C.; Wester, P.; Henein, M.Y. Carotid plaque echogenicity predicts cerebrovascular symptoms: A systematic review and meta-analysis. Eur. J. Neurol. 2016, 23, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, M.; Sundstrom, J.; Andren, B.; Larsson, A.; Lind, L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009, 205, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.C.; Korcarz, C.E.; Gepner, A.D.; Nye, R.; Young, R.L.; Matsuzaki, M.; Post, W.S.; Kaufman, J.D.; McClelland, R.L.; Stein, J.H. Carotid Artery Echolucency, Texture Features, and Incident Cardiovascular Disease Events: The MESA Study. J. Am. Heart Assoc. 2019, 8, e010875. [Google Scholar] [CrossRef] [PubMed]
- Lind, L. A detailed lipoprotein profile in relation to intima-media thickness and echogenicity of three major arteries. Clin. Physiol. Funct. Imaging 2019, 39, 415–421. [Google Scholar] [CrossRef]
- Mitchell, C.; Piper, M.E.; Korcarz, C.E.; Hansen, K.; Weber, J.; Fiore, M.C.; Baker, T.B.; Stein, J.H. Echogenicity of the carotid arterial wall in active smokers. J. Diagn. Med. Sonogr. 2018, 34, 161–168. [Google Scholar] [CrossRef]
- Jarhult, S.J.; Sundstrom, J.; Lind, L. Brachial artery hyperemic blood flow velocities are related to carotid atherosclerosis. Clin. Physiol. Funct. Imaging 2009, 29, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Andersson, J.; Larsson, A.; Sandhagen, B. Shear stress in the common carotid artery is related to both intima-media thickness and echogenecity. The Prospective Investigation of the Vasculature in Uppsala Seniors study. Clin. Hemorheol. Microcirc. 2009, 43, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 2011, 218, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Olsen, L.; Lind, L. Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci. Total Environ. 2012, 416, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, B.; Lind, P.M.; Lind, L. Serum levels of monobenzylphthalate (MBzP) is related to carotid atherosclerosis in the elderly. Environ. Res. 2014, 133, 348–352. [Google Scholar] [CrossRef]
- Ibrahimi, P.; Jashari, F.; Johansson, E.; Gronlund, C.; Bajraktari, G.; Wester, P.; Henein, M.Y. Common carotid intima-media features determine distal disease phenotype and vulnerability in asymptomatic patients. Int. J. Cardiol. 2015, 196, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.; Lind, L.; Soderberg, S.; Ingelsson, E. Associations of circulating adiponectin with measures of vascular function and morphology. J. Clin. Endocrinol. Metab. 2010, 95, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Altedorneburg, G.; Droste, D.W.; Haas, N.; Kemeny, V.; Nabavi, D.G.; Fuzesi, L.; Ringelstein, E.B. Preoperative B-mode ultrasound plaque appearance compared with carotid endarterectomy specimen histology. Acta Neurol. Scand. 2000, 101, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, D.; Hamsten, A.; Veglia, F.; de Faire, U.; Humphries, S.E.; Smit, A.J.; Giral, P.; Kurl, S.; Rauramaa, R.; Mannarino, E.; et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: Results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J. Am. Coll. Cardiol. 2012, 60, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, D.; Nyyssonen, K.; Rauramaa, R.; de Faire, U.; Hamsten, A.; Smit, A.J.; Mannarino, E.; Humphries, S.E.; Giral, P.; Grossi, E.; et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: The IMPROVE study. Eur. Heart J. 2010, 31, 614–622. [Google Scholar] [CrossRef]
- Andersson, J.; Sundstrom, J.; Gustavsson, T.; Hulthe, J.; Elmgren, A.; Zilmer, K.; Zilmer, M.; Lind, L. Echogenecity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 2009, 204, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Iyer, S.; Gardener, H.; Della-Morte, D.; Crisby, M.; Dong, C.; Cheung, K.; Mora-McLaughlin, C.; Wright, C.B.; Elkind, M.S.; et al. Cigarette Smoking and Carotid Plaque Echodensity in the Northern Manhattan Study. Cerebrovasc. Dis. 2015, 40, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sans, S.; Kesteloot, H.; Kromhout, D. The burden of cardiovascular diseases mortality in Europe. Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur. Heart J. 1997, 18, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.S.; Lee, Y.H.; Shin, M.H.; Choi, J.S.; Rhee, J.A.; Choi, S.W.; Ryu, S.Y.; Kim, B.H.; Nam, H.S.; Jeong, S.K.; et al. Effects of cumulative smoking exposure and duration of smoking cessation on carotid artery structure. Circ. J. 2012, 76, 2041–2047. [Google Scholar] [CrossRef]
- Frigerio, B.; Werba, J.P.; Amato, M.; Ravani, A.; Sansaro, D.; Coggi, D.; Vigo, L.; Tremoli, E.; Baldassarre, D. Traditional Risk Factors are Causally Related to Carotid Intima-Media Thickness Progression: Inferences from Observational Cohort Studies and Interventional Trials. Curr. Pharm. Des. 2020, 26, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sharrett, A.R.; Ding, J.; Criqui, M.H.; Saad, M.F.; Liu, K.; Polak, J.F.; Folsom, A.R.; Tsai, M.Y.; Burke, G.L.; Szklo, M. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: The Multiethnic Study of Atherosclerosis (MESA). Atherosclerosis 2006, 186, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Sundstrom, J.; Kurland, L.; Gustavsson, T.; Hulthe, J.; Elmgren, A.; Zilmer, K.; Zilmer, M.; Lind, L. The carotid artery plaque size and echogenicity are related to different cardiovascular risk factors in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Lipids 2009, 44, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Dong, C.; Crisby, M.; Gardener, H.; Cabral, D.; Elkind, M.S.V.; Gutierrez, J.; Sacco, R.L.; Rundek, T. Association of Carotid Plaque Morphology and Glycemic and Lipid Parameters in the Northern Manhattan Study. Front. Cardiovasc. Med. 2022, 9, 793755. [Google Scholar] [CrossRef]
- Sigurdardottir, V.; Fagerberg, B.; Wikstrand, J.; Schmidt, C.; Hulthe, J. Circulating oxidized low-density lipoprotein is associated with echolucent plaques in the femoral artery independently of hsCRP in 61-year-old men. Atherosclerosis 2007, 190, 187–193. [Google Scholar] [CrossRef]
- Craiem, D.; Chironi, G.; Graf, S.; Denarie, N.; Armentano, R.L.; Simon, A. Atheromatous plaque: Quantitative analysis of the echogenicity of different layers. Rev. Esp. Cardiol. 2009, 62, 984–991. [Google Scholar] [CrossRef]
- Jung, M.; Parrinello, C.M.; Xue, X.; Mack, W.J.; Anastos, K.; Lazar, J.M.; Selzer, R.H.; Shircore, A.M.; Plankey, M.; Tien, P.; et al. Echolucency of the carotid artery intima-media complex and intima-media thickness have different cardiovascular risk factor relationships: The Women’s Interagency HIV Study. J. Am. Heart Assoc. 2015, 4, e001405. [Google Scholar] [CrossRef]
- Mitchell, C.; Piper, M.E.; Smith, S.S.; Korcarz, C.E.; Fiore, M.C.; Baker, T.B.; Stein, J.H. Changes in carotid artery structure with smoking cessation. Vasc. Med. 2019, 24, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, H.; Kaneko, H.; Itoh, H.; Yoshida, Y.; Nakanishi, K.; Mizuno, Y.; Daimon, M.; Morita, H.; Yamamichi, N.; Komuro, I. Effect of cigarette smoking on carotid artery atherosclerosis: A community-based cohort study. Heart Vessel. 2020, 35, 22–29. [Google Scholar] [CrossRef]
- Karim, R.; Xu, W.; Kono, N.; Li, Y.; Yan, M.; Stanczyk, F.Z.; Hodis, H.N.; Mack, W.J. Comparison of Cardiovascular Disease Risk Factors Between 2 Subclinical Atherosclerosis Measures in Healthy Postmenopausal Women: Carotid Artery Wall Thickness and Echogenicity: Carotid Artery Wall Thickness and Echogenicity. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2022, 42, 35–44. [Google Scholar] [CrossRef]
- Sigurdardottir, V.; Fagerberg, B.; Wikstrand, J.; Schmidt, C.; Hulthe, J. Circulating oxidized LDL is associated with the occurrence of echolucent plaques in the carotid artery in 61-year-old men. Scand. J. Clin. Lab. Investig. 2008, 68, 292–297. [Google Scholar] [CrossRef]
- Asciutto, G.; Dias, N.V.; Persson, A.; Nilsson, J.; Goncalves, I. Treatment with betablockers is associated with higher grey-scale median in carotid plaques. BMC Cardiovasc. Disord. 2014, 14, 111. [Google Scholar] [CrossRef]
- Genkel, V.V.; Kuznetsova, A.S.; Lebedev, E.V.; Shaposhnik, I.I. Factors associated with atherosclerotic plaque echogenicity in patients aged 40-64 with carotid atherosclerosis. Kardiologiia 2021, 61, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, P.L.; Plavnik, F.L.; Scaciota, A.; Lima, J.O.; Miranda, R.B.; Ajzen, S.A. Relationship between cardiovascular risk factors and the echogenicity and pattern of the carotid intima-media complex in men. Sao Paulo Med. J. 2014, 132, 97–104. [Google Scholar] [CrossRef]
- Pham, D.V.; Park, P.H. Recent insights on modulation of inflammasomes by adipokines: A critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch. Pharm. Res. 2020, 43, 997–1016. [Google Scholar] [CrossRef]
- Marso, S.P.; Mehta, S.K.; Frutkin, A.; House, J.A.; McCrary, J.R.; Kulkarni, K.R. Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care 2008, 31, 989–994. [Google Scholar] [CrossRef][Green Version]
- Prahl, U.; Holdfeldt, P.; Bergstrom, G.; Fagerberg, B.; Hulthe, J.; Gustavsson, T. Percentage white: A new feature for ultrasound classification of plaque echogenicity in carotid artery atherosclerosis. Ultrasound Med. Biol. 2010, 36, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, C.C.; Veglia, F.; de Faire, U.; Kurl, S.; Smit, A.J.; Rauramaa, R.; Giral, P.; Amato, M.; Bonomi, A.; Ravani, A.; et al. Association of lifelong occupation and educational level with subclinical atherosclerosis in different European regions. Results from the IMPROVE study. Atherosclerosis 2018, 269, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.P.; Rose, K.M.; Catellier, D.J.; Kaufman, J.S.; Wyatt, S.B.; Diez-Roux, A.V.; Heiss, G. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Ann. Epidemiol. 2007, 17, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Camelo, L.V.; Giatti, L.; Chor, D.; Griep, R.H.; Bensenor, I.M.; Santos, I.S.; Kawachi, I.; Barreto, S.M. Associations of life course socioeconomic position and job stress with carotid intima-media thickness. The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Soc. Sci. Med. 2015, 141, 91–99. [Google Scholar] [CrossRef]
- Steptoe, A.; Hiltl, T.J.; Dowd, J.B.; Hamer, M. Socioeconomic status and central adiposity as determinants of stress-related biological responses relevant to cardiovascular disease risk. Brain Behav. Immun. 2019, 77, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, P.; Jashari, F.; Johansson, E.; Gronlund, C.; Bajraktari, G.; Wester, P.; Henein, M.Y. Vulnerable plaques in the contralateral carotid arteries in symptomatic patients: A detailed ultrasound analysis. Atherosclerosis 2014, 235, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Cires-Drouet, R.S.; Mozafarian, M.; Ali, A.; Sikdar, S.; Lal, B.K. Imaging of high-risk carotid plaques: Ultrasound. Semin. Vasc. Surg. 2017, 30, 44–53. [Google Scholar] [CrossRef]
- Karjalainen, J.P.; Toyras, J.; Riekkinen, O.; Hakulinen, M.; Jurvelin, J.S. Ultrasound backscatter imaging provides frequency-dependent information on structure, composition and mechanical properties of human trabecular bone. Ultrasound Med. Biol. 2009, 35, 1376–1384. [Google Scholar] [CrossRef]
- Ramnarine, K.V.; Garrard, J.W.; Kanber, B.; Nduwayo, S.; Hartshorne, T.C.; Robinson, T.G. Shear wave elastography imaging of carotid plaques: Feasible, reproducible and of clinical potential. Cardiovasc. Ultrasound 2014, 12, 49. [Google Scholar] [CrossRef]
| Standardized Beta | p Value | Partial R2 | |
|---|---|---|---|
| Latitude | −3.23 | <0.0001 | 8.7% |
| Diastolic blood pressure | 1.36 | <0.0001 | 1.7% |
| IMTmax (quartiles) | −0.96 | <0.0001 | 0.8% |
| Pack-yearscode | 0.85 | <0.0001 | 0.7% |
| Whole model | 12.0% |
| Standardized Beta | p Value | Partial R2 | |
|---|---|---|---|
| Latitude | −4.27 | <0.0001 | 11.1% |
| Height | 1.50 | <0.0001 | 2.0% |
| Systolic blood pressure | 1.44 | <0.0001 | 1.5% |
| PF CC-IMTmean (quartiles) | −1.85 | <0.0001 | 1.2% |
| Waist/hip ratio | −1.93 | <0.0001 | 1.0% |
| Fibrates | −0.95 | <0.0001 | 0.6% |
| Pack-yearscode | 0.93 | <0.0001 | 0.6% |
| MCV | −0.79 | <0.0001 | 0.5% |
| Alpha-2 inhibitors (sartans) | −0.79 | <0.0001 | 0.5% |
| Educational level (study years) | −0.79 | <0.0001 | 0.4% |
| Creatinine (log) | 0.96 | <0.0001 | 0.4% |
| Whole model | 19.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigerio, B.; Coggi, D.; Bonomi, A.; Amato, M.; Capra, N.; Colombo, G.I.; Sansaro, D.; Ravani, A.; Savonen, K.; Giral, P.; et al. Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data. Biomedicines 2024, 12, 737. https://doi.org/10.3390/biomedicines12040737
Frigerio B, Coggi D, Bonomi A, Amato M, Capra N, Colombo GI, Sansaro D, Ravani A, Savonen K, Giral P, et al. Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data. Biomedicines. 2024; 12(4):737. https://doi.org/10.3390/biomedicines12040737
Chicago/Turabian StyleFrigerio, Beatrice, Daniela Coggi, Alice Bonomi, Mauro Amato, Nicolò Capra, Gualtiero I. Colombo, Daniela Sansaro, Alessio Ravani, Kai Savonen, Philippe Giral, and et al. 2024. "Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data" Biomedicines 12, no. 4: 737. https://doi.org/10.3390/biomedicines12040737
APA StyleFrigerio, B., Coggi, D., Bonomi, A., Amato, M., Capra, N., Colombo, G. I., Sansaro, D., Ravani, A., Savonen, K., Giral, P., Gallo, A., Pirro, M., Gigante, B., Eriksson, P., Strawbridge, R. J., Mulder, D. J., Tremoli, E., Veglia, F., & Baldassarre, D., on behalf of the IMPROVE Study Group. (2024). Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data. Biomedicines, 12(4), 737. https://doi.org/10.3390/biomedicines12040737











