Diabetic Cardiomyopathy—From Basics through Diagnosis to Treatment
Abstract
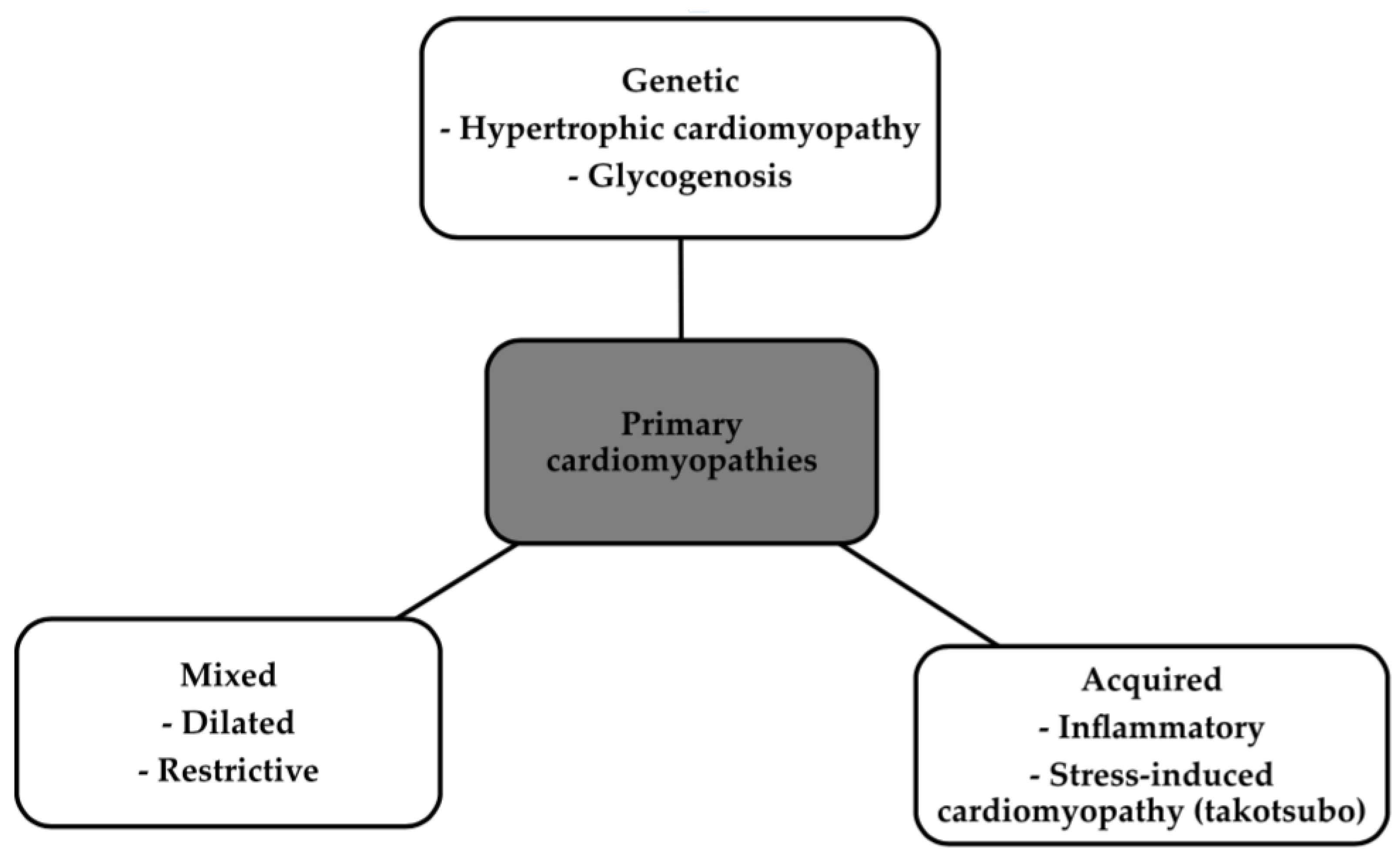
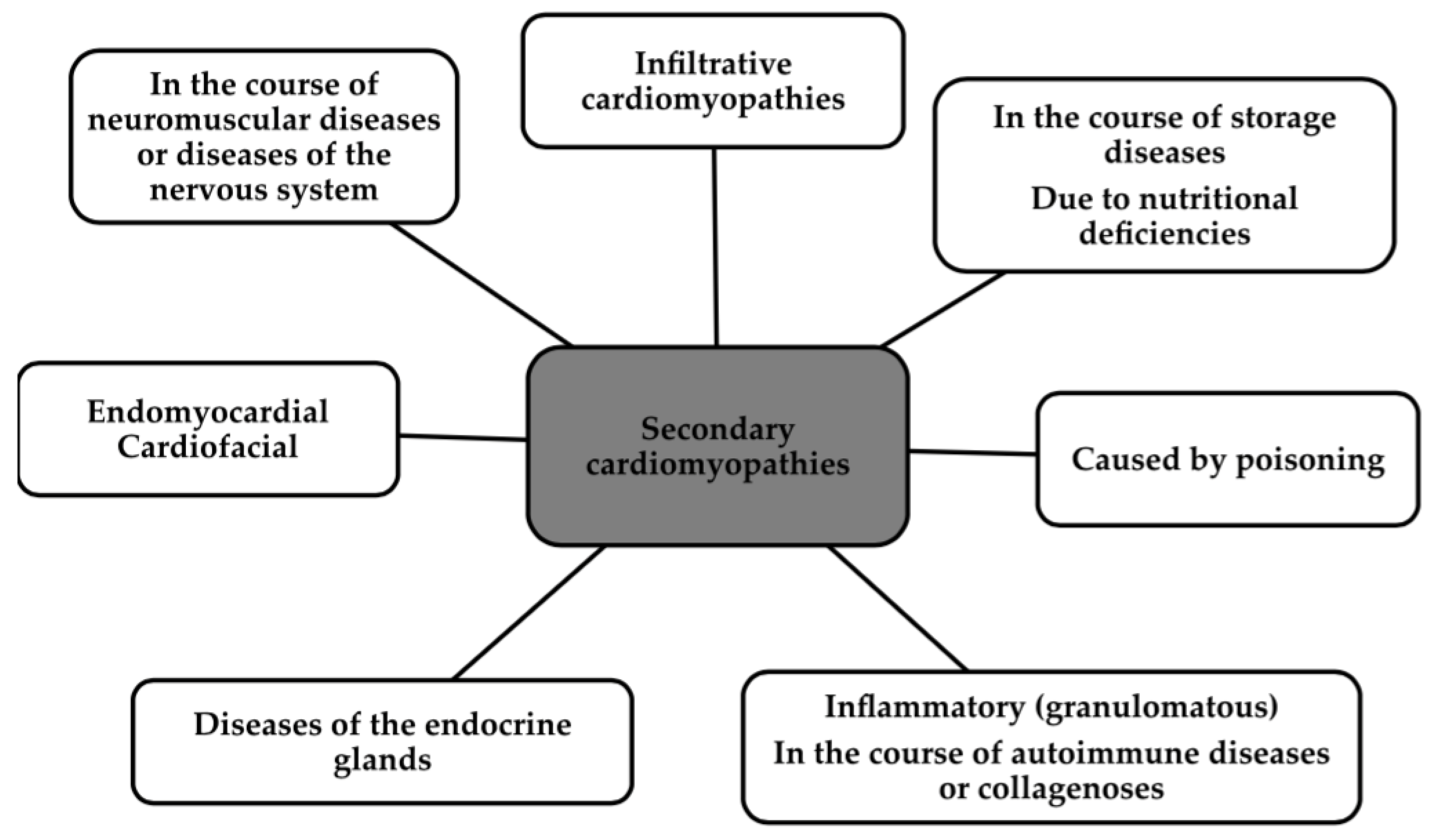
1. Introduction
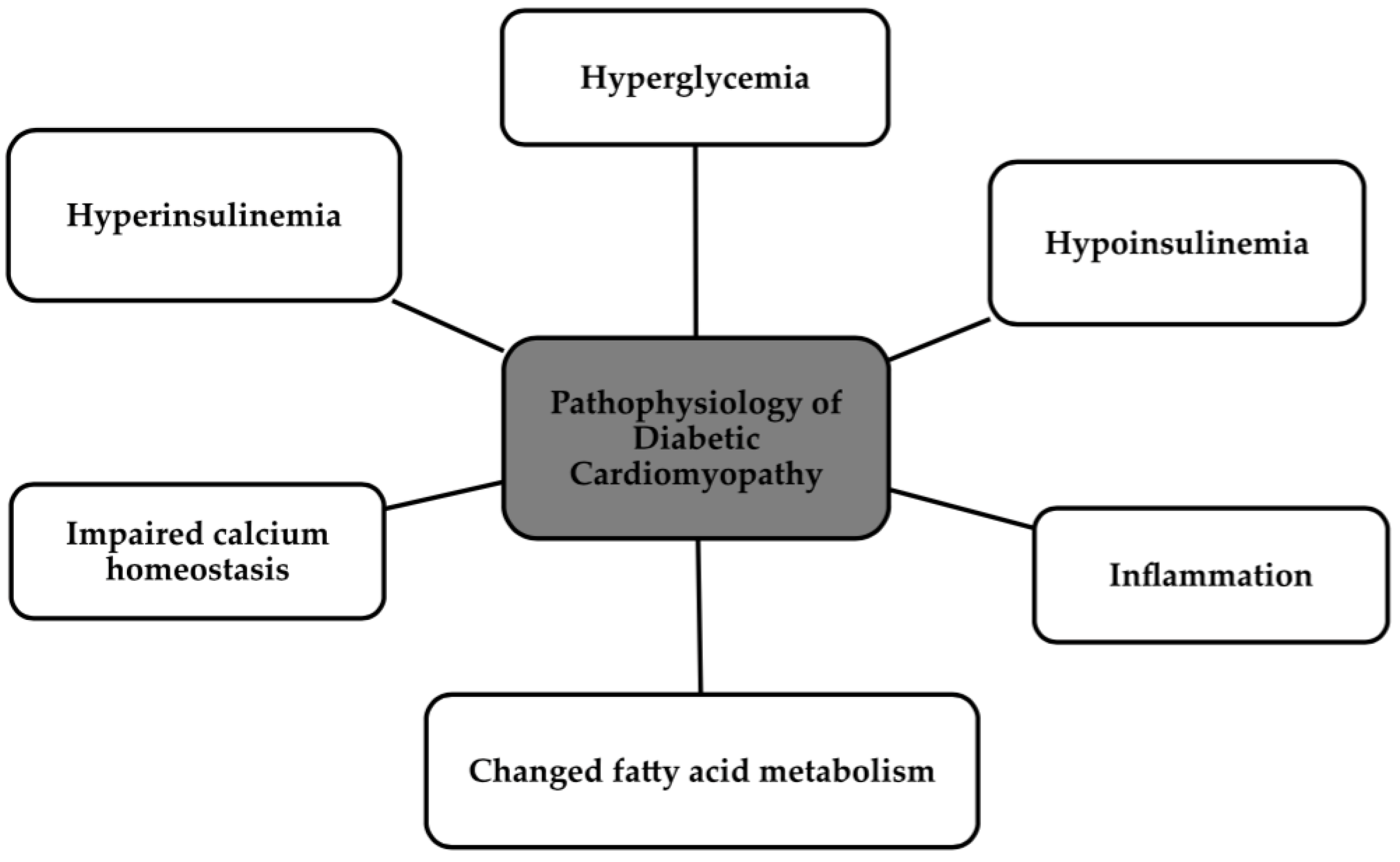
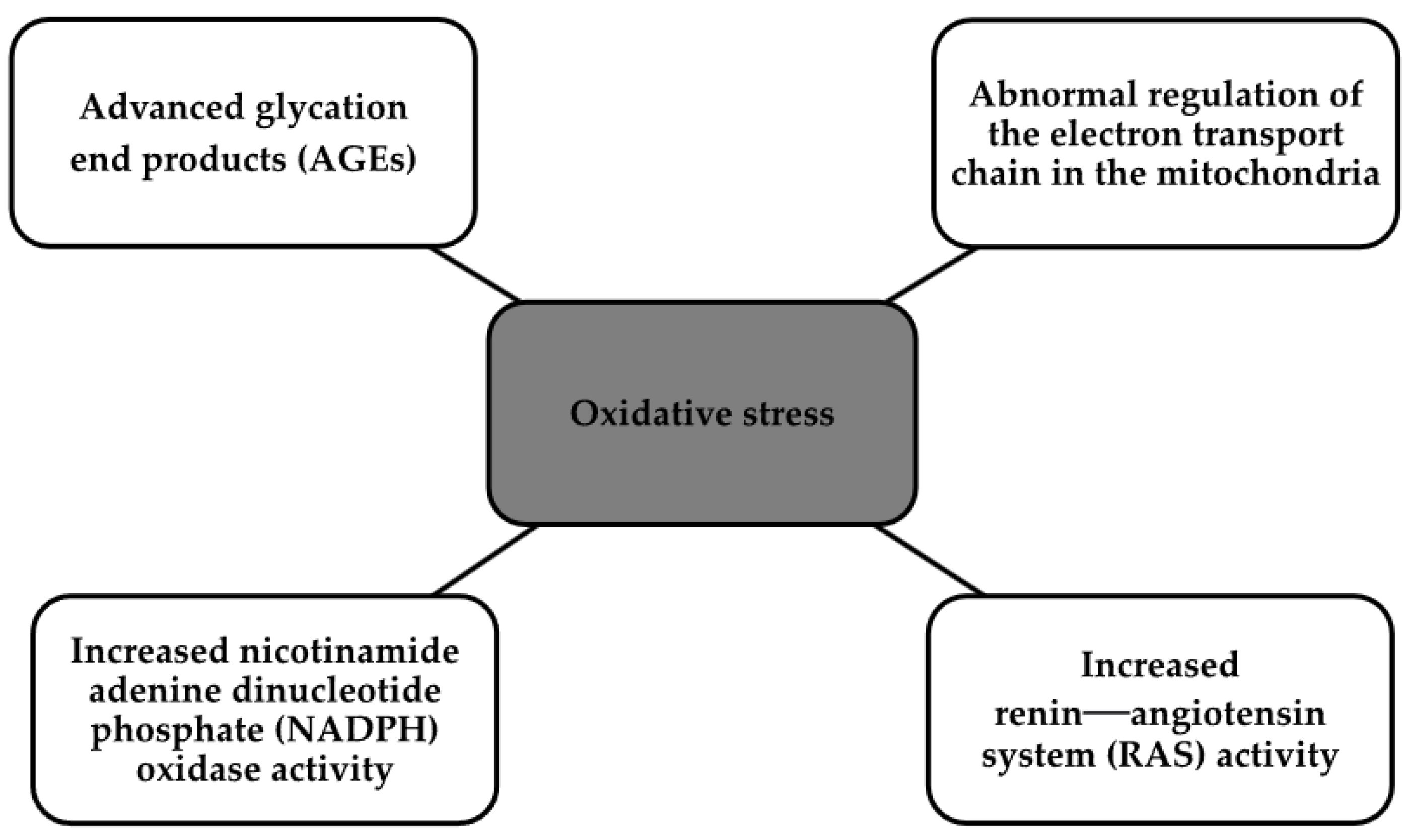
2. Pathophysiology
3. Epidemiology
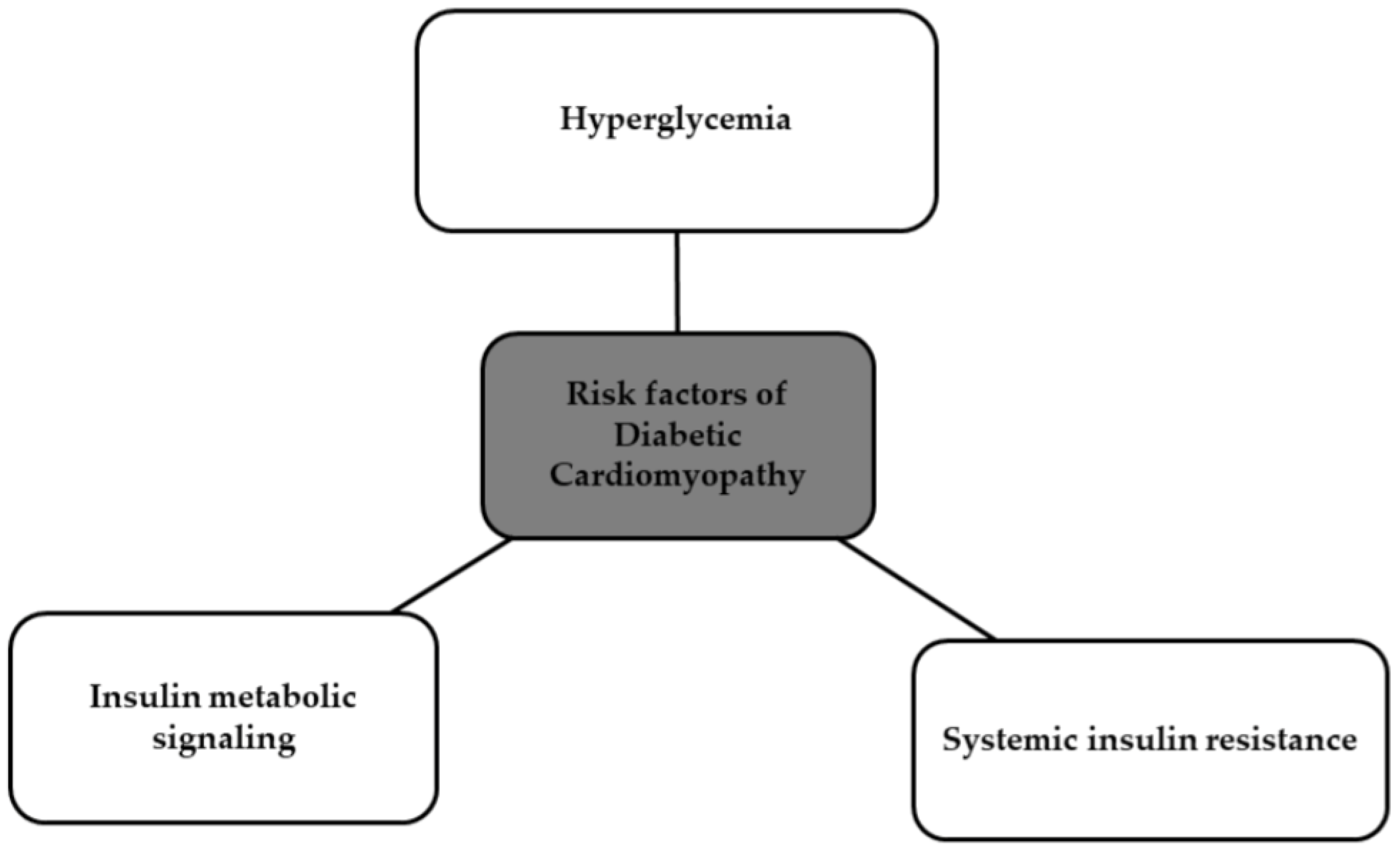
4. Risk Factors
5. Diagnosis
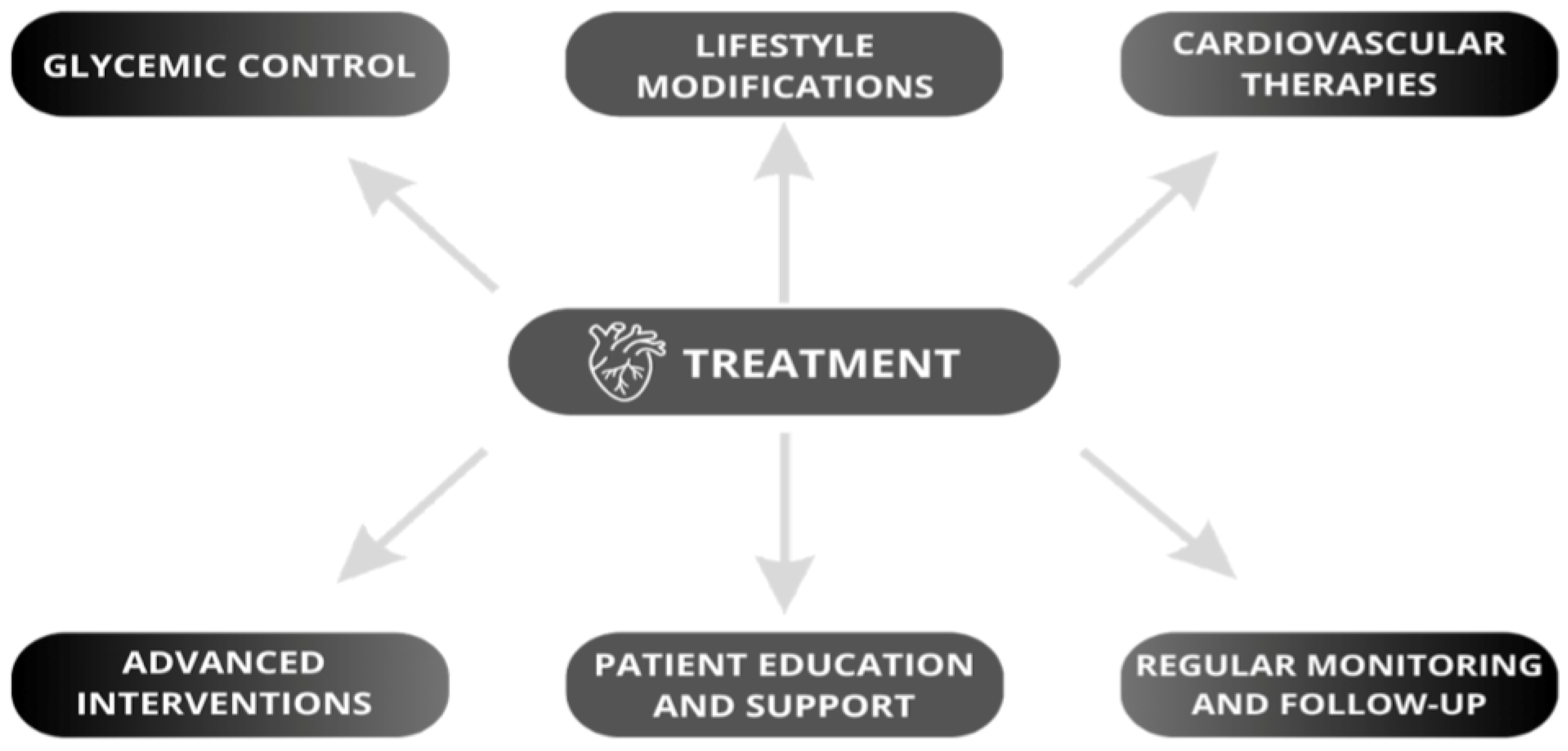
6. Treatment
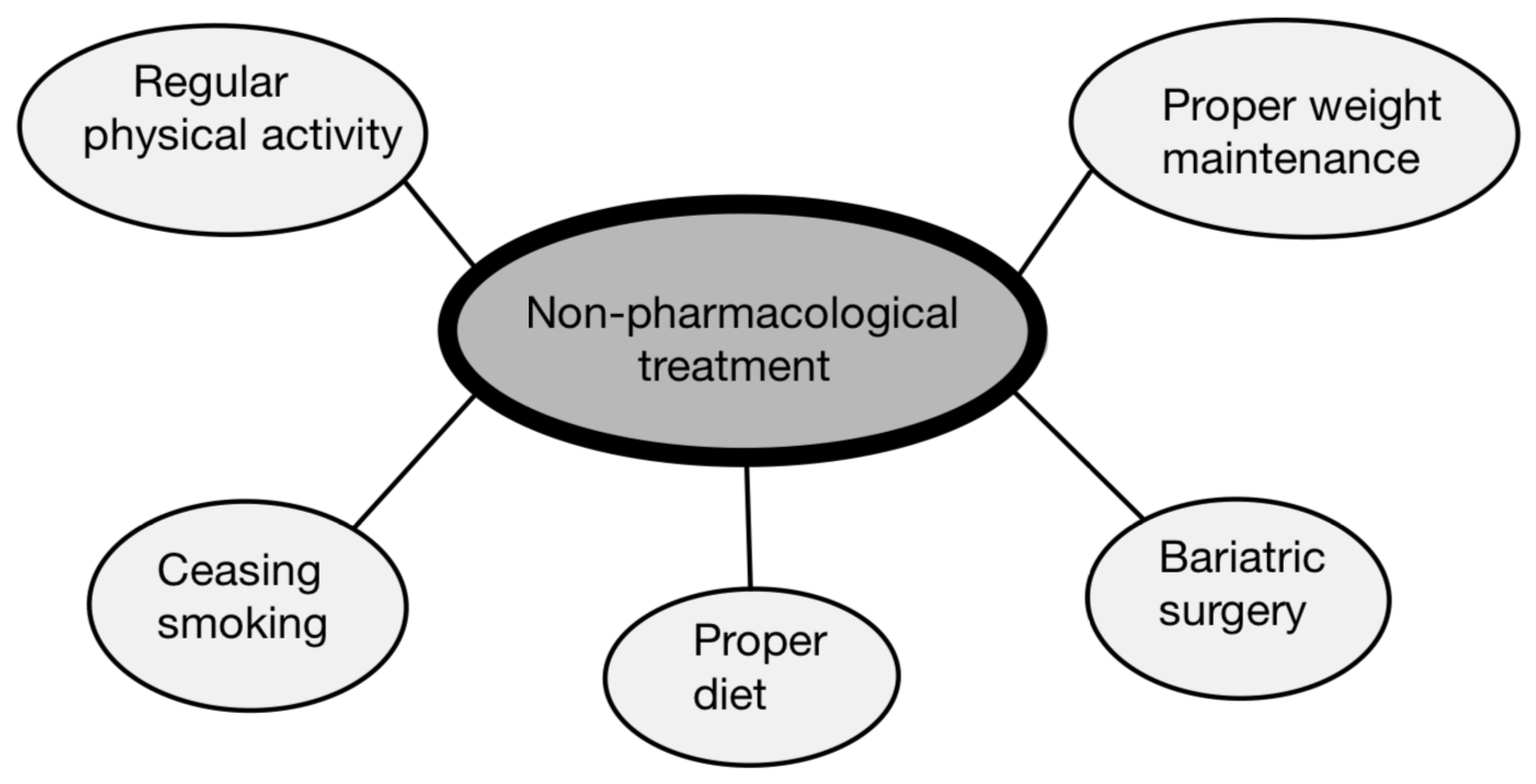
6.1. Non-Pharmacological Treatment
6.2. Hypoglycemic Drugs
6.2.1. Metformin
6.2.2. Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors
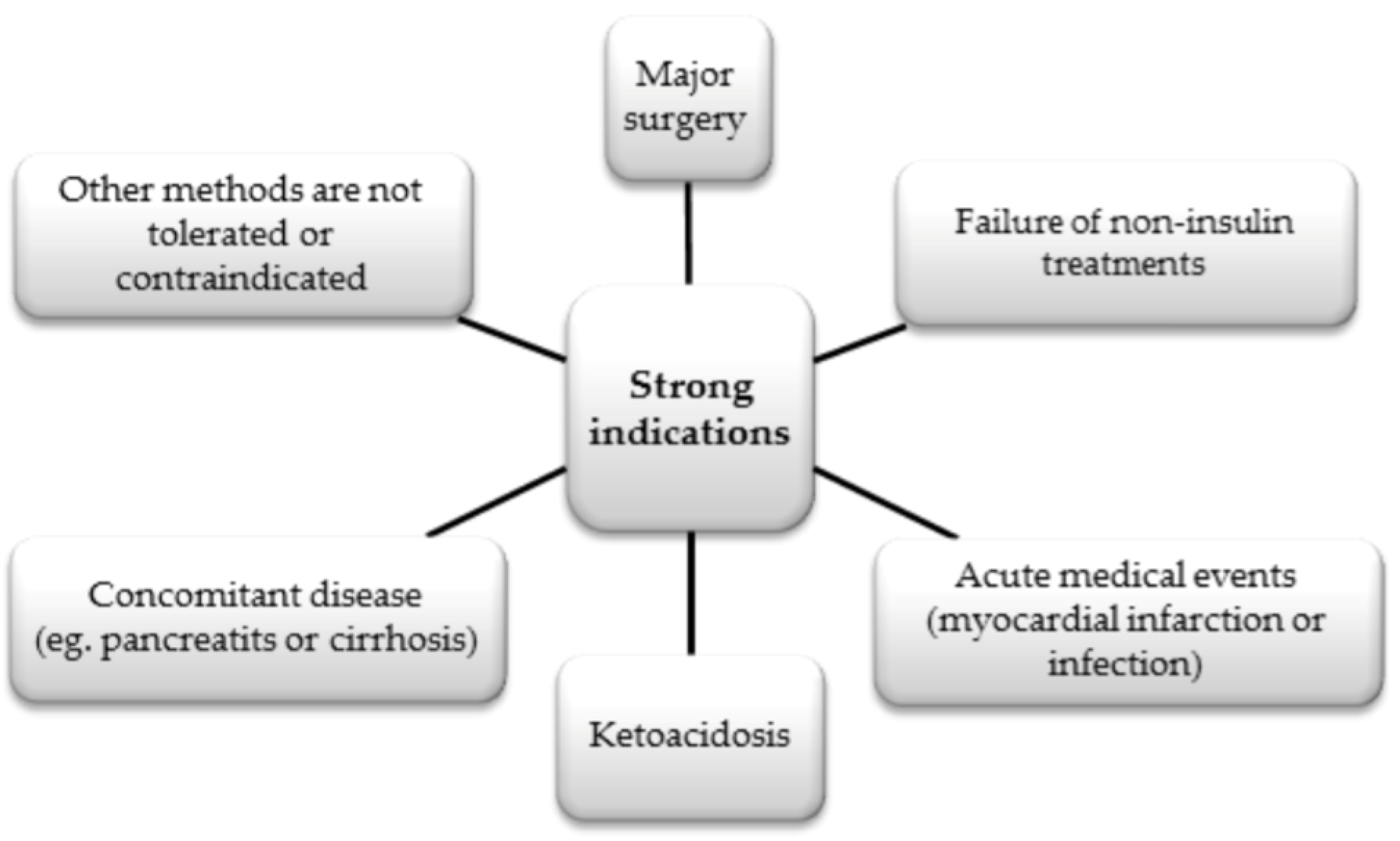
6.2.3. Insulin
6.2.4. Dipeptidylpeptidase-4 Inhibitors
6.2.5. Thiazolidinediones
6.2.6. Dual GIP/GLP-1 Receptor Agonist
6.3. Lipid-Lowering Drugs-Statins
7. The NLRP3 Inflammasome
8. Complications, Prognosis and Novel Therapeutic Options
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brieler, J.; Breeden, M.A.; Tucker, J. Cardiomyopathy: An Overview. Am. Fam. Physician 2017, 96, 640–646. [Google Scholar] [PubMed]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Sardu, C.; Mansueto, G.; Napoli, C.; Paolisso, G. Evidence for human diabetic cardiomyopathy. Acta Diabetol. 2021, 58, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Almorós, A.; Cepeda-Rodrigo, J.M.; Lorenzo, Ó. Diabetic cardiomyopathy. Rev. Clin. Esp. 2022, 222, 100–111. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, C.; Wang, D.W.; Li, H. Hyperglycemic memory in diabetic cardiomyopathy. Front. Med. 2022, 16, 25–38. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Wang, X.; Chen, Y.; Pang, P.; Yang, Q.; Lin, J.; Deng, S.; Wu, S.; Fan, G.; et al. Diabetic cardiomyopathy: Clinical phenotype and practice. Front. Endocrinol. 2022, 13, 1032268. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, J. Diabetic cardiomyopathy: Where we are and where we are going. Korean J. Intern. Med. 2017, 32, 404–421. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Trachanas, K.; Sideris, S.; Aggeli, C.; Poulidakis, E.; Gatzoulis, K.; Tousoulis, D.; Kallikazaros, I. Diabetic cardiomyopathy: From pathophysiology to treatment. Hellenic J. Cardiol. 2014, 55, 411–421. [Google Scholar] [PubMed]
- Hölscher, M.E.; Bode, C.; Bugger, H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int. J. Mol. Sci. 2016, 17, 2136. [Google Scholar] [CrossRef] [PubMed]
- Quinaglia, T.; Oliveira, D.C.; Matos-Souza, J.R.; Sposito, A.C. Diabetic cardiomyopathy: Factual or factoid? Rev. Assoc. Med. Bras. 2019, 65, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Calicchio, F.; Grassi, G.; Mancia, G. Diabetic cardiomyopathy: How can cardiac magnetic resonance help? Acta Diabetol. 2020, 57, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Virk, H.U.H.; Khalid, M.; Lavie, C.J.; Ventura, H.; Mukherjee, D.; Ramu, V.; Bhogal, S.; Kumar, G.; Shanmugasundaram, M.; et al. Diabetic cardiomyopathy—A comprehensive updated review. Prog. Cardiovasc. Dis. 2019, 62, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Athithan, L.; Gulsin, G.S.; McCann, G.P.; Levelt, E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J. Diabetes 2019, 10, 490–510. [Google Scholar] [CrossRef]
- Pan, K.L.; Hsu, Y.C.; Chang, S.T.; Chung, C.M.; Lin, C.L. The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. Int. J. Mol. Sci. 2023, 24, 8604. [Google Scholar] [CrossRef]
- Levelt, E.; Gulsin, G.; Neubauer, S.; McCann, G.P. Mechanisms in Endocrinology: Diabetic cardiomyopathy: Pathophysiology and potential metabolic interventions state of the art review. Eur. J. Endocrinol. 2018, 178, R127–R139. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Pan, J.; Lin, H.; Gu, J. Diabetic cardiomyopathy: A brief summary on lipid toxicity. ESC Heart Fail. 2023, 10, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; McMurray, J.J.V.; Lorenzo-Almorós, A.; Kristensen, S.L.; Sattar, N.; Jhund, P.S.; Petrie, M.C. Diabetic cardiomyopathy. Heart 2019, 105, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Cieluch, A.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options. Int. J. Mol. Sci. 2020, 21, 2852. [Google Scholar] [CrossRef]
- Phang, R.J.; Ritchie, R.H.; Hausenloy, D.J.; Lees, J.G.; Lim, S.Y. Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy. Cardiovasc. Res. 2023, 119, 668–690. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, S.; Chen, Y.; Shen, J.; Zheng, Y.; Liu, X.; Zhu, M.; Meng, G. Protective role of hydrogen sulfide against diabetic cardiomyopathy via alleviating necroptosis. Free Radic. Biol. Med. 2022, 181, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Ritchie, R.; Shaw, J.E.; Kaye, D. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Khan, M.S.; Patel, K.V.; Butler, J.; Tang, W.H.W.; Vaduganathan, M.; Lam, C.S.P.; Verma, S.; McGuire, D.K.; Pandey, A. Prevalence and Prognostic Implications of Diabetes with Cardiomyopathy in Community-Dwelling Adults. J. Am. Coll. Cardiol. 2021, 78, 1587–1598. [Google Scholar] [CrossRef]
- Peng, M.L.; Fu, Y.; Wu, C.W.; Zhang, Y.; Ren, H.; Zhou, S.S. Signaling Pathways Related to Oxidative Stress in Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 907757. [Google Scholar] [CrossRef]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef]
- Seferović, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Paiman, E.H.M.; van Eyk, H.J.; Bizino, M.B.; Dekkers, I.A.; de Heer, P.; Smit, J.W.A.; Jazet, I.M.; Lamb, H.J. Phenotyping diabetic cardiomyopathy in Europeans and South Asians. Cardiovasc. Diabetol. 2019, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Haji, M.; Erqou, S.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Type 1 diabetes and risk of heart failure: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023, 202, 110805. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Kirresh, A.; Ahmad, M. Diabetic Cardiomyopathy Patients Are More Complex and Require a Nuanced Approach. JACC Clin. Electrophysiol. 2020, 6, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Moliner, P.; Mauricio, D. Pathogenesis, Clinical Features and Treatment of Diabetic Cardiomyopathy. Adv. Exp. Med. Biol. 2018, 1067, 197–217. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kleissl-Muir, S.; Owen, A.; Rasmussen, B.; Zinn, C.; Driscoll, A. Effects of a low carbohydrate diet on heart failure symptoms and quality of life in patients with diabetic cardiomyopathy: A randomised controlled trial pilot study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Longo, M.; Scappaticcio, L.; Cirillo, P.; Maio, A.; Carotenuto, R.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Glycemic Control and the Heart: The Tale of Diabetic Cardiomyopathy Continues. Biomolecules 2022, 12, 272. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Tuñón, J.; Orejas, M.; Cortés, M.; Egido, J.; Lorenzo, Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 28. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Prins, J.B.; Marwick, T.H. Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocr. Rev. 2004, 25, 543–567. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Wilson, S.; Mone, P.; Lombardi, A.; Gambardella, J.; Santulli, G. Heart failure in diabetes. Metabolism 2021, 125, 154910. [Google Scholar] [CrossRef]
- Grubić Rotkvić, P.; Planinić, Z.; Liberati Pršo, A.M.; Šikić, J.; Galić, E.; Rotkvić, L. The Mystery of Diabetic Cardiomyopathy: From Early Concepts and Underlying Mechanisms to Novel Therapeutic Possibilities. Int. J. Mol. Sci. 2021, 22, 5973. [Google Scholar] [CrossRef]
- Seo, D.Y.; Ko, J.R.; Jang, J.E.; Kim, T.N.; Youm, J.B.; Kwak, H.B.; Bae, J.H.; Kim, A.H.; Ko, K.S.; Rhee, B.D.; et al. Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 6284. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, E.D.; Lăcătușu, C.M.; Floria, M.; Cazac, G.D.; Onofriescu, A.; Ceasovschih, A.; Crețu, I.; Mihai, B.M.; Șorodoc, L. Association of Inflammatory and Metabolic Biomarkers with Mitral Annular Calcification in Type 2 Diabetes Patients. J. Pers. Med. 2022, 12, 1484. [Google Scholar] [CrossRef]
- Falcão-Pires, I.; Leite-Moreira, A.F. Diabetic cardiomyopathy: Understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail. Rev. 2012, 17, 325–344. [Google Scholar] [CrossRef]
- Ghoreyshi-Hefzabad, S.M.; Jeyaprakash, P.; Gupta, A.; Vo, H.Q.; Pathan, F.; Negishi, K. Three-Dimensional Global Left Ventricular Myocardial Strain Reduced in All Directions in Subclinical Diabetic Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020811. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Varughese, G.I.; Sriraman, R.; Arunagirinathan, G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J. Diabetes 2013, 4, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, E.; Schwenk, R.W.; Glatz, J.F.; Luiken, J.J.; van Eys, G.J. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 219–225. [Google Scholar] [CrossRef]
- Chavali, V.; Tyagi, S.C.; Mishra, P.K. Predictors and prevention of diabetic cardiomyopathy. Diabetes Metab. Syndr. Obes. 2013, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, J.; Zheng, S.; Zhang, L.; Guo, X.; Zhang, J.; Xiao, X. Physical Exercise and Its Protective Effects on Diabetic Cardiomyopathy: What Is the Evidence? Front. Endocrinol. 2018, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Smail, M.; Singh, R.; Bidasee, K.; Howarth, F.; Hanoman, C.; Singh, J. Diabetic cardiomyopathy and the role of regular exercise in preventing the disease: A review. World Heart J. 2018, 9, 319–333. [Google Scholar]
- Mayyas, F.; Alzoubi, K.H. Cardiac effects of cigarette tobacco smoking in rat model of diabetes. Life Sci. 2018, 211, 279–285. [Google Scholar] [CrossRef]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Das, R.J.; Patowary, P.; Kaishap, P.P.; Bordoloi, S.; Das, J.; Sarma, H.; et al. Therapeutic Repurposing of Antidiabetic Drugs in Diabetes-associated Comorbidities. Current Drug Therapy 2024, 19, 178–194. [Google Scholar] [CrossRef]
- Seksaria, S.; Dutta, B.J.; Kaur, M.; Gupta, G.D.; Bodakhe, S.H.; Singh, A. Role of GLP-1 receptor agonist in diabetic cardio-renal disorder: Recent updates of clinical and pre-clinical evidence. Curr. Diabetes Rev. 2023. ahead of print. [Google Scholar] [CrossRef]
- Pavithra, N.; Chaitanya, M.V.N.L. Lipid Lowering Effect of Anti Diabetic Agents-Recent Research. Hypertension 2015, 1, 2. [Google Scholar]
- Vig, H.; Ravinandan, A.P.; Vishwas, H.N.; Tyagi, S.; Rathore, S.; Wal, A.; Wal, P. An Insight into the Pathogenesis of Diabetic Cardiomyopathy along with the Novel Potential Therapeutic Approaches. Curr. Diabetes Rev. 2024, 20, e020523216416. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, A.O. Increasing lipids with risk of worsening cardiac damage in 1595 adolescents: A 7-year longitudinal and mediation study. Atherosclerosis 2024, 389, 117440. [Google Scholar] [CrossRef] [PubMed]
- Bounader, K.; Flécher, E. End-stage heart failure: The future of heart transplant and artificial heart. Presse Med. 2023, 53, 104191. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J.; et al. Revolutionizing Cardiac Care: A Comprehensive Narrative Review of Cardiac Rehabilitation and the Evolution of Cardiovascular Medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Almorós, A.; Cepeda-Rodrigo, J.M.; Lorenzo, Ó. Diabetic cardiomyopathy. Rev. Clin. Esp. 2020. ahead of print. [Google Scholar] [CrossRef]
- Folsom, A.R.; Shah, A.M.; Lutsey, P.L.; Roetker, N.S.; Alonso, A.; Avery, C.L.; Miedema, M.D.; Konety, S.; Chang, P.P.; Solomon, S.D. American Heart Association’s Life’s Simple 7: Avoiding Heart Failure and Preserving Cardiac Structure and Function. Am. J. Med. 2015, 128, 970–976.e2. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Liang, Y.; Pechter, D.; Jones, L.W.; McAlister, F.A.; Clark, A.M. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J. Am. Coll. Cardiol. 2007, 49, 2329–2336. [Google Scholar] [CrossRef]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients with Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Eaton, C.B. Physical Activity in the Treatment and Prevention of Heart Failure: An Update. Curr. Sports Med. Rep. 2021, 20, 410–417. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Kleissl-Muir, S.; Rasmussen, B.; Owen, A.; Zinn, C.; Driscoll, A. Low Carbohydrate Diets for Diabetic Cardiomyopathy: A Hypothesis. Front. Nutr. 2022, 9, 865489. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Ma, X.; Zhang, Z.; Xu, Q.; Liu, C.; Li, B.; Dong, S.; Li, L.; Zhu, J.; et al. Bariatric surgery for diabetic comorbidities: A focus on hepatic, cardiac and renal fibrosis. Front. Pharmacol. 2022, 13, 1016635. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Lee, H.J. Association between persistent smoking after a diagnosis of heart failure and adverse health outcomes: A systematic review and meta-analysis. Tob. Induc. Dis. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef] [PubMed]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Gopal, K.; Chahade, J.J.; Kim, R.; Ussher, J.R. The Impact of Antidiabetic Therapies on Diastolic Dysfunction and Diabetic Cardiomyopathy. Front. Physiol. 2020, 11, 603247. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Fang, X.; Green, C.D.; Das, A. mTORC1 and SGLT2 Inhibitors-A Therapeutic Perspective for Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 15078. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H. SGLT2 Inhibitors: A Novel Player in the Treatment and Prevention of Diabetic Cardiomyopathy. Drug Des. Devel Ther. 2020, 14, 4775–4788. [Google Scholar] [CrossRef]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2021, 26, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Luo, X.; Liao, B.; Li, G.; Feng, J. Insights into SGLT2 inhibitor treatment of diabetic cardiomyopathy: Focus on the mechanisms. Cardiovasc. Diabetol. 2023, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ropero, A.; Badimon, J.J.; Santos-Gallego, C.G. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: The latest developments. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 1287–1302. [Google Scholar] [CrossRef]
- Wilson, L.M.; Castle, J.R. Recent Advances in Insulin Therapy. Diabetes Technol. Ther. 2020, 22, 929–936. [Google Scholar] [CrossRef]
- Cahn, A.; Miccoli, R.; Dardano, A.; Del Prato, S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Freeland, B.; Farber, M.S. A Review of Insulin for the Treatment of Diabetes Mellitus. Home Healthc. Now. 2016, 34, 416–423. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Pham, T.K.; Nguyen, T.H.T.; Yi, J.M.; Kim, G.S.; Yun, H.R.; Kim, H.K.; Won, J.C. Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp. Mol. Med. 2023, 55, 767–778. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, H.E.; Oh, I.S.; Hong, S.M.; Yu, S.H.; Lee, C.B.; Shin, J.Y. Cardiovascular safety of evogliptin in patients with type 2 diabetes: A nationwide cohort study. Diabetes Obes. Metab. 2021, 23, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
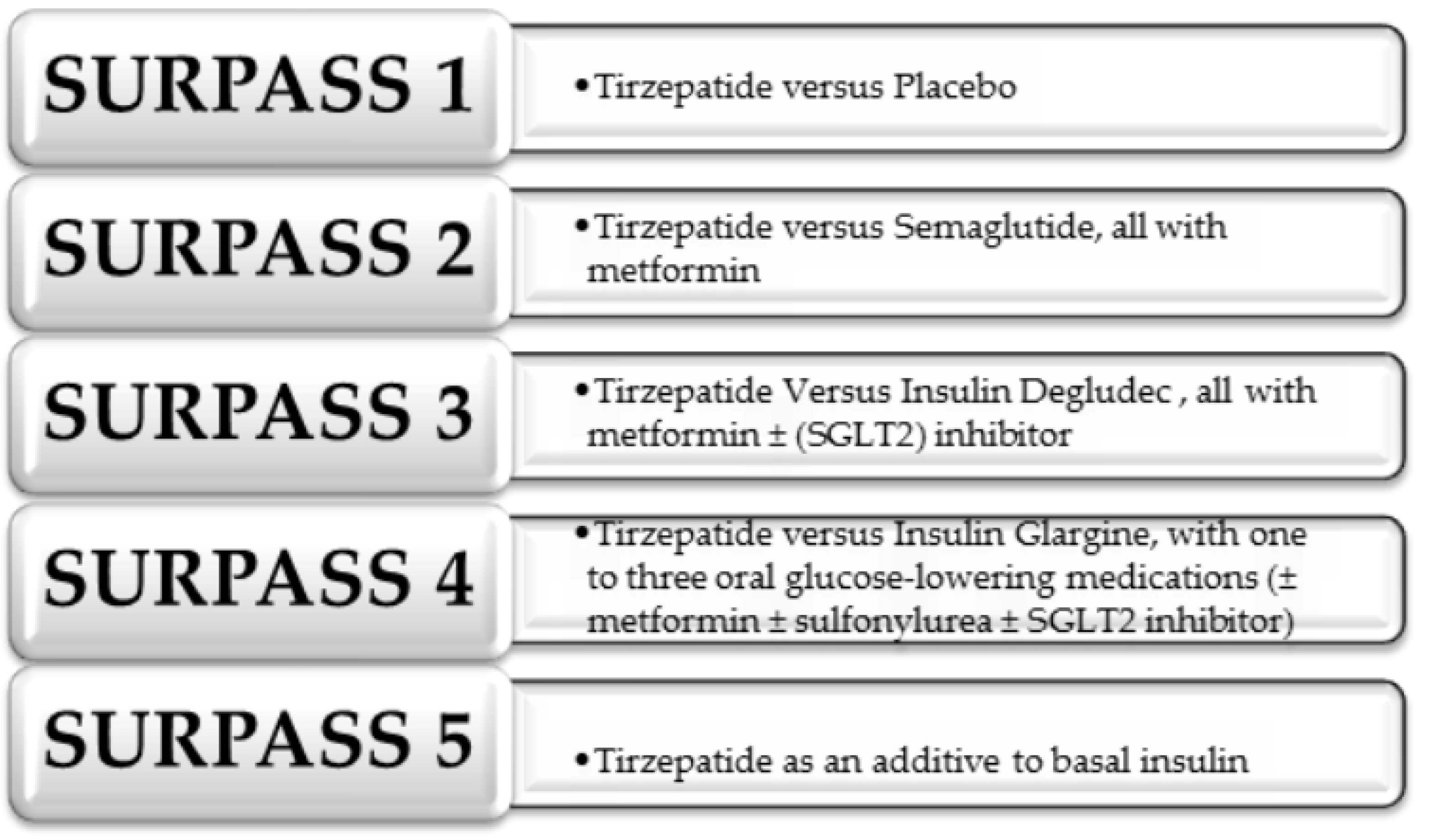
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021, 12, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Vadher, K.; Patel, H.; Mody, R.; Levine, J.A.; Hoog, M.; Cheng, A.Y.; Pantalone, K.M.; Sapin, H. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison. Diabetes Obes. Metab. 2022, 24, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Karagiannis, T.; Avgerinos, I.; Liakos, A.; Del Prato, S.; Matthews, D.R.; Tsapas, A.; Bekiari, E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: A systematic review and meta-analysis. Diabetologia 2022, 65, 1251–1261. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Boye, K.S.; Thieu, V.T.; Sapin, H.; Lee, C.J.; Landó, L.F.; Brown, K.; Bray, R.; Wiese, R.J.; Patel, H.; Rodríguez, Á.; et al. Patient-Reported Outcomes in People with Type 2 Diabetes Receiving Tirzepatide in the SURPASS Clinical Trial Programme. Diabetes Ther. 2023, 14, 1833–1852. [Google Scholar] [CrossRef]
- Lee, C.J.; Mao, H.; Thieu, V.T.; Landó, L.F.; Thomas, M.K. Tirzepatide as Monotherapy Improved Markers of Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes (SURPASS-1). J. Endocr. Soc. 2023, 7, bvad056. [Google Scholar] [CrossRef]
- Boye, K.S.; Sapin, H.; Dong, W.; Williamson, S.; Lee, C.J.; Thieu, V.T. Improved Glycaemic and Weight Management Are Associated with Better Quality of Life in People with Type 2 Diabetes Treated with Tirzepatide. Diabetes Ther. 2023, 14, 1867–1887. [Google Scholar] [CrossRef]
- Dutta, P.; Kumar, Y.; Babu, A.T.; Giri Ravindran, S.; Salam, A.; Rai, B.; Baskar, A.; Dhawan, A.; Jomy, M. Tirzepatide: A Promising Drug for Type 2 Diabetes and beyond. Cureus 2023, 15, e38379. [Google Scholar] [CrossRef]
- Avagimyan, A.; Fogacci, F.; Pogosova, N.; Kakrurskiy, L.; Kogan, E.; Urazova, O.; Kobalava, Z.; Mikhaleva, L.; Vandysheva, R.; Zarina, G.; et al. Diabetic Cardiomyopathy: 2023 Update by the International Multidisciplinary Board of Experts. Curr. Probl. Cardiol. 2024, 49, 102052. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Avagimyan, A.; Popov, S.; Shalnova, S. The Pathophysiological Basis of Diabetic Cardiomyopathy Development. Curr. Probl. Cardiol. 2022, 47, 101156. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Surma, S.; Reiner, Z.; Katsiki, N.; Penson, P.E.; Fras, Z.; Sahebkar, A.; Paneni, F.; Rizzo, M.; Kastelein, J. Personalized management of dyslipidemias in patients with diabetes-it is time for a new approach (2022). Cardiovasc. Diabetol. 2022, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martín, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Picatoste, B.; Ramírez, E.; Egido, J.; Tuñón, J.; Lorenzo, Ó. Targeting metabolic disturbance in the diabetic heart. Cardiovasc. Diabetol. 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Akhmedov, A.; Melina, G.; Mohammed, S.A.; Othman, A.; Ambrosini, S.; Wijnen, W.J.; Sada, L.; Ciavarella, G.M.; Liberale, L.; et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur. Heart J. 2019, 40, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243, Erratum in Circ. Res. 2018, 123, e20. [Google Scholar] [CrossRef]
- Okyay, K. Pleiotropic effects of statins: New evidences. Turk. Kardiyol. Dern. Ars. 2021, 49, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Riesen, W.F. Pleiotrope Effekte von Statinen—Was ist ihre klinische Bedeutung? [Pleiotropic Effects of Statins—What Is Their Clinical Significance?]. Praxis 2022, 110, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Liao, J.K. The Pleiotropic Effects of Statins—From Coronary Artery Disease and Stroke to Atrial Fibrillation and Ventricular Tachyarrhythmia. Curr. Vasc. Pharmacol. 2019, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.A.; Patel, B.; Khattar, R.S.; Malik, R.A. Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment. Clin. Sci. 2004, 107, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Riad, A.; Dhayat, N.; Spillmann, F.; Du, J.; Dhayat, S.; Westermann, D.; Hilfiker-Kleiner, D.; Noutsias, M.; Laufs, U.; et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 2007, 50, 1977–1986. [Google Scholar] [CrossRef]
- Carillion, A.; Feldman, S.; Na, N.; Biais, M.; Carpentier, W.; Birenbaum, A.; Cagnard, N.; Loyer, X.; Bonnefont-Rousselot, D.; Hatem, S.; et al. Atorvastatin reduces β-Adrenergic dysfunction in rats with diabetic cardiomyopathy. PLoS ONE 2017, 12, e0180103. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P. The NLRP3 inflammasome and diabetic cardiomyopathy: Editorial to: “Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model” by Beibei Luo et al. Cardiovasc. Drugs Ther. 2014, 28, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A.; Mahmoud, A.M. Simvastatin Ameliorates Diabetic Cardiomyopathy by Attenuating Oxidative Stress and Inflammation in Rats. Oxid. Med. Cell. Longev. 2017, 2017, 1092015. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323, Erratum in Eur. Heart J. 2020, 41, 4317. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Ouatu, A.; Buliga-Finis, O.N.; Floria, M.; Maranduca, M.A.; Serban, I.L. Portrayal of NLRP3 Inflammasome in Atherosclerosis: Current Knowledge and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 8162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 2018, 22, 4437–4448. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Song, C.; Hu, H.; Yin, K.; Huang, H.; Tang, H. The Role of NLRP3 Inflammasome in Diabetic Cardiomyopathy and Its Therapeutic Implications. Oxid. Med. Cell. Longev. 2022, 2022, 3790721. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S. NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention. Int. J. Mol. Sci. 2021, 22, 13228. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, M.; Zhang, Y.; An, F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE 2014, 9, e104771. [Google Scholar] [CrossRef]
- Wang, G.; Ma, T.Y.; Huang, K.; Zhong, J.H.; Lu, S.J.; Li, J.J. Role of pyroptosis in diabetic cardiomyopathy: An updated review. Front. Endocrinol. 2024, 14, 1322907. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Giannetta, E.; Isidori, A.M.; Galea, N.; Carbone, I.; Mandosi, E.; Vizza, C.D.; Naro, F.; Morano, S.; Fedele, F.; Lenzi, A. Chronic Inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: A randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 2012, 125, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
|---|---|---|---|---|
| Advancement of phase | Early phase | Middle phase | Middle/late phase | Late phase |
| Type of dysfunction | Diastolic dysfunction | Diastolic and systolic dysfunction | Diastolic and systolic dysfunction | Diastolic and systolic dysfunction |
| Anatomical changes | Hypertrophy, increased LV mass | Hypertrophy, increased LV mass and wall thickness, fibrosis, dilatation | Fibrosis, dilatation, microangiopathy | Fibrosis, dilatation, microangiopathy and macroangiopathy |
| Troponins | Not elevated | Not elevated | Elevated during inflammation/ischemia | Elevated during infarction or severe HF |
| HF symptoms in NYHA scale | NYHA I | NYHA II | NYHA II–III | NYHA II–IV |
| Method | Evaluation | Evaluated Criterion |
|---|---|---|
| Echocardiography | Functional | Mitral inflow for diastolic function |
| Tissue Doppler imaging for diastolic and systolic function | ||
| Structural | In two-dimensional echocardiography LV hypertrophy | |
| Cardiac PET | Metabolic and hemodynamic | Myocardial metabolic abnormality and disordered blood flow |
| Cardiac MRI | Functional | Late gadolium-enhancement for diastolic and systolic function |
| Structural | Myocardial steatosis, LV hypertrophy | |
| Metabolic | Magnetic resonance spectroscopy for myocardial TG content and PCr/ATP | |
| Coronary angiography | Functional and hemodynamic | Mean PCWP and LVEDP for diastolic function, microvascular coronary artery disease |
| Serology | Functional | mi-RNA for contractile function |
| BNP for diastolic and systolic function | ||
| Troponin for LV dysfunction | ||
| Structural | MMPs and TIMPs for myocardial fibrosis | |
| MAC biomarkers | Inflammatory and metabolic | Increase in TNF-alpha and HOMA-C peptide levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzioch, E.; Dąbek, B.; Balcerczyk-Lis, M.; Frąk, W.; Fularski, P.; Młynarska, E.; Rysz, J.; Franczyk, B. Diabetic Cardiomyopathy—From Basics through Diagnosis to Treatment. Biomedicines 2024, 12, 765. https://doi.org/10.3390/biomedicines12040765
Radzioch E, Dąbek B, Balcerczyk-Lis M, Frąk W, Fularski P, Młynarska E, Rysz J, Franczyk B. Diabetic Cardiomyopathy—From Basics through Diagnosis to Treatment. Biomedicines. 2024; 12(4):765. https://doi.org/10.3390/biomedicines12040765
Chicago/Turabian StyleRadzioch, Ewa, Bartłomiej Dąbek, Marta Balcerczyk-Lis, Weronika Frąk, Piotr Fularski, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2024. "Diabetic Cardiomyopathy—From Basics through Diagnosis to Treatment" Biomedicines 12, no. 4: 765. https://doi.org/10.3390/biomedicines12040765
APA StyleRadzioch, E., Dąbek, B., Balcerczyk-Lis, M., Frąk, W., Fularski, P., Młynarska, E., Rysz, J., & Franczyk, B. (2024). Diabetic Cardiomyopathy—From Basics through Diagnosis to Treatment. Biomedicines, 12(4), 765. https://doi.org/10.3390/biomedicines12040765