In our opinion, the data obtained in this study confirm that, as reported in the literature, the β-TCP matrices produced using our top-down DLP present promising properties for bone tissue engineering [
33]. In fact, we demonstrated that the purity of β-TCP powder and the physico-chemical properties and biocompatibility of matrices are not altered by our printing process [
34]. Our data also confirmed that DLP allows for reproducible and precise printing of phosphocalcic scaffolds. Our shaping technique therefore appears to be a solid basis for the development of phosphocalcic matrices with complex architectures incorporating different controlled macro-porosities and the microfluidic network needed to produce matrices for large-volume bone reconstruction.
4.1. β-TCP as a Bone Reconstruction Material
For decades, hydroxyapatite (HA) and β-TCP have demonstrated biocompatible and osteoconductive properties by allowing the attachment, migration, proliferation and differentiation of osteoblasts. Their osteoinductive properties, however, vary depending on their composition, geometry, specific surface area, and macro- and micro-porosity [
35]. Taking full control of matrix production from powder synthesis to final shaping is fundamental for further biomedical applications. This observation led us to develop our own product.
β-TCP has a less stable crystal structure than HA and is more degraded by osteoclasts [
36]. HA presents minimal resorption, whereas β-TCP can be completely resorbed into the reconstructed site and replaced by native bone, depending on its resorption speed. In our study, we confirmed that the matrices we produced possess resorption properties that vary according to the sintering temperature.
Although the resorption of β-TCP is beneficial for osteoinduction, it is detrimental for the homing of cells, bone ingrowth and, therefore, osteoconduction. There must therefore be a compromise between resorption and stability of the scaffolds [
37].
Calcium phosphate synthesis is not the unique parameter necessary for understanding biological properties. As we observed, an increase in the sintering temperature leads to a decrease in the micro-porosity of the matrices, as well as their resorption. This is in agreement with the findings of previous research that reported a reduction in micro-porosity associated with an increase in the grain size and the specific surface area [
38,
39]. Our first in vitro culture confirmed the biological impact of the sintering temperature, resulting in better cell proliferation associated with a higher sintering temperature.
We also tested the rinsing of our matrices to eliminate the non-adherent particles resulting from the sintering step. We observed a decrease in calcium release for all sintering temperatures. These results are in keeping with our second in vitro cultures, which achieved better results on rinsed matrices.
Studying the results of both culture tests, we can conclude that higher sintering temperatures are always beneficial for osteoblast cultures. That said, it is very difficult to directly transfer our in vitro observations to in vivo results. In vitro, the stability of β-TCP matrices is advantageous, improving the homing properties of their cells. However, these results may greatly differ from in vivo cultures, which are much more complex systems with autoregulation processes in which the osteoinductive properties of β-TCP cannot be ignored. That is why we focused our study on matrices sintered at 1050 °C, constituting a good compromise for future in vivo studies.
4.2. Purity and Biocompatibility of 3D-Printed Matrices
In most cases, Fused Deposition Modelling (FDM) is used to 3D print biomedical scaffolds [
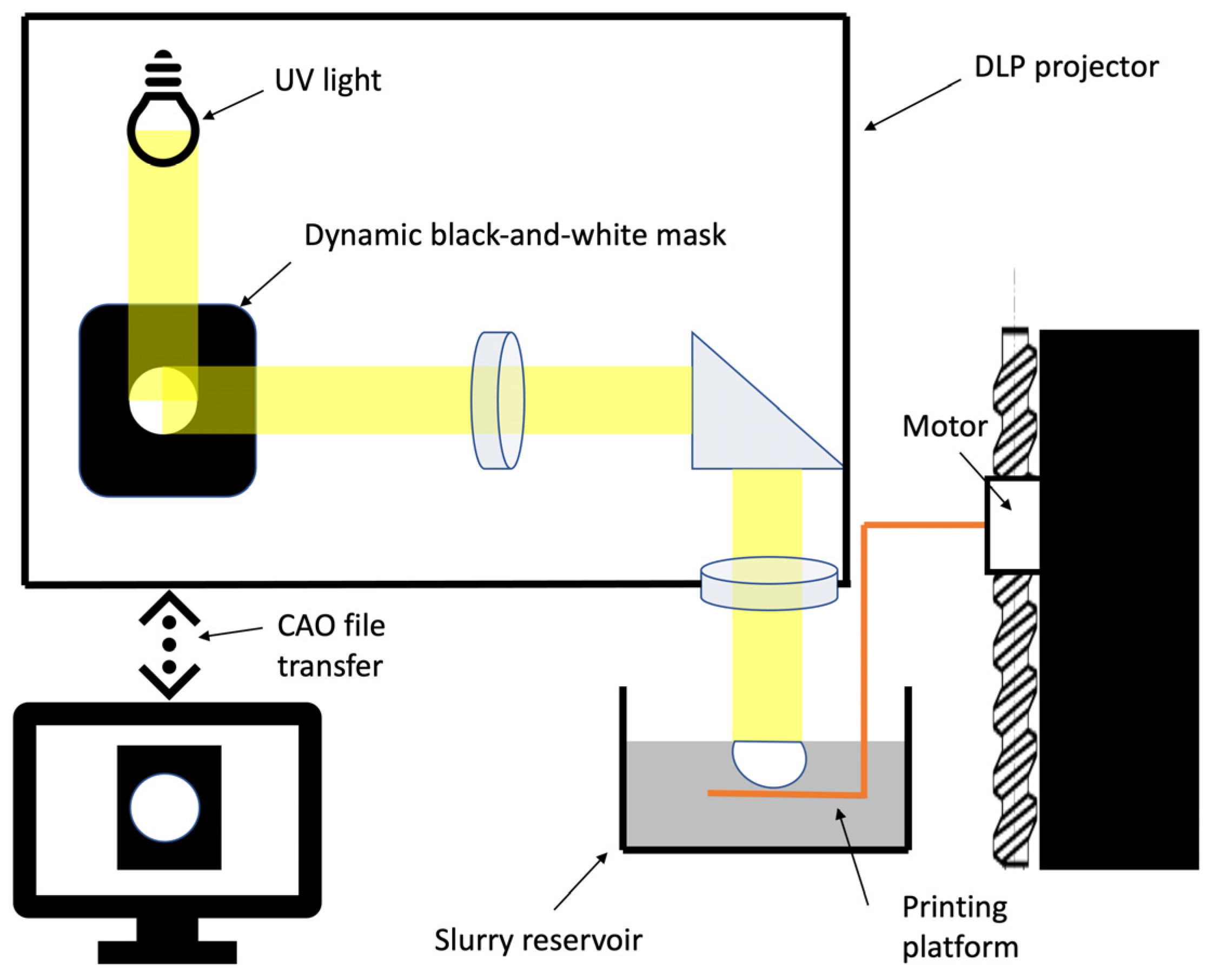
40]. The FDM technique does, however, present a number of limitations in terms of resolution; for example, its large nozzles (around 0.4–0.5 mm) and speed of production mean that each point of the printed matrix must be individually extruded. The DLP 3D-printing system, which uses layer-by-layer polymerisation, has the potential to produce at better speeds, and, depending on the size of the printing surface, multiple matrices can be produced simultaneously. Moreover, the resolution of FDM is not sufficiently high [
41]. DLP mixes phosphocalcic powders with polymer binders. Until recently, production techniques did not attempt to completely remove polymers to maintain the structural integrity of the matrices. Our study is, therefore, one of the first to report the production of 3D-printed pure phosphocalcic matrices [
19,
42,
43]. In fact, our production process combines de-binding and sintering processes in a single step that allows for the destruction and removal of biological components present in matrices, including polymer binders.
Thus, X-ray diffraction and infrared spectroscopy confirmed the industrial quality of our production. Looking for pollutants confirmed that both our synthesis and printing processes are safe and did not introduce pollutants into the matrices. Study of the open micro-porosity also demonstrates that the proposed printing processes did not modify properties of the β-TCP. Open porosity is mandatory in completely removing polymers and providing easy revascularisation of the matrices. Finally, the results of our osteoblast culture did not significantly differ between printed and casted scaffolds, demonstrating that they have the same biological properties and confirming the biocompatibility of our printing technique.
In our opinion, these elements demonstrate the efficiency of our technique in producing biocompatible 3D-printed phosphocalcic matrices.
Even though our study focused on β-TCP, other calcium phosphate synthetic apatites, like HA and biphasic calcium phosphate (BCP), a mixture of hydroxyapatite and β-TCP, can also be considered because of their specific biological and mechanical properties [
13,
33]. However, depending on the synthetic calcium apatite composition, specific formulations of polymer binders are necessary if we want to obtain high-density matrices with optimised properties [
44,
45].
4.3. DLP Printing for Clinical Applications Related to Tissue Engineering
Rapid prototyping processes are of interest with regard to controlling the reproducible micro-porosity of matrices compared to conventional methods [
23]. Indeed, conventional approaches result in variable and random interconnections due to the forming process. At the opposite end of the spectrum, as we experienced in our research, DLP enables us to produce reproducible matrices with controlled macro-porosity.
One of the difficulties involved in using DLP, however, finding a compromise between maximum powder loading and a viscosity compatible with the printing technique [
46]. That is why in this article, we studied the accuracy of our printed devices with 68% solid loading, which is equivalent to conventional shaping and higher than most DLP printers.
In our opinion, our study confirmed the potential of our shaping process because of its simplicity and accuracy. One element to consider in our production technique is the sintering phase. This results in a homogeneous contraction of the green parts and, thus, improves the precision of part printing. The controls we tested by scanning different parts produced using the same 3D model showed marginal variations on the same axis. Determining the contraction of parts as a function of sintering temperature is therefore essential if we want to produce digital models of the parts to be printed. However, our data also expose the limits of our process, which are connected to the viscosity of our slurry, light scattering within the ceramic and printing speed. Those parameters are in keeping with the findings of the literature, and we can apply corrections in the future to improve these results [
47]. Modulating the dimensions in the Z axis can therefore compensate for the deformation observed during our printing process. Furthermore, future modification of the light source during the printing process could also lead to an increase in our resolution, which we evaluated to be lower than 50 µm.
At present, most clinical practice involves the use of synthetic calcium phosphates in the form of powders or prefabricated blocks to fill bone defects. These, however, cannot perfectly adapt to reconstruct the defect, thus leading to instability and failure. Indeed, biomechanical stability is one of the fundamental elements involved during the bone healing process, and DLP printing of a custom implantable scaffold is of a high level of interest [
48,
49].
Furthermore, DLP allows us to design matrices with controlled macro-porosity that can be modified to mimic the characteristics of native bone, which has variable macro-porosity. The pores of the trabecular bone range in size from 200 to 700 µm and have a porosity ranging between 50 and 90%, whereas the cortical bone has less than 20% porosity and a pore range of 1100 µm. Furthermore, it has been demonstrated that variations in pore size seem to be essential to the biological properties of matrices [
37]. A study using DLP to produce macro-porous BCP matrices compared the impact of the size of macro-pores ranging from 0.8 to 1.4 mm on bone formation. The authors concluded that the formation of new bone was satisfying but that no significant variation was observed after 8 weeks of culturing [
19]. Other studies have reported that macro-pores with a diameter of up to 140 µm increase new bone formation and capillary density, that pores of up to 100 µm are important for bone oxygenation and that angiogenesis and pores of less than 1 µm play an important role in bioactivity [
50,
51].
In light of the above, the high-precision 3D printing of phosphocalcic bone matrices using techniques including our own is key to the development of bone tissue engineering. Such techniques allow for the precise control of the scaffolds’ architectures and optimise the physical and biological properties of scaffolds, enabling them to assume different shapes and have pores of a range of sizes.
Our study did not research the compressive strength of the matrices we produced. We arrived at this decision because the compressive strengths of phosphocalcic matrices increase with their sintering temperatures. In addition, the macro-porosity of the matrices and their various possible shapes have a direct impact on their mechanical strengths [
19].
In tissue engineering, vascularisation is considered to be essential [
52]. Most bone tissue engineering protocols use vascularisation brought by the surrounding tissues. Cells should be within 200 µm of a vessel in order to exchange oxygen and nutrients, thus theoretically limiting the size of functional bone scaffolds and preventing necrosis [
53]. Large reconstructions also need to both mimic bone and incorporate the vascularisation or fluidic network to make them useful in clinical applications. Multiple bioprinting strategies have therefore been developed to this end, including extrusion-based bioprinting, inkjet printing, acoustic wave patterning and light-based bioprinting [
54].
When it comes to phosphocalcic synthetic biomaterials, however, bioprinting is not compatible with the sintering process that is necessary for densification of the matrices. Another approach to mimic a functional vascularisation is to print a fluidic network into matrices and then culture endothelial cells on its walls. In that approach, the design of the network is essential to reduce the hypoxic areas inside matrix, as well as to contribute to a fluid flow with low turbulences, thus reducing thrombogenesis [
55]. Given these various constraints, DLP printing appears promising for producing phosphocalcic matrices with both controlled macro-porosity and a “vascular tree”. And this is precisely why we developed our shaping technique.
At present, the printing of phosphocalcic matrices is one of the most promising fields in bone tissue engineering. And given the accuracy of DLP, it could become the gold standard. However, printing is only one of the steps that can affect the biological and mechanical properties of matrices.
Given the complexity and number of parameters to be considered in producing these scaffolds, the complete, careful monitoring of all steps in the production of these matrices—from powder synthesis to final sintering—is essential. This is reflected in our decision to dedicate a section of our study to the characterisation of our production to confirm that we produced β-TCP and that our printing technique does not alter its properties.
We also studied the accuracy of our shaping technique using DLP. Our findings demonstrate that top-down DLP is an effective technique for producing precise phosphocalcic matrices and that the shrinkage of green parts linked to sintering is involved in increasing the printing resolution. These findings will enable us to devote future studies to exploring complex phosphocalcic matrices, with a special focus on the development of a functional vascular network.