Essential Nutrients and White Matter Hyperintensities: A Two-Sample Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Exposure and Outcome Data
2.2. Selection of Instrumental Variables (IVs)
2.3. Mendelian Randomization
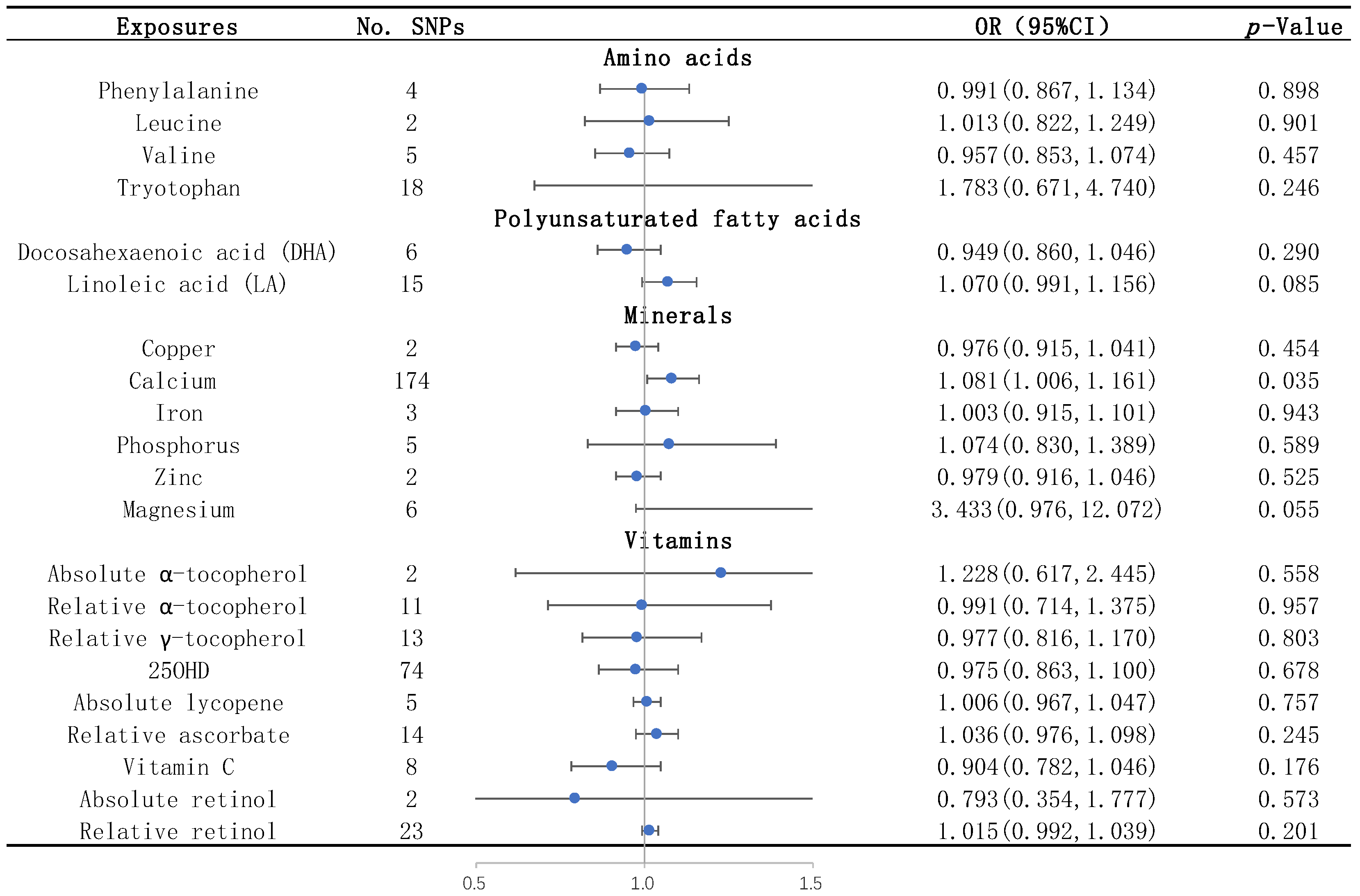
3. Results
3.1. Amino Acids
3.2. Unsaturated Fatty Acids
3.3. Mineral Elements
3.4. Vitamins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Persyn, E.; Hanscombe, K.B.; Howson, J.M.; Lewis, C.M.; Traylor, M.; Markus, H.S. Genome-wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat. Commun. 2020, 11, 2175. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Schilling, S.; Duperron, M.-G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-Analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Wong, S.M.; Uiterwijk, R.; Backes, W.H.; Jansen, J.F.A.; Jeukens, C.R.L.P.N.; van Oostenbrugge, R.J.; Staals, J. Blood-brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. 2019, 13, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.S.; Simpson, J.E.; Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Wharton, S.B.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497, Erratum in Lancet Neurol.2013, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Pasi, M.; van Uden, I.W.; Tuladhar, A.M.; de Leeuw, F.-E.; Pantoni, L. White Matter Microstructural Damage on Diffusion Tensor Imaging in Cerebral Small Vessel Disease: Clinical Consequences. Stroke 2016, 47, 1679–1684. [Google Scholar] [CrossRef]
- Viswanathan, A.; Godin, O.; Jouvent, E.; O’sullivan, M.; Gschwendtner, A.; Peters, N.; Duering, M.; Guichard, J.-P.; Holtmannspötter, M.; Dufouil, C.; et al. Impact of MRI markers in subcortical vascular dementia: A multi-modal analysis in CADASIL. Neurobiol. Aging 2010, 31, 1629–1636. [Google Scholar] [CrossRef]
- Zeestraten, E.A.; Lawrence, A.J.; Lambert, C.; Benjamin, P.; Brookes, R.L.; Mackinnon, A.D.; Morris, R.G.; Barrick, T.R.; Markus, H.S. Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology 2017, 89, 1869–1876. [Google Scholar] [CrossRef]
- Leitão, C.; Mignano, A.; Estrela, M.; Fardilha, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Effect of Nutrition on Aging—A Systematic Review Focusing on Aging-Related Biomarkers. Nutrients 2022, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Recalde, B.Y.; Mera, R.M. Dietary Oily Fish Intake is Inversely Associated with Severity of White Matter Hyperintensities of Presumed Vascular Origin. A Population-Based Study in Frequent Fish Consumers of Amerindian Ancestry. J. Stroke Cerebrovasc. Dis. 2021, 30, 105778. [Google Scholar] [CrossRef]
- Prinelli, F.; Fratiglioni, L.; Kalpouzos, G.; Musicco, M.; Adorni, F.; Johansson, I.; Marseglia, A.; Xu, W. Specific nutrient patterns are associated with higher structural brain integrity in dementia-free older adults. NeuroImage 2019, 199, 281–288. [Google Scholar] [CrossRef]
- Gu, Y.; Scarmeas, N. Diet and Neuroimaging Markers of Cerebrovascular Disease. Curr. Nutr. Rep. 2013, 2, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.; Silbert, L.; Howieson, D.; Dodge, H.; Traber, M.; Frei, B.; Kaye, J.; Shannon, J.; Quinn, J. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 2012, 78, 241–249. [Google Scholar] [CrossRef]
- Jensen, D.E.; Leoni, V.; Klein-Flügge, M.C.; Ebmeier, K.P.; Suri, S. Associations of dietary markers with brain volume and connectivity: A systematic review of MRI studies. Ageing Res. Rev. 2021, 70, 101360. [Google Scholar] [CrossRef]
- Lee, K.; Lim, C.-Y. Mendelian Randomization Analysis in Observational Epidemiology. J. Lipid Atheroscler. 2019, 8, 67–77. [Google Scholar] [CrossRef]
- Smith, G.D.; Timpson, N.; Ebrahim, S. Strengthening causal inference in cardiovascular epidemiology through Mendelian randomization. Ann. Med. 2008, 40, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, J.; Demirkan, A.; Würtz, P.; Draisma, H.H.; Haller, T.; Rawal, R.; Vaarhorst, A.; Kangas, A.J.; Lyytikäinen, L.-P.; Pirinen, M.; et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 2016, 7, 11122. [Google Scholar] [CrossRef]
- Shin, S.-Y.; The Multiple Tissue Human Expression Resource (MuTHER) Consortium; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.-C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLOS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef] [PubMed]
- Sinnott-Armstrong, N.; Tanigawa, Y.; Amar, D.; Mars, N.; Benner, C.; Aguirre, M.; Venkataraman, G.R.; Wainberg, M.; Ollila, H.M.; Kiiskinen, T.; et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021, 53, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Verwoert, G.C.; Hwang, S.-J.; Glazer, N.L.; Smith, A.V.; van Rooij, F.J.A.; Ehret, G.B.; Boerwinkle, E.; Felix, J.F.; Leak, T.S.; et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLOS Genet. 2010, 6, e1001045. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Glazer, N.L.; Köttgen, A.; Felix, J.F.; Hwang, S.-J.; Liu, Y.; Lohman, K.; Kritchevsky, S.B.; Hausman, D.B.; Petersen, A.-K.; et al. Common Genetic Variants Associate with Serum Phosphorus Concentration. J. Am. Soc. Nephrol. 2010, 21, 1223–1232. [Google Scholar] [CrossRef]
- Evans, D.M.; Zhu, G.; Dy, V.; Heath, A.C.; Madden, P.A.F.; Kemp, J.P.; McMahon, G.; St Pourcain, B.; Timpson, N.J.; Golding, J.; et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013, 22, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- D’adamo, C.R.; D’urso, A.; Ryan, K.A.; Yerges-Armstrong, L.M.; Semba, R.D.; Steinle, N.I.; Mitchell, B.D.; Shuldiner, A.R.; McArdle, P.F. A Common Variant in the SETD7 Gene Predicts Serum Lycopene Concentrations. Nutrients 2016, 8, 82. [Google Scholar] [CrossRef]
- Ferrucci, L.; Perry, J.R.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [Google Scholar] [CrossRef]
- Hazra, A.; Kraft, P.; Lazarus, R.; Chen, C.; Chanock, S.J.; Jacques, P.; Selhub, J.; Hunter, D.J. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum. Mol. Genet. 2009, 18, 4677–4687. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Imamura, F.; Stewart, I.D.; Day, F.R.; Pietzner, M.; Wheeler, E.; Lotta, L.A.; Gundersen, T.E.; et al. Plasma Vitamin C and Type 2 Diabetes: Genome-Wide Association Study and Mendelian Randomization Analysis in European Populations. Diabetes Care 2020, 44, 98–106. [Google Scholar] [CrossRef]
- Manousaki, D.; Mitchell, R.; Dudding, T.; Haworth, S.; Harroud, A.; Forgetta, V.; Shah, R.L.; Luan, J.; Langenberg, C.; Timpson, N.J.; et al. Genome-Wide Association Study for Vitamin D Levels Reveals 69 Independent Loci. Am. J. Hum. Genet. 2020, 106, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Major, J.M.; Yu, K.; Wheeler, W.; Zhang, H.; Cornelis, M.C.; Wright, M.E.; Yeager, M.; Snyder, K.; Weinstein, S.J.; Mondul, A.; et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 2011, 20, 3876–3883. [Google Scholar] [CrossRef]
- Long, T.; Hicks, M.; Yu, H.-C.; Biggs, W.H.; Kirkness, E.F.; Menni, C.; Zierer, J.; Small, K.S.; Mangino, M.; Messier, H.; et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017, 49, 568–578. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Silbert, L.C.; Lahna, D.; Promjunyakul, N.-O.; Boespflug, E.; Ohya, Y.; Higashiuesato, Y.; Nishihira, J.; Katsumata, Y.; Tokashiki, T.; Dodge, H.H. Risk Factors Associated with Cortical Thickness and White Matter Hyperintensities in Dementia Free Okinawan Elderly. J. Alzheimer’s Dis. 2018, 63, 365–372. [Google Scholar] [CrossRef]
- Payne, M.E.; Pierce, C.W.; McQuoid, D.R.; Steffens, D.C.; Anderson, J.J.B. Serum ionized calcium may be related to white matter lesion volumes in older adults: A pilot study. Nutrients 2013, 5, 2192–2205. [Google Scholar] [CrossRef]
- Payne, M.E.; Anderson, J.J.; Steffens, D.C. Calcium and vitamin D intakes may be positively associated with brain lesions in depressed and nondepressed elders. Nutr. Res. 2008, 28, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.E.; McQuoid, U.R.; Steffens, D.C.; Anderson, J.J.B. Elevated brain lesion volumes in older adults who use calcium supplements: A cross-sectional clinical observational study. Br. J. Nutr. 2014, 112, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Shibata, K.; Nomura, R.; Kawamoto, D.; Nagamine, R.; Imaizumi, K. Linoleic acid-rich fats reduce atherosclerosis development beyond its oxidative and inflammatory stress-increasing effect in apolipoprotein E-deficient mice in comparison with saturated fatty acid-rich fats. Br. J. Nutr. 2005, 94, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, A.; Basu, S.; Mutanen, M. A high linoleic acid diet increases oxidative stress in vivo and affects nitric oxide metabolism in humans. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y. Linoleic acid–good or bad for the brain? NPJ Sci. Food 2020, 4, 1, Erratum in NPJ Sci. Food.2020, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Kern, S.; Blennow, K.; Zetterberg, H.; Waern, M.; Guo, X.; Börjesson-Hanson, A.; Skoog, I.; Östling, S. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 2016, 87, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-Z.; Wang, Z.-X.; Wang, Z.-T.; Hou, X.-H.; Shen, X.-N.; Ou, Y.-N.; Dong, Q.; Tan, L.; Yu, J.-T. Serum Calcium Predicts Cognitive Decline and Clinical Progression of Alzheimer’s Disease. Neurotox. Res. 2020, 39, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Garmo, H.; Malmström, H.; Hammar, N.; Jungner, I.; Walldius, G.; Van Hemelrijck, M. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis 2016, 251, 85–93. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J.; Ishani, A.; Kalra, P.A. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 556–563. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Burgess, S.; Boncoraglio, G.B.; Jern, C.; Michaëlsson, K.; Markus, H.S.; Malik, R.; Chauhan, G.; Sargurupremraj, M.; et al. Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology 2019, 92, e944–e950. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, R.; Guo, Y.; Li, Q.; Zhou, H.; Yu, S.; Liang, H.; Li, Z. An Updated Mendelian Randomization Analysis of the Association between Serum Calcium Levels and the Risk of Alzheimer’s Disease. Front. Genet. 2021, 12, 731391. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, N.; Ishizaka, Y.; Takahashi, E.; Tooda, E.-I.; Hashimoto, H.; Nagai, R.; Yamakado, M. Serum calcium concentration and carotid artery plaque: A population-based study. J. Cardiol. 2002, 39, 151–157. [Google Scholar] [PubMed]
- Rubin, M.R.; Rundek, T.; McMahon, D.J.; Lee, H.-S.; Sacco, R.L.; Silverberg, S.J. Carotid artery plaque thickness is associated with increased serum calcium levels: The Northern Manhattan study. Atherosclerosis 2007, 194, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Erbay, S.; Han, R.; Baccei, S.; Krakov, W.; Zou, K.H.; Bhadelia, R.; Polak, J. Intracranial carotid artery calcification on head CT and its association with ischemic changes on brain MRI in patients presenting with stroke-like symptoms: Retrospective analysis. Neuroradiology 2006, 49, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fanning, N.; Walters, T.; Fox, A.; Symons, S. Association between calcification of the cervical carotid artery bifurcation and white matter ischemia. AJNR Am. J. Neuroradiol. 2006, 27, 378–383. [Google Scholar]
- Kim, B.J.; Lee, S.-H.; Kim, C.K.; Ryu, W.-S.; Kwon, H.-M.; Choi, S.-Y.; Yoon, B.-W. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ. J. 2011, 75, 451–456. [Google Scholar] [CrossRef]

| Exposures | Methods | No. SNPs | OR(95%CI) | p-Value |
|---|---|---|---|---|
| Amino acids | ||||
| Phenylalanine | Simple median | 4 | 0.972 (0.832, 1.136) | 0.721 |
| Weighted median | 4 | 0.991 (0.843, 1.166) | 0.917 | |
| MR Egger | 4 | 1.053 (0.641, 1.730) | 0.857 | |
| IVW | 4 | 0.991 (0.867, 1.134) | 0.898 | |
| Leucine | IVW | 2 | 1.013 (0.822, 1.249) | 0.901 |
| Valine | Simple median | 5 | 1.094 (0.894, 1.338) | 0.383 |
| Weighted median | 5 | 0.893 (0.777, 1.025) | 0.108 | |
| MR Egger | 5 | 0.608 (0.330, 1.119) | 0.208 | |
| IVW | 5 | 0.957 (0.853, 1.074) | 0.457 | |
| Tryptophan | Simple median | 18 | 0.589 (0.164, 2.118) | 0.417 |
| Weighted median | 18 | 0.587 (0.173, 1.993) | 0.393 | |
| MR Egger | 18 | 4060.115 (0.009, 1,924,702,705.747) | 0.231 | |
| IVW | 18 | 1.783 (0.671, 4.740) | 0.246 | |
| Polyunsaturated fatty acids | ||||
| Docosahexaenoic acid (DHA) | Simple median | 6 | 0.914 (0.813, 1.026) | 0.129 |
| Weighted median | 6 | 0.927 (0.829, 1.037) | 0.188 | |
| MR Egger | 6 | 1.380 (0.846, 2.249) | 0.267 | |
| IVW | 6 | 0.949 (0.860, 1.046) | 0.290 | |
| Linoleic acid (LA) | Simple median | 15 | 1.064 (0.979, 1.155) | 0.143 |
| Weighted median | 15 | 1.045 (0.971, 1.125) | 0.241 | |
| MR Egger | 15 | 1.125 (0.941, 1.345) | 0.217 | |
| IVW | 15 | 1.070 (0.991, 1.156) | 0.085 | |
| Minerals | ||||
| Copper | IVW | 2 | 0.976 (0.915, 1.041) | 0.454 |
| Calcium | Simple median | 174 | 1.070 (0.969, 1.182) | 0.178 |
| Weighted median | 174 | 1.058 (0.954, 1.172) | 0.286 | |
| MR Egger | 174 | 1.119 (0.905, 1.383) | 0.302 | |
| IVW | 174 | 1.081 (1.006, 1.161) | 0.035 | |
| Iron | Simple median | 3 | 1.047 (0.949, 1.153) | 0.360 |
| Weighted median | 3 | 1.043 (0.958, 1.137) | 0.332 | |
| MR Egger | 3 | 1.223 (0.869, 1.723) | 0.455 | |
| IVW | 3 | 1.003 (0.915, 1.101) | 0.943 | |
| Phosphorus | Simple median | 5 | 1.037 (0.731, 1.469) | 0.840 |
| Weighted median | 5 | 1.129 (0.834, 1.528) | 0.433 | |
| MR Egger | 5 | 2.946 (0.754, 11.518) | 0.218 | |
| IVW | 5 | 1.074 (0.830, 1.389) | 0.589 | |
| Zinc | IVW | 2 | 0.979 (0.916, 1.046) | 0.525 |
| Magnesium | Simple median | 6 | 1.462 (0.216, 9.907) | 0.697 |
| Weighted median | 6 | 1.882 (0.389, 9.102) | 0.432 | |
| MR Egger | 6 | 20.027 (0.540, 742.534) | 0.179 | |
| IVW | 6 | 3.433 (0.976, 12.072) | 0.055 | |
| Vitamins | ||||
| Absolute α-tocopherol | IVW | 2 | 1.228 (0.617, 2.445) | 0.558 |
| Relative α-tocopherol | Simple median | 11 | 0.886 (0.573, 1.369) | 0.586 |
| Weighted median | 11 | 1.041 (0.679, 1.596) | 0.854 | |
| MR Egger | 11 | 1.091 (0.543, 2.193) | 0.811 | |
| IVW | 11 | 0.991 (0.714, 1.375) | 0.957 | |
| Relative γ-tocopherol | Simple median | 13 | 0.923 (0.716, 1.190) | 0.536 |
| Weighted median | 13 | 1.014 (0.803, 1.281) | 0.905 | |
| MR Egger | 13 | 1.150 (0.774, 1.708) | 0.504 | |
| IVW | 13 | 0.977 (0.816, 1.170) | 0.803 | |
| 25OHD | Simple median | 74 | 1.030 (0.869, 1.221) | 0.736 |
| Weighted median | 74 | 0.987 (0.851, 1.144) | 0.862 | |
| MR Egger | 74 | 0.942 (0.784, 1.132) | 0.525 | |
| IVW | 74 | 0.975 (0.863, 1.100) | 0.678 | |
| Absolute lycopene | Simple median | 5 | 0.988 (0.937, 1.043) | 0.671 |
| Weighted median | 5 | 1.002 (0.956, 1.051) | 0.919 | |
| MR Egger | 5 | 1.008 (0.936, 1.085) | 0.844 | |
| IVW | 5 | 1.006 (0.967, 1.047) | 0.757 | |
| Relative ascorbate | Simple median | 14 | 0.984 (0.893, 1.085) | 0.751 |
| Weighted median | 14 | 1.029 (0.946, 1.120) | 0.508 | |
| MR Egger | 14 | 1.059 (0.954, 1.176) | 0.304 | |
| IVW | 14 | 1.036 (0.976, 1.098) | 0.245 | |
| Vitamin C | Simple median | 8 | 0.899 (0.750, 1.078) | 0.252 |
| Weighted median | 8 | 0.875 (0.728, 1.052) | 0.157 | |
| MR Egger | 8 | 0.866 (0.576, 1.302) | 0.514 | |
| IVW | 8 | 0.904 (0.782, 1.046) | 0.176 | |
| Absolute retinol | IVW | 2 | 0.793 (0.354, 1.777) | 0.573 |
| Relative retinol | Simple median | 23 | 1.013 (0.980, 1.046) | 0.451 |
| Weighted median | 23 | 1.012 (0.981, 1.044) | 0.458 | |
| MR Egger | 23 | 0.987 (0.926, 1.052) | 0.690 | |
| IVW | 23 | 1.015 (0.992, 1.039) | 0.201 | |
| Exposures | MR–Egger | Cochran’s Q | ||
|---|---|---|---|---|
| Intercept | p-Value | Q | p-Value | |
| Amino acids | ||||
| Phenylalanine | −0.005 | 0.827 | 0.782 | 0.854 |
| Leucine | NA | NA | 1.745 | 0.187 |
| Valine | 0.043 | 0.235 | 4.319 | 0.365 |
| Tryptophan | −0.041 | 0.262 | 22.173 | 0.178 |
| Polyunsaturated fatty acids | ||||
| Docosahexaenoic acid (DHA) | −0.044 | 0.201 | 5.892 | 0.317 |
| Linoleic acid (LA) | −0.008 | 0.548 | 39.887 | 0.000 |
| Minerals | ||||
| Copper | NA | NA | 0.002 | 0.960 |
| Calcium | −0.001 | 0.735 | 202.022 | 0.065 |
| Iron | −0.044 | 0.450 | 3.898 | 0.142 |
| Phosphorus | −0.046 | 0.236 | 4.294 | 0.368 |
| Zinc | NA | NA | 0.080 | 0.778 |
| Magnesium | −0.013 | 0.365 | 4.390 | 0.495 |
| Vitamins | ||||
| Absolute α-tocopherol | NA | NA | 1.590 | 0.207 |
| Relative α-tocopherol | −0.003 | 0.766 | 6.729 | 0.751 |
| Relative γ-tocopherol | −0.007 | 0.385 | 13.765 | 0.316 |
| 25OHD | 0.001 | 0.627 | 136.057 | 0.000 |
| Absolute lycopene | −0.001 | 0.957 | 1.325 | 0.857 |
| Relative ascorbate | −0.003 | 0.621 | 6.807 | 0.912 |
| Vitamin C | 0.002 | 0.830 | 3.263 | 0.860 |
| Absolute retinol | NA | NA | 3.111 | 0.078 |
| Relative retinol | 0.007 | 0.362 | 15.129 | 0.857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xia, K.; Li, J.; Liu, Y.; Zhou, Y.; Zhang, L.; Tang, L.; Zeng, X.; Fan, D.; Yang, Q. Essential Nutrients and White Matter Hyperintensities: A Two-Sample Mendelian Randomization Study. Biomedicines 2024, 12, 810. https://doi.org/10.3390/biomedicines12040810
Wang Z, Xia K, Li J, Liu Y, Zhou Y, Zhang L, Tang L, Zeng X, Fan D, Yang Q. Essential Nutrients and White Matter Hyperintensities: A Two-Sample Mendelian Randomization Study. Biomedicines. 2024; 12(4):810. https://doi.org/10.3390/biomedicines12040810
Chicago/Turabian StyleWang, Zhengrui, Kailin Xia, Jiayi Li, Yanru Liu, Yumou Zhou, Linjing Zhang, Lu Tang, Xiangzhu Zeng, Dongsheng Fan, and Qiong Yang. 2024. "Essential Nutrients and White Matter Hyperintensities: A Two-Sample Mendelian Randomization Study" Biomedicines 12, no. 4: 810. https://doi.org/10.3390/biomedicines12040810
APA StyleWang, Z., Xia, K., Li, J., Liu, Y., Zhou, Y., Zhang, L., Tang, L., Zeng, X., Fan, D., & Yang, Q. (2024). Essential Nutrients and White Matter Hyperintensities: A Two-Sample Mendelian Randomization Study. Biomedicines, 12(4), 810. https://doi.org/10.3390/biomedicines12040810







