The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions
Abstract
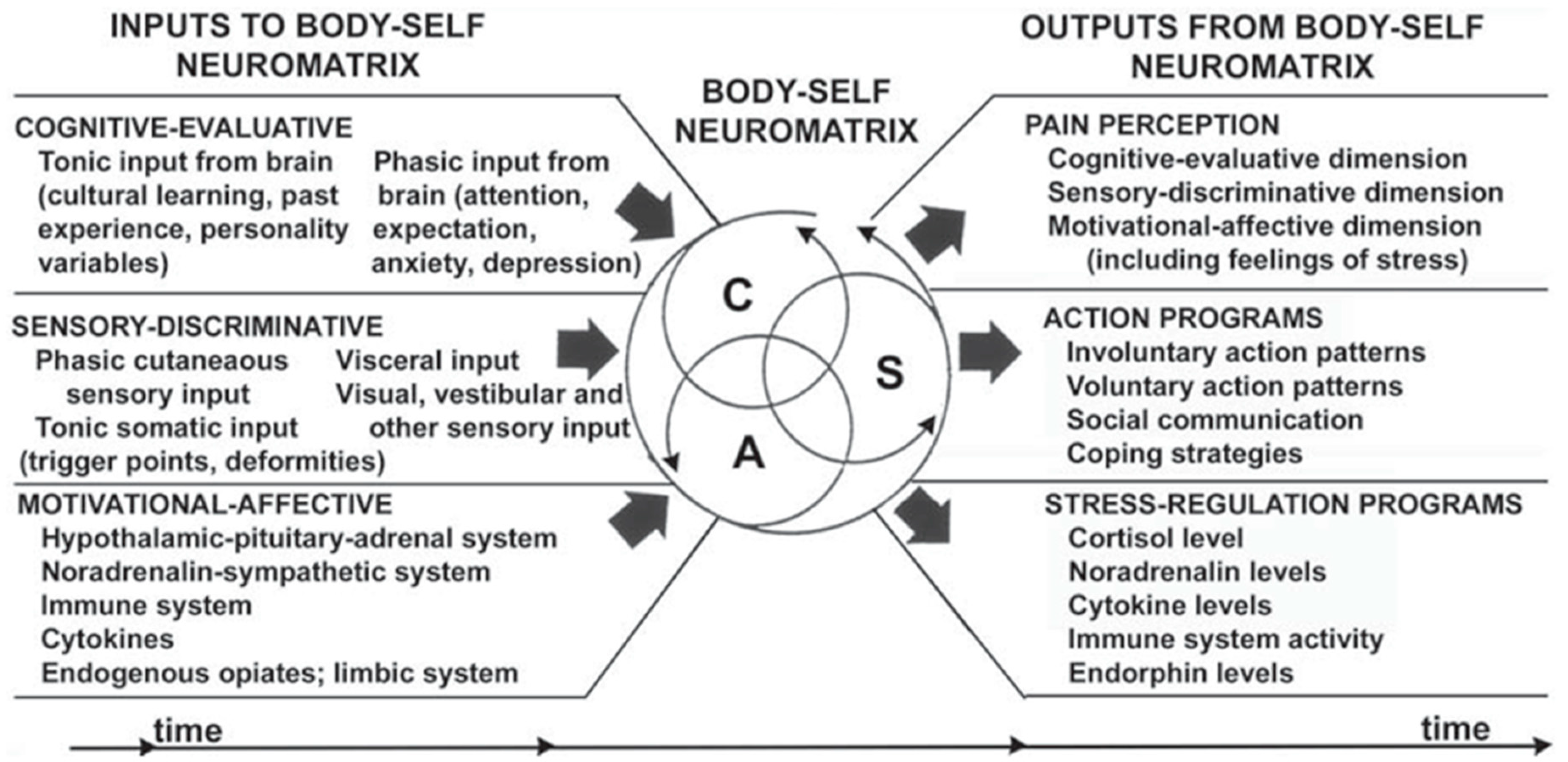
1. Pain as a Sensory, Affective and Cognitive Experience
“Animal affective states are elicited by rewards and punishers or their predictors. A reward is anything for which an animal will work, and a punisher is anything that it will work to escape or avoid. Rewards or the absence of punishers, and associated predictions thereof, induce positive affect. Punishers or the absence of rewards, and associated predictions thereof, induce negative affect. Short-term emotion-like states follow immediately from individual rewarding or punishing events, whilst cumulative experience of events influences longer-term mood-like states” [7].
2. Fibromyalgia
2.1. Fibromyalgia Diagnosis and Characterization
2.2. Fibromyalgia Treatment
2.3. Purpose
3. Preclinical Models of Fibromyalgia
3.1. Reserpine
Reserpine Evaluation
3.2. Acidic Saline
Acidic Saline Evaluation
3.3. Fatigue-Enhanced Muscle Pain
Evaluation of Fatigue-Enhanced Muscle Pain Model
3.4. Subchronic Swim Stress
3.4.1. Forced Swim Test Analyses
3.4.2. Subchronic Swim Evaluation
3.5. Cold Stress
Cold Stress Evaluation
3.6. Sound Stress
Sound Stress Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Reference | Behavioral Measures | Affective | Cognitive | Treatment for Affect/Cognition |
|---|---|---|---|---|
| Reserpine | ||||
| Arora et al. (2011) [38] | MPWT * Tail-flick latency * FST * | * | - | Curcumin * |
| Hubner de Souza et al. (2014) [39] | MPWT * TWL * FST * | * | - | Pha1b ✓ Pregabalin ✓ Diclofenac ✓ |
| Tamano et al. (2016) [40] | MPWT * FST * | * | - | Duloxetine - Milnacipran - |
| Mohammed (2016) [41] | FST * | * | - | Laser irradiation * |
| Shibrya et al. (2017) [42] | FST * | * | - | Laser irradiation * Duloxetine * |
| Moghazy et al. (2017) [47] | FST * | * | - | Cerebrolysin * Citalopram * ATP * |
| Siemian et al. (2019) [43] | MPWT * FST * | * | - | I2R agonists (2-BFI ✓, phenyzoline ✓, CR4056 *) Imipramine * |
| Fusco et al. (2019) [44] | MPWT * TWL * Tail-flick latency * FST * | * | - | Melatonin * Folic acid * Melatonin + folic acid * |
| Dagnino et al. (2020) [45] | MPWT * FST * | * | - | B1R Knockout * B1R antagonist R-715 * Pregabalin ✓ |
| Miyahara et al. (2021) [48] | TWL * FST * | * | - | - |
| Khadrawy et al. (2021) [46] | FST * | * | - | Cur-IONPs ✓ |
| Ogino et al. (2013) [85] | MPT * Locomotor activity ✓ | ✓ | - | 5-HT2C agonists (YM348 ✓, lorcaserin ✓, vabicaserin ✓) 5-HT1A and 5-HT2A agonists (buspirone -, TCB2 -) |
| Xu et al. (2013) [49] | MPWT * Tail-flick latency * TST * FST * Locomotor activity ✓ | * | - | Ferulic acid * Imipramine * |
| Antkiewicz-Michaluk et al. (2014) [50] | FST * Locomotor activity * | * | - | TIQ * 1MeTIQ * |
| Klein et al. (2014) [51] | MPWT * TWL * OFT * TST * FST * | * | - | RvD1 ✓ RvD2 ✓ AT-RvD1 ✓ |
| Blasco-Serra et al. (2015) [73] | MPWT * MPT * NSFT * Locomotor activity ✓ | * | - | - |
| Wu et al. (2015) [79] | PWL * EZM ✓ OFT * | * | - | Electroacupuncture * |
| Klein et al. (2016) [52] | MPWT * TWL * OFT * FST * | * | - | Resveratrol * Rice oil ✓ Resveratrol + rice oil * Pregabalin ✓ |
| Oliveira et al. (2016) [53] | MPWT * TWL * OFT # FST * NSFT * Splash test # | * | - | OMePhSe2 supplemented diet * |
| Khadrawy et al. (2017) [87] | OFT # | # | - | THC # |
| Wu et al. (2017) [22] | MPWT * OFT * EZM * | * | - | Electroacupuncture (EA) * 5-HT resynthesis inhibitor pCPA ✓ EA + pCPA ✓ |
| Favero et al. (2017) [75] | Locomotor activity * | * | - | Melatonin * |
| Blasco-Serra et al. (2017) [37] | NSFT * | * | - | Duloxetine * Desvenlafaxine * |
| Sousa et al. (2018) [54] | TWL * FST * OFT * | * | - | α- (phenylselanyl) acetophenone * Imipramine * |
| Nagakura et al. (2018) [77] | MPWT * Locomotor activity * Catalepsy * | * | - | - |
| Dagnino et al. (2019) [55] | MPWT * TWL * FST * EPM ✓ Rota-rod * Grip strength * Inverted screen * | * | - | NOPr agonist N/OFQ * Selective peptide NOPr antagonist UFP-101 * Pregabalin * |
| Favero et al. (2019) [74] | Locomotor activity * | * | - | Melatonin * |
| Roversi et al. (2019) [56] | FST * Splash test * SPT * | * | - | Tactile stimulus * Imipramine * |
| Brusco et al. (2019) [57] | MPWT * Acetone * Overt nociception * Burrowing * Thigmotaxis * FST * | * | - | B1R Knockout - B2R Knockout - B1R antagonist (DALBk *, SSR240612 ✓) B2R antagonist (Icatibant ✓, FR173657 ✓) Pregabalin ✓ |
| Nagakura et al. (2019) [81] | MPWT * Grimace * | * | - | Gabapentin * Duloxetine * Diclofenac ✓ Buprenorphine ✓ Diazepam ✓ |
| Yao et al. (2020) [58] | MPWT * TWL * Tail-flick latency* RotaRod * FST * OFT * TST * | * | - | Fisetin * Pregabalin * |
| Tanei et al. (2020) [82] | Grimace * | * | - | - |
| Fischer et al. (2020) [59] | MPWT * TWL * Tail-flick latency * Muscle strength * Capsaicin * Thigmotaxis * FST * | * | - | TRPV1 antagonists (α-spinasterol *, SB-366791 *) Pregabalin - Amitriptyline * |
| Brum et al. (2020) [60] | MPWT * Acetone * Grip strength * FST* Catalepsy ✓ Locomotor activity ✓ Thigmotaxis * | * | - | CoQ10 * |
| Kang et al. (2020) [61] | MPWT * RotaRod FST * OFT * | * | - | Yukmijihwang-won (YJ-01 *, YJ-06 *) Gabapentin * Fluoxetine * Fluoxetine + YJ-01 * Fluoxetine + YJ-06 * 2,2,2-tribromoethanol - |
| El-Marasy et al. (2021) [62] | OFT * FST * | * | - | Cerebrolysin * Fluoxetine * |
| Salat & Furgala-Wojas (2021) [78] | MPWT * TWL ✓ FST * Four-plate test * Locomotor activity * Grip strength ✓ | * | - | Vortioxetine * Ropinirole * |
| Mendes et al. (2021) [63] | MPWT * TWL * SPT * Locomotor activity * FST * | * | - | Pha1b * Pregabalin ✓ Diclofenac ✓ |
| Ferrarini et al. (2021) [72] | MPWT * Acetone * Grimace * TST ✓ OFT ✓ | * | - | Strength exercise * Aerobic exercise * Pregabalin * |
| Elkholy et al. (2021) [86] | TWL * OFT # | # | - | Encapsulated cationic liposome (beta-carotene #, lutein #) |
| Martins et al. (2022) [64] | MPWT * Tail-flick latency * FST * Splash test ✓ EPM ✓ OFT ✓ | * | - | Pramipexole * |
| Álvarez-Pérez et al. (2022) [65] | MPWT * TWL * OFT * Dark/light box * FST * | * | - | Pregabalin - |
| Kuzay et al. (2022) [66] | FST * TST * NSFT * SPT * | * | - | Citalopram * Thymoquinone (TQ) * Citalopram + TQ * |
| Zhao et al. (2022) [67] | MPWT * TWL * SPT * OFT * FST * | * | - | - |
| Souza et al. (2013) [83] | Olfactory fear conditioning * Step-down inhibitory avoidance * Olfactory discrimination ✓ OFT ✓ EPM ✓ | ✓ | * | Environmental enrichment * |
| Kaur et al. (2019) [68] | MPWT * PAM * Inclined plane * FST * OFT * MWM * Passive avoidance * EPM * | * | * | Gabapentin * Imperatorin * |
| Singh et al. (2020) [69] | MPWT * PAM * OFT * FST * MWM * | * | * | Gabapentin * Esculetin * |
| Kaur et al. (2020) [76] | MPWT * PAM * Inclined plane * OFT * MWM * Passive avoidance * | * | * | Angelica archangelica * Gabapentin * |
| Singh et al. (2021) [70] | MPWT * PAM * FST * MWM * | * | * | Gabapentin * Daphnetin * |
| Hernandez-Leon et al. (2019) [80] | MPT * MPWT * Acetone * Sleep (W *, SWS-I *, SWS-II *, REM *) | # | # | Fluoxetine * |
| Murai et al. (2019) [88] | MPT * Sleep (NREM #, REM #, sleep interruptions #) | # | # | GABAb receptor positive allosteric modulator (ASP8062 #) Baclofen # GABAb antagonist (CGP55845) – |
| Blasco-Serra et al. (2020) [89] | Sleep (SWS-I *, SWS-II *, REM *) Atonia * | # | # | - |
| Acidic Saline | ||||
| Liu et al. (2014) [97] | MPWT * EPM * OFT * SCT * SPT * FST * | * | - | - |
| Liu et al. (2017) [93] | MPWT * EPM * SPT * FST * | * | - | Pregabalin * Duloxetine * Diazepam * |
| Murasawa et al. (2020) [95] | MPWT * OFT * EPM * | * | - | Mirogabalin * |
| Lottering & Lin (2021) [98] | MPWT * TWL * OFT * FST * | * | - | TRPV1 Knockout * Electroacupuncture * |
| Wang et al. (2021) [96] | MPWT * EPM * Burying * | * | - | Mossy cell activation * |
| Álvarez-Pérez et al. (2022) [65] | MPWT * TWL * OFT ✓ Dark/light box ✓ FST * | * | - | Pregabalin * |
| Pratt et al. (2013) [99] | PEAP ✓ Learned avoidance ✓ Avoidance of voluntary activity ✓ | ✓ | ✓ | - |
| Heimfarth et al. (2020) [94] | MPWT * Grip strength ✓ OFT ✓ NOR # EPM * | * | # | Myrtenol ✓ Myrtenol + β-ciclodextrin (βCD) * Pregabalin # |
| Murasawa et al. (2021) [100] | MPWT * Y-maze * NOR * MWM * Step-through passive avoidance* | - | * | Mirogabalin * |
| Sutton & Opp (2014a) [101] | MPWT * Sleep (transitions*, W ✓, NREM ✓, REM *) | # | # | - |
| Sutton & Opp (2014b) [103] | MPWT * Sleep fragmentation * | # | # | - |
| Wei et al. (2019) [102] | Sleep (W *, NREM1 *, NREM2 *, transition sleep *, REM ✓) | # | # | - |
| Fatigue-Enhanced Muscle Pain | ||||
| Pratt et al. (2013) [99] | PEAP ✓ Learned avoidance ✓ Avoidance of voluntary activity ✓ | ✓ | ✓ | - |
| Subchronic Swim Stress | ||||
| Dhir & Kulkarni (2008) [120] | Tail-flick latency * RotaRod * FST (model induction) * EPM ✓ Locomotor activity # Mirror chamber * | # | - | Venlafaxine * |
| Sachdeva et al. (2010) [115] | Tail-flick latency * FST (model induction) * Fatigue (grooming initiation) * Mirror chamber * EPM * | * | - | Epigallocatechin gallate (EGCG) * |
| Trivedi & Sharma (2011) [117] | Tail-flick latency * FST (model induction) * Locomotion * RotaRod * Mirror chamber * EPM ✓ | * | - | Glycyrrhiza glabra* Fluoxetine* |
| Saha (2011) [116] | Escape behavior * OFT ✓ EPM ✓ | * | - | - |
| Li et al. (2017) [113] | MPWT * Inclined plane ✓ FST * SPT * EPM ✓ | * | - | Imipramine * Ifenprodil * |
| Nazeri et al. (2018) [114] | Orofacial formalin ✓ test EPM * RotaRod ✓ Wire grip ✓ | * | - | - |
| Chen et al. (2018) [112] | SPT * FST * TST * Inclined plane ✓ | * | - | - |
| Zhang et al. (2020) [127] | MPWT * FST (model induction) * TWL * TST * | * | - | - |
| Xue et al. (2020) [121] | MPWT * TWL * EPM * OFT * SPT ✓ FST (model induction) * | # | # | - |
| Bagues et al. (2022) [111] | Orofacial formalin ✓ Paw formalin * Hypertonic saline stimulation * EPM ✓ Locomotor activity * | * | - | Morphine * |
| Nazeri et al. (2014; 2016) [118,119] | TWL * Tail-flick latency * Passive avoidance * OFT ✓ | ✓ | * | L-Arginine * L-NAME * |
| Cold Stress | ||||
| Nishiyori et al. (2011) [33] | MPWT * TWL * TST ✓ EPM ✓ Locomotor activity ✓ | ✓ | - | Milnacipran - Amitriptyline - Mianserin - Paroxetine - |
| Montserrat-de la Paz et al. (2015) [138] | MPWT * TWL * Tail-flick latency * Hole-board test * Traction test * Evasion test * | * | - | Gabapentin * |
| Lee et al. (2018) [137] | MPWT * TWL * Tail-flick latency * TST * | # | - | Valeriana fauriei * |
| Nasu et al. (2019) [139] | FST * | * | - | Neurotropin * Imipramine * |
| Sound Stress | ||||
| Green et al. (2011) [143] | Visceral hyperalgesia * Spinal hyperalgesia * EPM * | * | - | - |
| Golzio dos Santos et al. (2020) [145] | EPM ✓ OFT # | # | - | Riparin III |
| Hung et al. (2020) [144] | MPWT * MWT * TWL * OFT * EPM * Grip force * | * | - | Pregabalin – Morphine - Diclofenac - |
| Viero et al. (2022) [146] | MPWT * Periorbital thresholds * Grimace * OFT * | * | - | CGRP receptor antagonist (olcegepant; BIBN4096BS *) |
| Model | Methods |
|---|---|
| Reserpine/Biogenic Amine Depletion [29] | Subcutaneous reserpine (1 mg/kg) daily, for 3 consecutive days |
| Acidic saline [30] | Two intramuscular injections of 4.0 pH saline into the gastrocnemius, 5 days apart |
| Fatigue-enhanced muscle pain [31,32] | Fatigue protocol (wheel running or direct muscle stimulation) followed by muscular insult (0.03% carrageenan, or 2 injections of 5.0 pH saline, 5 days apart) |
| Subchronic Swim Stress [36] | Forced swimming in cylindrical tube for 3 consecutive days (10 min on day 1, 20 min on days 2 and 3) |
| Cold Stress [33] | Overnight exposure to cold (−3 °C) for 15 h, then alterations in temperature every 30 min between room temperature (22 °C) and cold temperature (−3 °C) for 5 days |
| Sound Stress [34,35] | 5 or 10 s 105 dB tone of mixed frequencies (11–19 kHz), every minute at random times, for a period of 30 min on days 1, 3, and 4. |
References
- International Association for the Study of Pain: IASP Announces Revised Definition of Pain. Available online: https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/ (accessed on 6 November 2023).
- Lipowski, Z.J. Chronic idiopathic pain syndrome. Ann. Med. 1990, 22, 213–217. [Google Scholar] [CrossRef]
- Melzack, R.; Casey, K.L. Sensory, motivational, and central control determinants of pain: A new conceptual model. Ski. Senses 1968, 1, 423–443. [Google Scholar]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82, S121–S126. [Google Scholar] [CrossRef]
- Melzack, R. Pain and the Neuromatrix in the Brain. J. Dent. Educ. 2001, 65, 1378–1382. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/patients/drug-development-process/step-2-preclinical-research (accessed on 7 August 2023).
- Mendl, M.; Paul, E.S. Animal affect and decision-making. Neurosci. Biobehav. Rev. 2020, 112, 144–163. [Google Scholar] [CrossRef]
- Salcido, C.A.; Geltmeier, M.K.; Fuchs, P.N. Pain and decision-making: Interrelated through homeostasis. Open Pain J. 2018, 11, 31–40. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/arthritis/basics/fibromyalgia.htm (accessed on 7 August 2023).
- Lacasse, A.; Bourgault, P.; Choinière, M. Fibromyalgia-related costs and loss of productivity: A substantial societal burden. BMC Musculoskelet. Disord. 2016, 17, 168. [Google Scholar] [CrossRef]
- The National Fibromyalgia Association. Available online: https://www.fmaware.org/fibromyalgia-prevalence/ (accessed on 7 August 2023).
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Singh, G.; Kaul, S. Anxiety and depression are common in fibromyalgia patients and correlate with symptom severity score. Indian J. Rheumatol. 2018, 13, 168. [Google Scholar] [CrossRef]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Kravitz, H.M.; Katz, R.S. Fibrofog and fibromyalgia: A narrative review and implications for clinical practice. Rheumatol. Int. 2015, 35, 1115–1125. [Google Scholar] [CrossRef]
- Mease, P.; Arnold, L.M.; Choy, E.H.; Clauw, D.J.; Crofford, L.; Glass, J.M.; Martin, S.A.; Morea, J.; Simon, L.; Strand, V.; et al. Fibromyalgia syndrome module at OMERACT 9: Domain construct. J. Rheumatol. 2009, 36, 2318–2329. [Google Scholar] [CrossRef]
- Walker, M.P. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 2009, 1156, 168–197. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chang, L.Y.; Lee, H.C.; Fang, S.C.; Tsai, P.S. Sleep disturbances in fibromyalgia: A meta-analysis of case-control studies. J. Psychosom. Res. 2017, 96, 89–97. [Google Scholar] [CrossRef]
- Forte, M.L.; Butler, M.; Andrade, K.E.; Vincent, A.; Schousboe, J.T.; Kane, R.L. Treatments for Fibromyalgia in Adult Subgroups; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015.
- American College of Rheumatology. Available online: https://rheumatology.org/patients/fibromyalgia (accessed on 7 August 2023).
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Farag, H.M.; Yunusa, I.; Goswami, H.; Sultan, I.; Doucette, J.A.; Eguale, T. Comparison of Amitriptyline and US Food and Drug Administration–Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2022, 5, e221293. [Google Scholar] [CrossRef]
- Skaer, T.L. Fibromyalgia: Disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics 2014, 32, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Garijo, I.; Jimenez-Del-Barrio, S.; Mingo-Gomez, T.; Medrano-de-la-Fuente, R.; Ceballos-Laita, L. Effectiveness of non-pharmacological conservative therapies in adults with fibromyalgia: A systematic review of high-quality clinical trials. J. Back Musculoskelet. Rehabil. 2022, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef]
- Sluka, K.A.; Kalra, A.; Moore, S.A. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2001, 24, 37–46. [Google Scholar] [CrossRef]
- Yokoyama, T.; Lisi, T.L.; Moore, S.A.; Sluka, K.A. Muscle fatigue increases the probability of developing hyperalgesia in mice. J. Pain 2007, 8, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Rasmussen, L.A. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain 2010, 148, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Nishiyori, M.; Uchida, H.; Nagai, J.; Araki, K.; Mukae, T.; Kishioka, S.; Ueda, H. Permanent relief from intermittent cold stress-induced fibromyalgia-like abnormal pain by repeated intrathecal administration of antidepressants. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef]
- Khasar, S.G.; Dina, O.A.; Green, P.G.; Levine, J.D. Sound stress–induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J. Pain 2009, 10, 1073–1077. [Google Scholar] [CrossRef]
- Khasar, S.G.; Green, P.G.; Levine, J.D. Repeated sound stress enhances inflammatory pain in the rat. Pain 2005, 116, 79–86. [Google Scholar] [CrossRef]
- Quintero, L.; Moreno, M.; Avila, C.; Arcaya, J.; Maixner, W.; Suarez-Roca, H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacology Biochem. Behav. 2000, 67, 449–458. [Google Scholar] [CrossRef]
- Blasco-Serra, A.; González-Soler, E.M.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. A standardization of the novelty-suppressed feeding test protocol in rats. Neurosci. Lett. 2017, 658, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Kuhad, A.; Tiwari, V.; Chopra, K. Curcumin ameliorates reserpine-induced pain–depression dyad: Behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology 2011, 36, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Hubner de Souza, A.; da Costa Lopes, A.M.; Castro, C.J., Jr.; Pereira, E.M.R.; Klein, C.P.; da Silva, C.A., Jr.; da Silva, J.F.; Ferreira, J.; Gomez, M.V. The effects of Phα1β, a spider toxin, calcium channel blocker, in a mouse fibromyalgia model. Toxicon 2014, 81, 37–42. [Google Scholar] [CrossRef]
- Tamano, R.; Ishida, M.; Asaki, T.; Hasegawa, M.; Shinohara, S. Effect of spinal monoaminergic neuronal system dysfunction on pain threshold in rats, and the analgesic effect of serotonin and norepinephrine reuptake inhibitors. Neurosci. Lett. 2016, 615, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S. Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med. Sci. 2016, 31, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Shibrya, E.E.; Radwan, R.R.; Abd El Fattah, M.A.; Shabaan, E.A.; Kenawy, S.A. Evidences for amelioration of reserpine-induced fibromyalgia in rat by low dose of gamma irradiation and duloxetine. Int. J. Radiat. Biol. 2017, 93, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Siemian, J.N.; Shang, L.; Seaman, R.W., Jr.; Zhu, Q.; Zhang, Y.; Li, J.X. Effects of imidazoline I2 receptor agonists on reserpine-induced hyperalgesia and depressive-like behavior in rats. Behav. Pharmacol. 2019, 30, 429. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin plus folic acid treatment ameliorates reserpine-induced fibromyalgia: An evaluation of pain, oxidative stress, and inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Azevedo, V.M.; Oliboni, P.; Campos, M.M.; de Sousa Maciel, I. Kinin B1 receptor is involved in mechanical nociception in a fibromyalgia-like model in mice. J. Reprod. Neurosci. 2020, 1, 1431. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef]
- Moghazy, A.M.; El Moneim Saad, A.A.; Haridy, S.A. The potential antidepressant effect of adenosine triphosphate and cerebrolysin on reserpine induced depression in male rats. Int. J. Adv. Res. 2019, 7, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Nishimaru, H.; Matsumoto, J.; Setogawa, T.; Taguchi, T.; Ono, T.; Nishijo, H. Involvement of parvalbumin-positive neurons in the development of hyperalgesia in a mouse model of fibromyalgia. Front. Pain Res. 2021, 2, 627860. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Shao, T.; Ruan, L.; Wang, L.; Sun, J.; Li, J.; Zhu, X.; O’Donnell, J.M.; Pan, J. Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: Behavioral and neurobiological analyses. Metab. Brain Dis. 2013, 28, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz-Michaluk, L.; Wąsik, A.; Możdżeń, E.; Romańska, I.; Michaluk, J. Antidepressant-like effect of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: Behavioral and neurochemical studies in the rat. Neurotox. Res. 2014, 26, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Sperotto, N.D.; Maciel, I.S.; Leite, C.E.; Souza, A.H.; Campos, M.M. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 2014, 86, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Rodrigues, M.C.; Binda, N.; Montijo, D.D.; Gomez, M.V.; Souto, A.A.; de Souza, A.H. Coadministration of Resveratrol and Rice Oil Mitigates Nociception and Oxidative State in a Mouse Fibromyalgia-Like Model. Pain Res. Treat. 2016, 2016, 3191638. [Google Scholar] [CrossRef]
- Oliveira, C.E.S.; Sari, M.H.M.M.; Zborowski, V.A.; Prado, V.C.; Nogueira, C.W.; Zeni, G. Pain-depression dyad induced by reserpine is relieved by p, p′-methoxyl-diphenyl diselenide in rats. Eur. J. Pharmacol. 2016, 791, 794–802. [Google Scholar] [CrossRef]
- Sousa, F.S.S.; Birmann, P.T.; Baldinotti, R.; Fronza, M.G.; Balaguez, R.; Alves, D.; Bruning, C.A.; Savegnago, L. α-(phenylselanyl) acetophenone mitigates reserpine-induced pain–depression dyad: Behavioral, biochemical and molecular docking evidences. Brain Res. Bull. 2018, 142, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; da Silva, R.B.M.; Chagastelles, P.C.; Pereira, T.C.B.; Venturin, G.T.; Greggio, S.; Costa da Costa, J.; Bogo, M.R.; Campos, M.M. Nociceptin/orphanin FQ receptor modulates painful and fatigue symptoms in a mouse model of fibromyalgia. Pain 2019, 160, 1383–1401. [Google Scholar] [CrossRef]
- Roversi, K.; de David Antoniazzi, C.T.; Milanesi, L.H.; Rosa, H.Z.; Kronbauer, M.; Rossato, D.R.; Duarte, T.; Duarte, M.M.; Burger, M.E. Tactile stimulation on adulthood modifies the HPA axis, neurotrophic factors, and GFAP signaling reverting depression-like behavior in female rats. Mol. Neurobiol. 2019, 56, 6239–6250. [Google Scholar] [CrossRef]
- Brusco, I.; Justino, A.B.; Silva, C.R.; Fischer, S.; Cunha, T.M.; Scussel, R.; Machado-de-Avila, R.A.; Ferreira, J.; Oliveira, S.M. Kinins and their B1 and B2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem. Pharmacol. 2019, 168, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, L.; Kandhare, A.D.; Mukherjee Kandhare, A.A.; Bodhankar, S.L. Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp. Ther. Med. 2020, 19, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.P.M.; Brusco, I.; Brum, E.S.; Fialho, M.F.P.; Camponogara, C.; Scussel, R.; Machado-De-Ávila, R.A.; Trevisan, G.; Oliveira, S.M. Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem. Int. 2020, 134, 104673. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brum, E.; Fialho, M.F.P.; Fischer, S.P.M.; Hartmann, D.D.; Gonçalves, D.F.; Scussel, R.; Machado-de-Ávila, R.A.; Dalla Corte, C.L.; Soares, F.A.A.; Oliveira, S.M. Relevance of mitochondrial dysfunction in the reserpine-induced experimental fibromyalgia model. Mol. Neurobiol. 2020, 57, 4202–4217. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lee, J.; Choi, J.G.; Kim, J.; Kim, J.Y.; Park, J.B.; Jung, I.C.; Kim, H.W. Traditional Herbal Medicine Yukmijihwang-won Alleviates Reserpine-Induced Pain and Depression-Like Behavior in Mice. J. Orient. Neuropsychiatry 2020, 31, 269–278. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; El Awdan, S.A.; Hassan, A.; Ahmed-Farid, O.A.; Ogaly, H.A. Anti-depressant effect of cerebrolysin in reserpine-induced depression in rats: Behavioral, biochemical, molecular and immunohistochemical evidence. Chem.-Biol. Interact. 2021, 334, 109329. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.P.G.; Dos Santos, D.C.; Rezende, M.J.S.; Ferreira, L.C.A.; Rigo, F.K.; de Castro Junior, C.J.; Gomez, M.V. Effects of intravenous administration of recombinant Phα1β toxin in a mouse model of fibromyalgia. Toxicon 2021, 195, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.; Paes, R.S.; Baldasso, G.M.; Ferrarini, E.G.; Scussel, R.; Zaccaron, R.P.; Machado-de-Ávila, R.A.; Silveira, P.C.L.; Dutra, R.C. Pramipexole, a dopamine D3/D2 receptor-preferring agonist, attenuates reserpine-induced fibromyalgia-like model in mice. Neural Regen. Res. 2022, 17, 450. [Google Scholar] [CrossRef]
- Álvarez-Pérez, B.; Deulofeu, M.; Homs, J.; Merlos, M.; Vela, J.M.; Verdú, E.; Boadas-Vaello, P. Long-lasting reflexive and nonreflexive pain responses in two mouse models of fibromyalgia-like condition. Sci. Rep. 2022, 12, 9719. [Google Scholar] [CrossRef]
- Kuzay, D.; Dileköz, E.; Özer, Ç. Effects of thymoquinone in a rat model of reserpine-induced depression. Braz. J. Pharm. Sci. 2022, 58, e19847. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, W.; Lu, Y.; Gao, X.; Wang, A.; Zhang, S.; Du, Y.; Wang, Y.; Li, L. Alterations of monoamine neurotransmitters, HPA-axis hormones, and inflammation cytokines in reserpine-induced hyperalgesia and depression comorbidity rat model. BMC Psychiatry 2022, 22, 419. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, L.; Singh, N.; Bhatti, M.S.; Bhatti, R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: Role of NMDA/NFkB mediated downstream signaling. Biochem. Pharmacol. 2019, 166, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kaur, A.; Garg, S.; Singh, A.P.; Bhatti, R. Protective effect of esculetin, natural coumarin in mice model of fibromyalgia: Targeting pro-inflammatory cytokines and MAO-A. Neurochem. Res. 2020, 45, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kaur, A.; Singh, A.P.; Bhatti, R. Daphnetin, a natural coumarin averts reserpine-induced fibromyalgia in mice: Modulation of MAO-A. Exp. Brain Res. 2021, 239, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- West, A.P. Neurobehavioral studies of forced swimming: The role of learning and memory in the forced swim test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1990, 14, 863–877, IN3–IN4. [Google Scholar] [CrossRef]
- Ferrarini, E.G.; Gonçalves, E.C.D.; Menegasso, J.F.; Rabelo, B.D.; Felipetti, F.A.; Dutra, R.C. Exercise reduces pain and deleterious histological effects in fibromyalgia-like model. Neuroscience 2021, 465, 46–59. [Google Scholar] [CrossRef]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; Gonzalez-Soler, E.M.; Martinez-Exposito, F.; Blasco-Ausina, C.; Martinez-Bellver, S.; Cervera-Fern, A.; Teruel-Marti, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [CrossRef]
- Favero, G.; Bonomini, F.; Franco, C.; Rezzani, R. Mitochondrial dysfunction in skeletal muscle of a fibromyalgia model: The potential benefits of melatonin. Int. J. Mol. Sci. 2019, 20, 765. [Google Scholar] [CrossRef]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral supplementation of melatonin protects against fibromyalgia-related skeletal muscle alterations in reserpine-induced myalgia rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Bhatti, M.S.; Bhatti, R. Optimization of extraction conditions of Angelica archangelica extract and activity evaluation in experimental fibromyalgia. J. Food Sci. 2020, 85, 3700–3710. [Google Scholar] [CrossRef]
- Nagakura, Y.; Ohsaka, N.; Azuma, R.; Takahashi, S.; Takebayashi, Y.; Kawasaki, S.; Murai, S.; Miwa, M.; Saito, H. Monoamine system disruption induces functional somatic syndromes associated symptomatology in mice. Physiol. Behav. 2018, 194, 505–514. [Google Scholar] [CrossRef]
- Sałat, K.; Furgała-Wojas, A. Serotonergic neurotransmission system modulator, vortioxetine, and dopaminergic D2/D3 receptor agonist, ropinirole, attenuate fibromyalgia-like symptoms in mice. Molecules 2021, 26, 2398. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Jiang, Y.L.; He, X.F.; Zhao, X.Y.; Shao, X.M.; Du, J.Y.; Fang, J.Q. Effects of electroacupuncture with dominant frequency at SP 6 and ST 36 based on meridian theory on pain-depression dyad in rats. Evid. Based Complement. Altern. Med. 2015, 2015, 732845. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; Fernández-Guasti, A.; Martínez, A.; Pellicer, F.; González-Trujano, M.E. Sleep architecture is altered in the reserpine-induced fibromyalgia model in ovariectomized rats. Behav. Brain Res. 2019, 364, 383–392. [Google Scholar] [CrossRef]
- Nagakura, Y.; Miwa, M.; Yoshida, M.; Miura, R.; Tanei, S.; Tsuji, M.; Takeda, H. Spontaneous pain-associated facial expression and efficacy of clinically used drugs in the reserpine-induced rat model of fibromyalgia. Eur. J. Pharmacol. 2019, 864, 172716. [Google Scholar] [CrossRef] [PubMed]
- Tanei, S.; Miwa, M.; Yoshida, M.; Miura, R.; Nagakura, Y. The method simulating spontaneous pain in patients with nociplastic pain using rats with fibromyalgia-like condition. MethodsX 2020, 7, 100826. [Google Scholar] [CrossRef]
- Souza, R.R.; França, S.L.; Bessa, M.M.; Takahashi, R.N. The usefulness of olfactory fear conditioning for the study of early emotional and cognitive impairment in reserpine model. Behav. Process. 2013, 100, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Jiang, Y.L.; He, X.F.; Zhao, X.Y.; Shao, X.M.; Sun, J.; Shen, Z.; Shou, S.Y.; Wei, J.J.; Ye, J.Y.; et al. 5HT in the dorsal raphe nucleus is involved in the effects of 100Hz electroacupuncture on the pain-depression dyad in rats. Exp. Ther. Med. 2017, 14, 107–114. [Google Scholar] [CrossRef]
- Ogino, S.; Nagakura, Y.; Tsukamoto, M.; Watabiki, T.; Ozawa, T.; Oe, T.; Shimizu, Y.; Ito, H. Systemic administration of 5-HT2C receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol. Biochem. Behav. 2013, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Cationic liposome-encapsulated carotenoids as a potential treatment for fibromyalgia in an animal model. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2021, 1867, 166150. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Sawie, H.G.; Abdel-Salam, O.M.; Hosny, E.N. Cannabis exacerbates depressive symptoms in rat model induced by reserpine. Behav. Brain Res. 2017, 324, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Kondo, Y.; Akuzawa, S.; Mihara, T.; Shiraishi, N.; Kakimoto, S.; Matsumoto, M. A novel GABAB receptor positive allosteric modulator, ASP8062, exerts analgesic effects in a rat model of fibromyalgia. Eur. J. Pharmacol. 2019, 865, 172750. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Serra, A.; Alfosea-Cuadrado, G.; Cervera-Ferri, A.; González-Soler, E.M.; Lloret, A.; Martínez-Ricós, J.; Teruel-Martí, V.; Valverde-Navarro, A.A. Hippocampal oscillatory dynamics and sleep atonia are altered in an animal model of fibromyalgia: Implications in the search for biomarkers. J. Comp. Neurol. 2020, 528, 1367–1391. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Price, M.P.; Breese, N.M.; Stucky, C.L.; Wemmie, J.A.; Welsh, M.J. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 2003, 106, 229–239. [Google Scholar] [CrossRef] [PubMed]
- DeSantana, J.M.; da Cruz, K.M.; Sluka, K.A. Animal models of fibromyalgia. Arthritis Res. Ther. 2013, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Sluka, K.A. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci. Lett. 2009, 457, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chen, S.D.; Chuang, Y.C.; Shaw, F.Z. Pregabalin, duloxetine, and diazepam selectively modulate acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Neuropsychiatry 2017, 7, 849–861. [Google Scholar] [CrossRef]
- Heimfarth, L.; dos Anjos, K.S.; de Carvalho, Y.M.B.G.; dos Santos, B.L.; Serafini, M.R.; de Carvalho Neto, A.G.; Nunes, P.S.; Filho, J.I.A.B.; da Silva, S.P.; Ribeiro, A.M.; et al. Characterization of β-cyclodextrin/myrtenol complex and its protective effect against nociceptive behavior and cognitive impairment in a chronic musculoskeletal pain model. Carbohydr. Polym. 2020, 244, 116448. [Google Scholar] [CrossRef]
- Murasawa, H.; Kobayashi, H.; Yasuda, S.; Saeki, K.; Domon, Y.; Arakawa, N.; Kubota, K.; Kitano, Y. Anxiolytic-like effects of mirogabalin, a novel ligand for α 2 δ ligand of voltage-gated calcium channels, in rats repeatedly injected with acidic saline intramuscularly, as an experimental model of fibromyalgia. Pharmacol. Rep. 2020, 72, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Y.; Wu, J.-W.; Cheng, J.-K.; Tamas, G.; Nakazawa, K. Elevation of hilar mossy cell activity suppresses hippocampal excitability and avoidance behavior. Cell Rep. 2021, 36, 109702. [Google Scholar] [CrossRef]
- Liu, Y.T.; Shao, Y.W.; Yen, C.T.; Shaw, F.Z. Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiol. Behav. 2014, 131, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lottering, B.; Lin, Y.W. TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII using electroacupuncture treatment for chronic pain and depression comorbidity in a murine model. Int. J. Mol. Sci. 2021, 22, 5028. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.; Fuchs, P.N.; Sluka, K.A. Assessment of avoidance behaviors in mouse models of muscle pain. Neuroscience 2013, 248, 54–60. [Google Scholar] [CrossRef]
- Murasawa, H.; Pawlak, A.; Kobayashi, H.; Saeki, K.; Yasuda, S.I.; Kitano, Y. Mirogabalin, a novel ligand for α2δ subunit of voltage-gated calcium channels, improves cognitive impairments in repeated intramuscular acidic saline injection model rats, an experimental model of fibromyalgia. Biomed. Pharmacother. 2021, 139, 111647. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.C.; Opp, M.R. Musculoskeletal sensitization and sleep: Chronic muscle pain fragments sleep of mice without altering its duration. Sleep 2014, 37, 505–513. [Google Scholar] [CrossRef]
- Wei, T.Y.; Young, C.P.; Liu, Y.T.; Xu, J.H.; Liang, S.F.; Shaw, F.Z.; Kuo, C.E. Development of a rule-based automatic five-sleep-stage scoring method for rats. Biomed. Eng. Online 2019, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.C.; Opp, M.R. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep 2014, 37, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Gibson-Corley, K.; Frey-Law, L.; Sluka, K.A. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain 2013, 154, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Danielson, J.; Rasmussen, L.; Dasilva, L.F. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med. Sci. Sports Exerc. 2012, 44, 420. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; O’Donnell, J.M.; Danielson, J.; Rasmussen, L.A. Regular physical activity prevents development of chronic pain and activation of central neurons. J. Appl. Physiol. 2013, 114, 725–733. [Google Scholar] [CrossRef]
- Quintero, L.; Cardenas, R.; Suarez-Roca, H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain 2011, 152, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Quintero, L.; Cuesta, M.C.; Silva, J.A.; Arcaya, J.L.; Pinerua-Suhaibar, L.; Maixner, W.; Suarez-Roca, H. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain Res. 2003, 965, 259–268. [Google Scholar] [CrossRef]
- Suarez-Roca, H.; Silva, J.A.; Arcaya, J.L.; Quintero, L.; Maixner, W.; Piňerua-Shuhaibar, L. Role of μ-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav. Brain Res. 2006, 167, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Roca, H.; Quintero, L.; Arcaya, J.L.; Maixner, W.; Rao, S.G. Stress-induced muscle and cutaneous hyperalgesia: Differential effect of milnacipran. Physiol. Behav. 2006, 88, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bagues, A.; Girón, R.; Abalo, R.; Goicoechea, C.; Martín-Fontelles, M.I.; Sánchez-Robles, E.M. Short-term stress significantly decreases morphine analgesia in trigeminal but not in spinal innervated areas in rats. Behav. Brain Res. 2022, 435, 114046. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Cai, J.; Wei, T.J.; Liu, L.Y.; Zhao, H.Y.; Liu, B.H.; Jing, H.B.; Jin, Z.R.; Liu, M.; et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav. Brain Res. 2018, 338, 134–142. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, L.Y.; Chen, L.; Cai, J.; Wan, Y.; Xing, G.G. Chronic stress exacerbates neuropathic pain via the integration of stress-affect–related information with nociceptive information in the central nucleus of the amygdala. Pain 2017, 158, 717–739. [Google Scholar] [CrossRef]
- Nazeri, M.; Zarei, M.R.; Pourzare, A.R.; Ghahreh-Chahi, H.R.; Abareghi, F.; Shabani, M. Evidence of altered trigeminal nociception in an animal model of fibromyalgia. Pain Med. 2018, 19, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Kuhad, A.; Tiwari, V.; Arora, V.; Chopra, K. Protective effect of epigallocatechin gallate in murine water-immersion stress model of chronic fatigue syndrome. Basic Clin. Pharmacol. Toxicol. 2010, 106, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Saha, K. Changes in GRK3 and Norepinephrine Responsiveness in Locus Coeruleus Neurons are Associated with Learned Helplessness After Repeated Forced Swim Stress. Ph.D. Thesis, University of Houston, Houston, TX, USA, 2011. [Google Scholar]
- Trivedi, R.; Sharma, K. Hydroalcoholic extract of Glycyrrhiza glabra linn. attentuates chronic fatigue stress induced behavioral alterations in mice. Int. J. Pharm. Biol. Arch. 2011, 2, 996–1001. [Google Scholar]
- Nazeri, M.; Razavinasab, M.; Abareghi, F.; Shabani, M. Role of nitric oxide in altered nociception and memory following chronic stress. Physiol. Behav. 2014, 129, 214–220. [Google Scholar] [CrossRef]
- Nazeri, M.; Shabani, M.; Parsania, S.; Golchin, L.; Razavinasab, M.; Abareghi, F.; Kermani, M. Simultaneous impairment of passive avoidance learning and nociception in rats following chronic swim stress. Adv. Biomed. Res. 2016, 5, 93. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Venlafaxine reverses chronic fatigue-induced behavioral, biochemical and neurochemical alterations in mice. Pharmacol. Biochem. Behav. 2008, 89, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wei, S.-Q.; Wang, P.-X.; Wang, W.-Y.; Liu, E.-Q.; Traub, R.J.; Cao, D.-Y. Down-regulation of spinal 5-HT2A and 5-HT2C receptors contributes to somatic hyperalgesia induced by orofacial inflammation combined with stress. Neuroscience 2020, 440, 196–209. [Google Scholar] [CrossRef]
- Okamoto, K.; Thompson, R.; Katagiri, A.; Bereiter, D.A. Estrogen status and psychophysical stress modify temporomandibular joint input to medullary dorsal horn neurons in a lamina-specific manner in female rats. Pain 2013, 154, 1057–1064. [Google Scholar] [CrossRef]
- Cao, D.Y.; Bai, G.; Ji, Y.; Karpowicz, J.; Traub, R.J. Histone hyperacetylation modulates spinal type II metabotropic glutamate receptor alleviating stress-induced visceral hypersensitivity in female rats. Mol. Pain 2016, 12, 1744806916660722. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Hu, B.; Li, J.; Traub, R.J. Opposing roles of estradiol and testosterone on stress-induced visceral hypersensitivity in rats. J. Pain 2018, 19, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kurose, M.; Shimizu, S.; Hasegawa, M.; Ikeda, N.; Yamamura, K.; Takagi, R.; Okamoto, K. Inhibitory effects of fluoxetine, an antidepressant drug, on masseter muscle nociception at the trigeminal subnucleus caudalis and upper cervical spinal cord regions in a rat model of psychophysical stress. Exp. Brain Res. 2018, 236, 2209–2221. [Google Scholar] [CrossRef]
- Nakatani, Y.; Kakihara, Y.; Shimizu, S.; Kurose, M.; Sato, T.; Kaneoke, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Japanese Rice Wine can reduce psychophysical stress-induced depression-like behaviors and Fos expression in the trigeminal subnucleus caudalis evoked by masseter muscle injury in the rats. Biosci. Biotechnol. Biochem. 2019, 83, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kanter, K.; Chen, J.; Kim, S.; Wang, Y.; Adeyemi, C.; O’Buckley, S.C.; Nackley, A.G. Low COMT and Stress Potentiate Functional Pain and Depressive Behavior, Especially in Female Mice. Pain 2020, 161, 446. [Google Scholar] [CrossRef]
- Kita, T.; Hata, T.; Iida, J.; Yoneda, R.; Isida, S. Decrease in pain threshold in SART stressed mice. Jpn. J. Pharmacol. 1979, 29, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Nishiyori, M.; Ueda, H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol. Pain 2008, 4, 1744–8069. [Google Scholar] [CrossRef]
- Nasu, T.; Taguchi, T.; Mizumura, K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur. J. Pain 2010, 14, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Itoh, E.; Kawabata, A. Changes in CNS levels of serotonin and its metabolite in SART-stressed (repeatedly cold-stressed) rats. Jpn. J. Pharmacol. 1991, 56, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Omiya, Y.; Goto, K.; Ishige, A.; Komatsu, Y. Changes in analgesia-producing mechanism of repeated cold stress loading in mice. Pharmacol. Biochem. Behav. 2000, 65, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, Y.; Satoh, M. Participation of spinal cord substance P in hyperalgesia induced by repeated cold stress. Regul. Pept. 1993, 46, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; KuraishiKuraishi, Y.; SatohSatoh, M. Effects of intrathecally injected glutamate and substance P antagonists on repeated cold stress-induced hyperalgesia in rats. Biol. Pharm. Bull. 1995, 18, 42–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satoh, M.; Kuraishi, Y.; Kawamura, M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: Comparison with carrageenan-induced hyperalgesia. Pain 1992, 49, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Ohtani-Kaneko, R.; Naiki, M.; Okada, T.; Masuko, K.; Yudoh, K.; Suematsu, N.; Okamoto, K.; Nishioka, K.; Kato, T. Involvement of post-translational modification of neuronal plasticity-related proteins in hyperalgesia revealed by a proteomic analysis. Proteomics 2008, 8, 1706–1719. [Google Scholar] [CrossRef]
- Lee, H.; Im, J.; Won, H.; Kim, J.Y.; Kim, H.K.; Kwon, J.T.; Kim, Y.O.; Lee, S.; Cho, I.H.; Lee, S.W.; et al. Antinociceptive effect of Valeriana fauriei regulates BDNF signaling in an animal model of fibromyalgia. Int. J. Mol. Med. 2018, 41, 485–492. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; García-Giménez, M.D.; Ángel-Martín, M.; Fernández-Arche, A. Validation and additional support for an experimental animal model of fibromyalgia. Mod. Rheumatol. 2015, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Nasu, T.; Kubo, A.; Queme, L.F.; Mizumura, K. A single administration of Neurotropin reduced the elongated immobility time in the forced swimming test of rats exposed to repeated cold stress. Behav. Pharmacol. 2019, 30, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.B.; Corley, K.C.; Phan, T.H.; Boadle-Biber, M.C. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res. 1990, 516, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Brum, E.S.; Becker, G.; Fialho, M.F.P.; Oliveira, S.M. Animal models of fibromyalgia: What is the best choice? Pharmacol. Ther. 2022, 230, 107959. [Google Scholar] [CrossRef] [PubMed]
- Dina, O.A.; Levine, J.D.; Green, P.G. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur. J. Pain 2011, 15, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Alvarez, P.; Gear, R.W.; Mendoza, D.; Levine, J.D. Further validation of a model of fibromyalgia syndrome in the rat. J. Pain 2011, 12, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-H.; Lee, C.-H.; Tsai, M.-H.; Chen, C.-H.; Lin, H.-F.; Hsu, C.-Y.; Lai, C.-L.; Chen, C.-C. Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16: 0 mediates psychological stress-induced fibromyalgia-like pain. Ann. Rheum. Dis. 2020, 79, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Golzio dos Santos, S.; Fernandes Gomes, I.; Fernandes de Oliveira Golzio, A.M.; Lopes Souto, A.; Scotti, M.T.; Fechine Tavares, J.; Gutierrez, S.J.C.; de Almeida, R.N.; Barbosa-Filho, J.M.; da Silva, M.S. Psychopharmacological effects of riparin III from Aniba riparia (Nees) Mez.(Lauraceae) supported by metabolic approach and multivariate data analysis. BMC Complement. Med. Ther. 2020, 20, 149. [Google Scholar] [CrossRef]
- Viero, F.; Rodrigues, P.; Frare, J.; da Silva, N.; Ferreira, M.; da Silva, A.; Pereira, G.C.; Ferreira, J.; Pillat, M.; Bochi, G.; et al. Unpredictable Sound Stress Model Causes Migraine-like Behaviors in Mice with Sexual Dimorphism. Front. Pharmacol. 2022, 13, 911105. [Google Scholar] [CrossRef]
| Affective | Cognitive | Treatment for Affect/Cognition | |
|---|---|---|---|
| Reserpine | |||
| Significant: | [22,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,72,73,74,75,76,77,78,79,80,81,82] | [68,69,70,76,83] | Antidepressants: [37,42,43,47,49,54,56,59,61,62,66,78,80,81] Parkinson’s DA agonist: [64,78] Anticonvulsants: [55,58,61,68,69,70,72,76,81] Neurorecovery/neuroprotective: [47,50,62] Organics: [38,44,49,52,58,60,61,63,66,68,69,70,74,75,76] Environment/Diet/Exercise: [53,56,72,83] Alternative Measures: [22,41,42,84] Other: [43,45,47,54,55,57,59,61] |
| Non-significant: | [83,85] | - | Anticonvulsants: [39,45,52,57,63,65] NSAID: [39,63,81] Opioid Partial Agonist: [81] Organics: [39,46,52] Alternative Measures: [22] Other: [22,43,51,57,85] |
| Inconclusive: | [80,86,87,88] | [80,88] | Benzodiazepines: [88] Organics: [86,87] Other: [88] |
| Not Investigated: Antidepressants: [40] Anticonvulsants: [59] Other: [57,61,85,88] | |||
| Affective | Cognitive | Sleep | Treatment for Affect/Cognition | |
|---|---|---|---|---|
| Acidic Saline | ||||
| Significant: | [65,93,94,95,96,97] | [100] | [101,102,103] | Antidepressants: [97] Benzodiazepines: [97] Anticonvulsants: [65,93,94,97,99] Alternative Devices: [98] Organics: [93] Other: [93,94,95,96,97,99] |
| Non-significant: | [96] | [99] | - | Organics: [94] |
| Inconclusive: | - | [94] | - | - |
| Affective | Cognitive | Treatment for Affect/Cognition | |
|---|---|---|---|
| Fatigue-Enhanced Muscle Pain | |||
| Non-significant: | [99] | [99] | - |
| Affective | Cognitive | Treatment for Affect/Cognition | |
|---|---|---|---|
| Subchronic Swim Stress | |||
| Significant: | [111,112,113,114,115,116,117] | [118,119] | Antidepressants: [113,117,120] Narcotic analgesic: [111] NMDA receptor antagonist: [113] NOS antagonist: [118,119] Organics: [115,117,118,119] |
| Non-significant: | [118,119] | - | - |
| Inconclusive: | [120,121] | - | - |
| Affective | Cognitive | Treatment for Affect/Cognition | |
|---|---|---|---|
| Cold Stress | |||
| Significant: | [137,138,139] | - | Antidepressants: [139] Anticonvulsants: [138] Non-protein extract: [139] Organics: [137] |
| Non-significant: | [129] | - | - |
| Not investigated: Antidepressants: [129] | |||
| Affective | Treatment for Affect/Cognition | |
|---|---|---|
| Sound Stress | ||
| Significant: | [143,144,146] | CGRP antagonist: [146] Alkamide-type alkaloid: [145] |
| Inconclusive: | [145] | - |
| Not investigated: Anticonvulsants: [144] Narcotic analgesics: [144] NSAIDs: [144] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenbright, C.M.; Bertlesman, A.M.; Russell, I.M.; Greer, T.L.; Peng, Y.B.; Fuchs, P.N. The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions. Biomedicines 2024, 12, 778. https://doi.org/10.3390/biomedicines12040778
Argenbright CM, Bertlesman AM, Russell IM, Greer TL, Peng YB, Fuchs PN. The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions. Biomedicines. 2024; 12(4):778. https://doi.org/10.3390/biomedicines12040778
Chicago/Turabian StyleArgenbright, Cassie M., Alysia M. Bertlesman, Izabella M. Russell, Tracy L. Greer, Yuan B. Peng, and Perry N. Fuchs. 2024. "The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions" Biomedicines 12, no. 4: 778. https://doi.org/10.3390/biomedicines12040778
APA StyleArgenbright, C. M., Bertlesman, A. M., Russell, I. M., Greer, T. L., Peng, Y. B., & Fuchs, P. N. (2024). The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions. Biomedicines, 12(4), 778. https://doi.org/10.3390/biomedicines12040778





