NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics
Abstract
1. Introduction
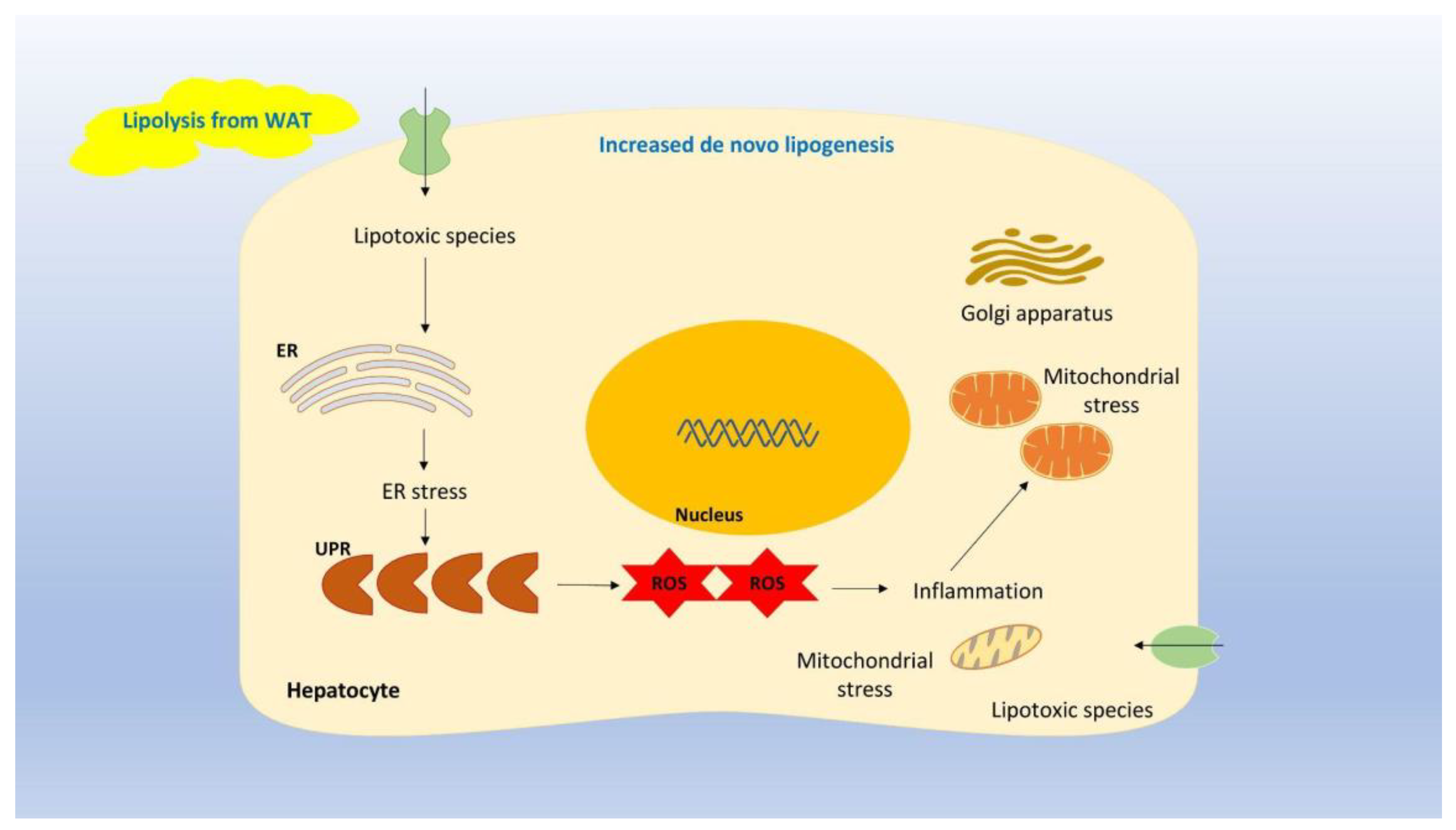
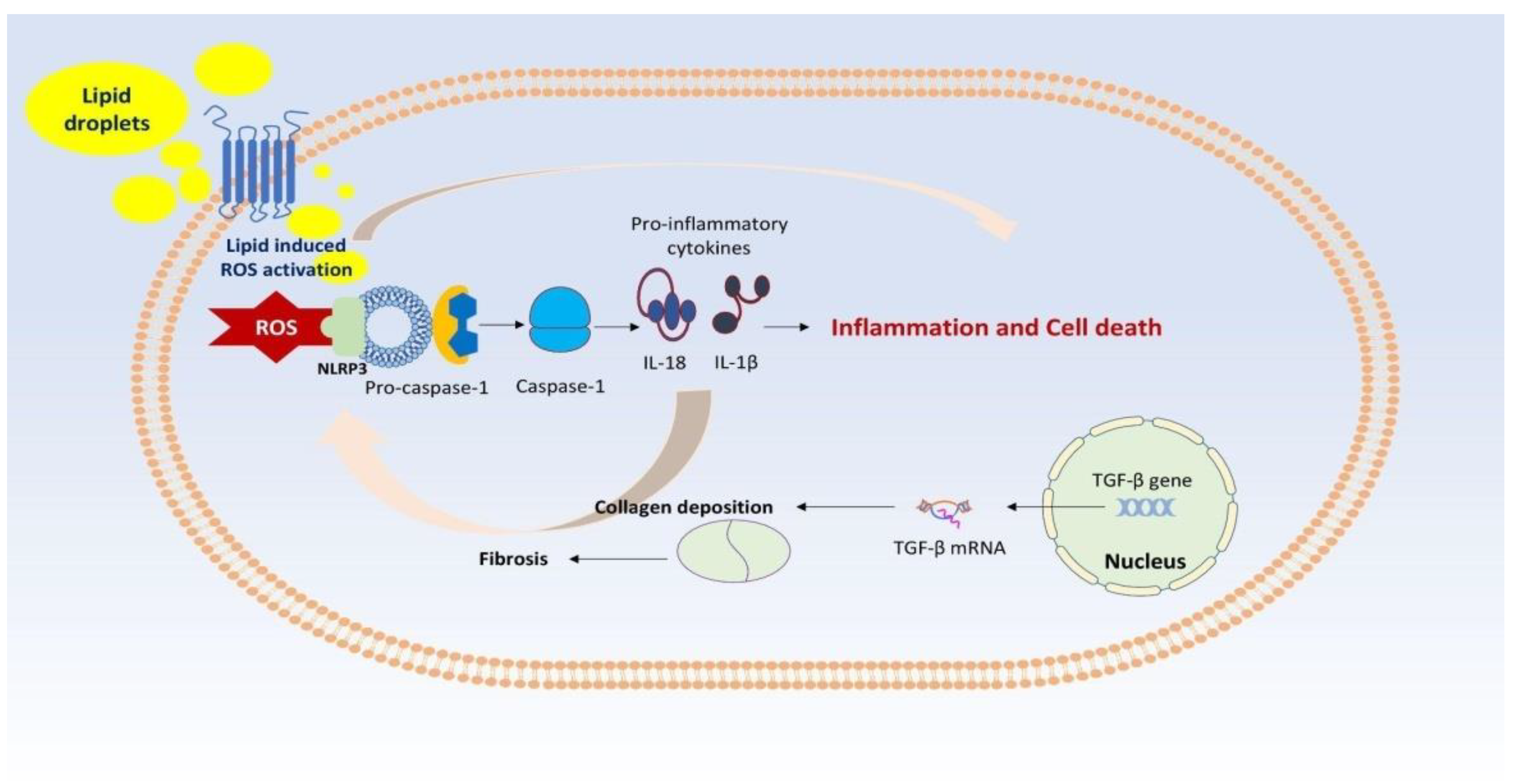
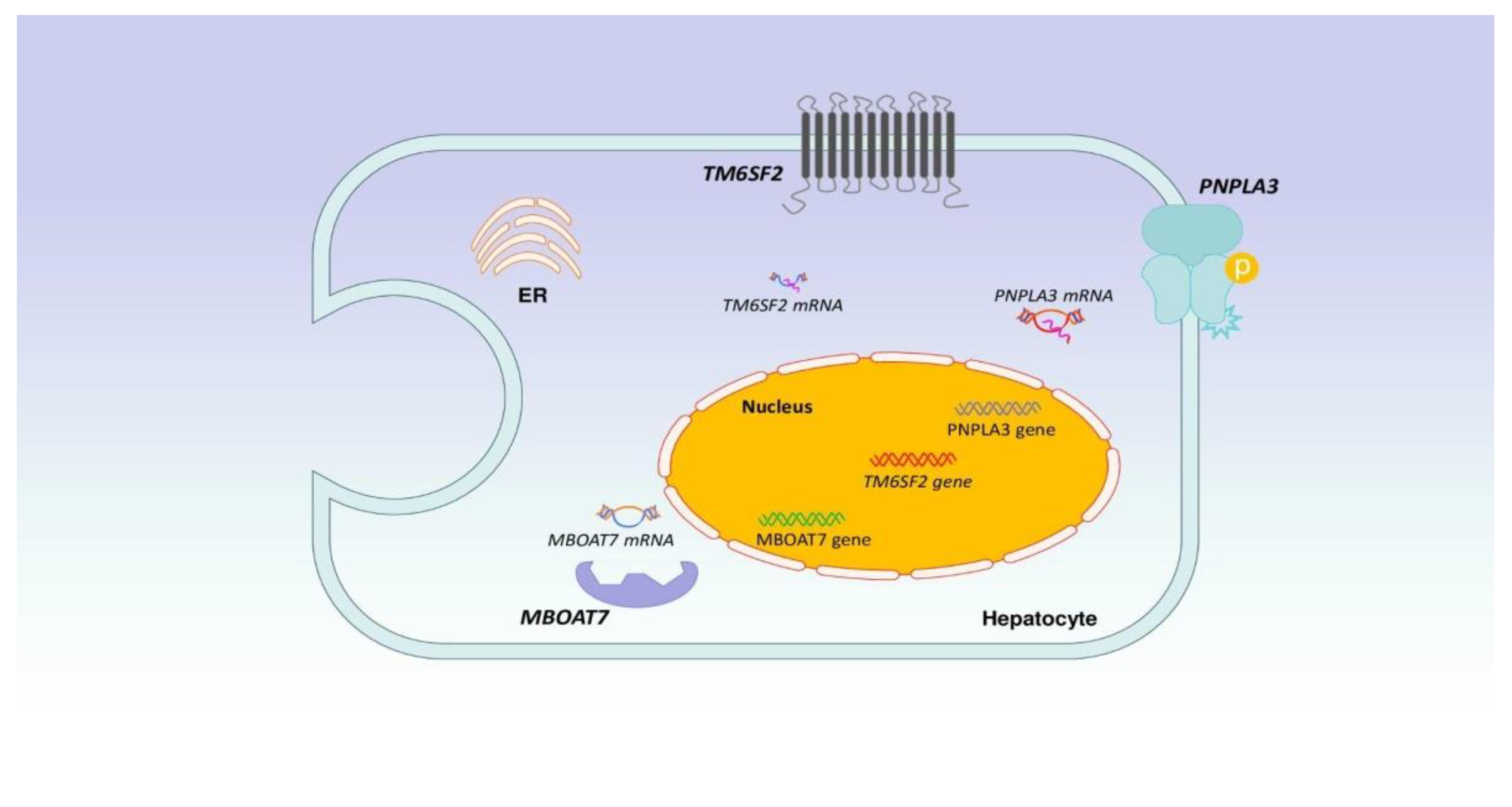
2. NAFLD Pathogenesis
3. NAFLD Monitoring in the Clinical Setting
3.1. Scores Based upon Serum Biomarkers
3.2. Scores Based upon Imaging Modalities
3.3. A Combination of Serum Biomarkers and Imaging Modalities
4. Prevention of NAFLD
5. NAFLD Treatment Options
6. Current Concepts and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD prevalence: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholicfatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Setiawan, V.W.; Ng, C.H.; Lim, W.H.; Muthiah, M.D.; Tan, E.X.; Dan, Y.Y.; Roberts, L.R.; Loomba, R.; Huang, D.Q. Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH. Hepatology 2023, 77, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell. Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef] [PubMed]
- Wieland, A.; Kohli, R. Non-Alcoholic Steatohepatitis as a Growing Indication for Liver Transplantation: The Evolving Gender and Ethnic Trends. Am. J. Gastroenterol. 2018, 113, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Vipani, A.; Bresee, C.; Todo, T.; Kim, I.K.; Alkhouri, N.; Setiawan, V.W.; Tran, T.; Ayoub, W.S.; Lu, S.C.; et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am. J. Gastroenterol. 2018, 113, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e585. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, S.; Valenti, L. Hepatic fat as clinical outcome and therapeutic target for nonalcoholic fatty liver disease. Liver Int. 2019, 39, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Chan, H.L.Y.; Chien, R.N.; Chuang, W.L.; Fung, J.; Goh, G.B.; Hu, T.H.; Huang, J.F.; Jang, B.K.; Jun, D.W.; et al. Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment. Pharmacol. Ther. 2020, 51, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Pan, L.; Ran, S.; Wang, M.; Huang, S.; Zhao, M.; Cao, Z.; Yao, Z.; Xu, L.; Yang, Q.; et al. Prediction of MAFLD and NAFLD using different screening indexes: A cross-sectional study in U.S. adults. Front. Endocrinol. 2023, 14, 1083032. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. NAFLD, MAFLD and obesity: Brothers in arms? Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dalbeni, A. NAFLD/MAFLD: New Evidence. Int. J. Mol. Sci. 2023, 24, 7241. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD). Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signaling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Iñiguez, I.; Panduro, A.; Roman, S.; González-Aldaco, K. What do we know about nutrient-based strategies targeting molecular mechanisms associated with obesity-related fatty liver disease? Ann. Hepatol. 2023, 28, 100874. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shen, H.; Li, X.; Wang, H. Endoplasmic reticulum stress in innate immune cells—A significant contribution to non-alcoholic fatty liver disease. Front. Immunol. 2022, 13, 951406. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Baiceanu, A.; Mesdom, P.; Lagouge, M.; Foufelle, F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat. Rev. Endocrinol. 2016, 12, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Alpini, G.; Francis, H. Updates on Dietary Models of Nonalcoholic Fatty Liver Disease: Current Studies and Insights. Gene Expr. 2018, 18, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Umar, M.I.; Imran, S.; Javaid, F.; Syed, S.K.; Riaz, R.; Hassan, W. TGF-β1 signaling can worsen NAFLD with liver fibrosis backdrop. Exp. Mol. Pathol. 2022, 124, 104733. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, B. Unraveling the pathogenesis of non-alcoholic fatty liver diseases through genome-wide association studies. J. Gastroenterol. Hepatol. 2023, 38, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Unalp-Arida, A.; Ruhl, C.E. Patatin-Like Phospholipase Domain-Containing Protein 3 I148M and Liver Fat and Fibrosis Scores Predict Liver Disease Mortality in the U.S. Population. Hepatology 2020, 71, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Pipitone, R.M.; Longo, M.; Malvestiti, F.; Amorini, A.M.; Distefano, A.; Casirati, E.; Ciociola, E.; Iraci, N.; Leggio, L.; et al. SIRT5 rs12216101 T>G variant is associated with liver damage and mitochondrial dysfunction in patients with non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Z.; Li, Y.T.; Wu, W.R.; Shi, D.; Fang, D.Q.; Yang, L.Y.; Bian, X.Y.; Wu, J.J.; Wang, Q.; Jiang, X.W. Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced non-alcoholic steatohepatitis. World. J. Gastroenterol. 2018, 24, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- Negi, C.K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Liu, J.; Dalamaga, M. What are the key points in the association between the gut microbiome and nonalcoholic fatty liver disease? Metabol. Open 2019, 1, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease. Current evidence and perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Nagashimada, M.; Honda, M. Effect of Microbiome on Non-Alcoholic Fatty Liver Disease and the Role of Probiotics, Prebiotics, and Biogenics. Int. J. Mol. Sci. 2021, 22, 8008. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Gibson, D.L.; Hekmatdoost, A. Non alcoholic fatty liver disease, the gut microbiome and diet. Adv. Nutr. 2017, 8, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Tincopa, M.A.; Loomba, R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol. 2023, 8, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef] [PubMed]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Biernacki, D.M.; Kalavalapalli, S.; Lomonaco, R.; Subbarayan, S.K.; Lai, J.; Tio, F.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019, 42, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of nonalcoholic fatty liver disease: Clinical prediction rules and blood based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xi, Y.; Raji, A.; Crutchlow, M.; Fernandes, G.; Engel, S.S.; Zhang, X. Overall and subgroup prevalence of non-alcoholic fatty liver disease and prevalence of advanced fibrosis in the United States: An updated national estimate in National Health and Nutrition Examination Survey (NHANES) 2011–2018. Ann. Hepatol. 2024, 29, 101154. [Google Scholar] [CrossRef] [PubMed]
- Raverdy, V.; Tavaglione, F.; Chatelain, E.; Caiazzo, R.; Saponaro, C.; Lassailly, G.; Verkindt, H.; Baud, G.; Marciniak, C.; Chetboun, M.; et al. Performance of non-invasive tests for liver fibrosis and resolution after bariatric surgery. Metabolism 2024, 12, 155790. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Schuh, A.; Longo, L.; Valentini, B.B.; Galvão, G.S.; Luchese, E.; Pinzon, C.; Bartels, R.; Álvares-da-Silva, M.R. New FIB-4 and NFS cutoffs to guide sequential non-invasive assessment of liver fibrosis by magnetic resonance elastography in NAFLD. Ann. Hepatol. 2023, 28, 100774. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Castera, L.; Wong, V.W. Noninvasive Assessment of Liver Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Patel, P.; Parkes, J.; Rosenberg, W. Derivation and performance of standardized enhanced liver fibrosis (ELF) test thresholds for the detection and prognosis of liver fibrosis. J. Appl. Lab. Med. 2019, 3, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ratziu, V.; Boursier, J.; Francque, S.; Bedossa, P.; Majd, Z.; Cordonnier, G.; Sudrik, F.B.; Darteil, R.; Liebe, R.; et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Hajji, Y.; Magnanensi, J.; Petit, S.; Majd, Z.; Delecroix, E.; Rosenquist, C.; Hum, D.; Staels, B.; et al. NIS2+TM as a screening tool to optimize patient selection in metabolic dysfunction-associated steatohepatitis clinical trials. J. Hepatol. 2024, 80, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ratziu, V.; Magnanensi, J.; Hajji, Y.; Deledicque, S.; Majd, Z.; Rosenquist, C.; Hum, D.W.; Staels, B.; Anstee, Q.M.; et al. NIS2+™, an optimisation of the blood-based biomarker NIS4® technology for the detection of at-risk NASH: A prospective derivation and validation study. J. Hepatol. 2023, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Tavaglione, F.; Jamialahmadi, O.; De Vincentis, A.; Qadri, S.; Mowlaei, M.E.; Mancina, R.M.; Ciociola, E.; Carotti, S.; Perrone, G.; Bruni, V.; et al. Development and Validation of a Score for Fibrotic Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1523–1532.e1. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Loomba, R.; Cui, J.; Wolfson, T.; Haufe, W.; Hooker, J.; Szeverenyi, N.; Ang, B.; Bhatt, A.; Wang, K.; Aryafar, H.; et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am. J. Gastroenterol. 2016, 111, 986–994. [Google Scholar] [CrossRef]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology 2014, 60, 1920–1928. [Google Scholar] [CrossRef]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.A.; Bettencourt, R.; Changchien, C.; Brenner, D.A.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R. MRI-Proton Density Fat Fraction Treatment Response Criteria in Nonalcoholic Steatohepatitis. Hepatology 2021, 73, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Munaganuru, N.; Jung, J.; Yonan, A.Q.; Loomba, R.R.; Bettencourt, R.; Ajmera, V.; Valasek, M.A.; Behling, C.; Sirlin, C.B.; et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut 2022, 71, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Ajmera, V.; Loomba, R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat. Rev. Endocrinol. 2022, 18, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Truong, E.; Gornbein, J.A.; Saouaf, R.; Guindi, M.; Todo, T.; Noureddin, N.; Yang, J.D.; Harrison, S.A.; Alkhouri, N. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J. Hepatol. 2022, 76, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Truong, E.; Gornbein, J.A.; Yang, J.D.; Noureddin, N.; Harrison, S.A.; Alkhouri, N.; Noureddin, M. MRI-AST (MAST) Score Accurately Predicts Major Adverse Liver Outcome, Hepatocellular Carcinoma, Liver Transplant, and Liver-Related Death. Clin. Gastroenterol. Hepatol. 2023, 21, 2570–2577.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Tamaki, N.; Imajo, K.; Yoneda, M.; Sutter, N.; Jung, J.; Lin, T.; Tu, X.M.; Bergstrom, J.; Nguyen, K.; et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J. Hepatol. 2022, 77, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.; Nguyen, K.; Tamaki, N.; Sharpton, S.; Bettencourt, R.; Loomba, R. Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease. Therap. Adv. Gastroenterol. 2022, 29, 17562848221093869. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Harrison, S.A.; Alkhouri, N. MEFIB vs. MAST and FAST: Not a competition but useful tools. J. Hepatol. 2024, 80, e35–e36. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Castille, J.M.; Poynard, T.; Soldevila-Pico, C.; Clark, V.C.; Firpi-Morell, R.J.; Lai, J.; Cusi, K. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J. Investig. Med. 2019, 67, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, X.; Li, H.; Zhang, X.; Chen, X.; Lu, K.; Chu, C. Effects of various interventions on non-alcoholic fatty liver disease (NAFLD): A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1180016. [Google Scholar] [CrossRef] [PubMed]
- Sandby, K.; Geiker, N.R.W.; Dalamaga, M.; Grønbæk, H.; Magkos, F. Efficacy of Dietary Manipulations for Depleting Intrahepatic Triglyceride Content: Implications for the Management of Non-alcoholic Fatty Liver Disease. Curr. Obes. Rep. 2021, 10, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Perna, S.; Allehdan, S.; Rafique, A.; Saad, S.; AlGhareeb, F.; Rondanelli, M.; Tayyem, R.F.; Marino, M.; Martini, D.; et al. Does the Mediterranean Diet Have Any Effect on Lipid Profile, Central Obesity and Liver Enzymes in Non-Alcoholic Fatty Liver Disease (NAFLD) Subjects? A Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2023, 15, 2250. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hassani Zadeh, S.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Effect of Mediterranean diet on liver enzymes: A systematic review and meta-analysis of randomized controlled trials. Br. J. Nutr. 2022, 128, 1231–1239. [Google Scholar] [CrossRef]
- Lange, M.; Nadkarni, D.; Martin, L.; Newberry, C.; Kumar, S.; Kushner, T. Intermittent fasting improves hepatic end points in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Commun. 2023, 7, e0212. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, D. The Paleolithic Diet. Cureus 2023, 15, e34214. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; Stomby, A.; Waling, M.; Isaksson, A.; Söderström, I.; Ryberg, M.; Svensson, M.; Hauksson, J.; Olsson, T. A heterogeneous response of liver and skeletal muscle fat to the combination of a Paleolithic diet and exercise in obese individuals with type 2 diabetes: A randomized controlled trial. Diabetologia 2018, 61, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Moriarty, P.; et al. Nutraceutical approaches to non-alcoholic fatty liver disease (NAFLD): A position paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, Y.; Peng, J.; Zhang, K.; Wang, L.; Feng, T.; Nhamdriel, T.; Fan, G. Phytochemicals for the treatment of metabolic diseases: Evidence from clinical studies. Biomed. Pharmacother. 2023, 165, 115274. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Z.; Duan, F.; Jia, Z.; Chen, X.; Li, S. The promising role of probiotics/prebiotics/synbiotics in energy metabolism biomarkers in patients with NAFLD: A systematic review and meta-analysis. Front. Public Health 2022, 10, 862266. [Google Scholar] [CrossRef]
- Nie, Q.; Li, M.; Huang, C.; Yuan, Y.; Liang, Q.; Ma, X.; Qiu, T.; Li, J. The clinical efficacy and safety of berberine in the treatment of non-alcoholic fatty liver disease: A meta-analysis and systematic review. J. Transl. Med. 2024, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Ding, S.B.; Chu, X.L.; Jin, Y.X.; Jiang, J.J.; Zhao, X.; Yu, M. Epigallocatechin gallate alleviates high-fat diet-induced hepatic lipotoxicity by targeting mitochondrial ROS-mediated ferroptosis. Front. Pharmacol. 2023, 14, 1148814. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced effect of curcumin and resveratrol on mitigating hepatic steatosis in metabolic associated fatty liver disease via the PI3K/AKT/mTOR and HIF-1/VEGF cascade. Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef]
- Banerjee, G.; Papri, S.R.; Satapathy, S.K.; Banerjee, P. Akkermansia muciniphila—A Potential Next-generation Probiotic for Non-alcoholic Fatty Liver Disease. Curr. Pharm. Biotechnol. 2023, 25, 426–433. [Google Scholar] [CrossRef]
- Hu, W.; Gao, W.; Liu, Z.; Fang, Z.; Wang, H.; Zhao, J.; Zhang, H.; Lu, W.; Chen, W. Specific Strains of Faecalibacterium prausnitzii Ameliorate Nonalcoholic Fatty Liver Disease in Mice in Association with Gut Microbiota Regulation. Nutrients 2022, 14, 2945. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.; Jin, X.; Zhou, Q. Efficacy of traditional Chinese medicine combined with Silibinin on nonalcoholic fatty liver disease: A meta-analysis and systematic review. Medicine 2024, 103, e37052. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.; Vanacore, D.; Diano, N.; Nicolucci, C.; Errico, S.; Dallio, M.; Federico, A.; Loguercio, C.; Stiuso, P. Ameliorative effect of Silybin on bisphenol A induced oxidative stress, cell proliferation and steroid hormones oxidation in HepG2 cell cultures. Sci. Rep. 2019, 9, 3228. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Peng, Y.; Zhang, L.; Ba, Y.; Jin, G.; Liu, G. Effect of different exercise modalities on nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Sci. Rep. 2024, 14, 6212. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.; Luo, W.; Sheng, Y. Effect of exercise intervention on clinical parameters in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Eur. J. Gastroenterol. Hepatol. 2024, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Yoo, J.J.; Cho, Y.; Kang, S.H.; Ahn, S.B.; Lee, H.W.; Jun, D.W.; Song, D.S.; Choi, M. Effect of exercise-based interventions in nonalcoholic fatty liver disease: A systematic review with meta-analysis. Dig. Liver Dis. 2023, 55, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Banini, B.A.; Do, A.; Gunderson, C.; Zaman, S.; Lim, J.K. Exercise Does Not Independently Improve Histological Outcomes in Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Deng, H.; Yang, L.; Li, L.; Lin, J.; Zheng, P.; Ji, G. Vitamin E for people with non-alcoholic fatty liver disease. Cochrane Database Syst. Rev. 2022, 2022, CD015033. [Google Scholar] [CrossRef]
- Pockros, P.J.; Fuchs, M.; Freilich, B.; Schiff, E.; Kohli, A.; Lawitz, E.J.; Hellstern, P.A.; Owens-Grillo, J.; Van Biene, C.; Shringarpure, R.; et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019, 39, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Ratziu, V.; Loomba, R.; Anstee, Q.M.; Kowdley, K.V.; Rinella, M.E.; Sheikh, M.Y.; Trotter, J.F.; Knapple, W.; Lawitz, E.J.; et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J. Hepatol. 2023, 79, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Tang, A.S.P.; Xiao, J.; Wong, Z.Y.; Yong, J.N.; Fu, C.E.; Zeng, R.W.; Tan, C.; Wong, G.H.Z.; Teng, M.; et al. Safety and tolerability of obeticholic acid in chronic liver disease: A pooled analysis of 1878 individuals. Hepatol. Commun. 2023, 7, e0005. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Bays, H.E.; Miller, M.; Cain, J.E.; 3rd Wasilewska, K.; Andrawis, N.S.; Parli, T.; Feng, S.; Sterling, L.; Tseng, L.; et al. The FGF21 analog pegozafermin in severe hypertriglyceridemia: A randomized phase 2 trial. Nat. Med. 2023, 29, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Pegozafermin for NASH—A Sprint to Start a Marathon. N. Engl. J. Med. 2023, 389, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 14, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Resmetirom safe for nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, H.; Zhang, H.; Chen, X.; Chen, J.; Xu, Z.; You, H.; Dong, R.; Peng, Y.; Li, J.; et al. ZSP1601, a novel pan-phosphodiesterase inhibitor for the treatment of NAFLD, A randomized, placebo-controlled phase Ib/IIa trial. Nat. Commun. 2023, 12, 14–6409. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Chubb, D.; Kemp, W.; Majeed, A.; Lubel, J.S.; Hodge, A.; Roberts, S.K. Understanding NAFLD: From Case Identification to Interventions, Outcomes, and Future Perspectives. Nutrients 2023, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Ajmera, V.H.; Puri, P.; Hsu, C.L.; Bassirian, S.; Mgdsyan, M.; Singh, S.; Faulkner, C.; Valasek, M.A.; Rizo, E.; et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut 2019, 68, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, J.; Eslam, M. Biomarkers of Metabolic (Dysfunction)-associated Fatty Liver Disease: An Update. J. Clin. Transl. Hepatol. 2022, 10, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi-Suzuki, M.; Cusi, K.; Bril, F.; Gong, Y.; Langaee, T.; Frye, R.F. A Genetic Score Associates with Pioglitazone Response in Patients with Non-alcoholic Steatohepatitis. Front. Pharmacol. 2018, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Guo, X.; Tan, J.; Lauzon, M.; Taylor, K.D.; Loomba, R.; Cummings, O.W.; Pillai, S.; Bhatnagar, P.; Kowdley, K.V.; et al. A Pilot Genome-Wide Analysis Study Identifies Loci Associated with Response to Obeticholic Acid in Patients with NASH. Hepatol. Commun. 2019, 3, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Stefan, N. Clustering NAFLD: Phenotypes of nonalcoholic fatty liver disease and their differing trajectories. Hepatol. Commun. 2023, 24, e0112. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Valenti, L.; Wong, V.W.; Fouad, Y.M.; Yilmaz, Y.; Kim, W.; Sebastiani, G.; Younossi, Z.M.; Hernandez-Gea, V.; Zheng, M.H. Decompensation in cirrhosis: Unraveling the evolving natural history of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 6–56. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Raufman, J.P. Uncoupler therapy for NAFLD: Is flushing a possible harbinger of a safety concern? Lancet Gastroenterol. Hepatol. 2024, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Kotsiliti, E. Nanoparticles in NAFLD therapeutics. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 414. [Google Scholar] [CrossRef] [PubMed]
| Substance/Food | Recommendation/ Daily Dose | Mechanism of Action | Remarks |
|---|---|---|---|
| Artichoke Leaf Extract [81] | Very Low 1000–2000 mg | Lipid-lowering activity, mechanism largely unknown, possible via inhibition of HMG-CoA reductase | ↓ LDL ↓ TGs ↓ AST and ALT AEs: very rare (flatulence) |
| Astaxanthin [82] | Very Low 5–20 mg | Mechanisms largely unknown Amelioration in liver fibrosis and inflammation | Generally safe. Further studies in humans are needed. |
| Berberine, can be found in roots and plants, especially Berberis genus [84] | Moderate 500–1500 mg | Antioxidant effects, effect on FXR and NF-κB, Activation of LDLRs, Inhibition of PCSK9 | ↓ liver steatosis ↓ IR ↓ LDL AEs: mild gastrointestinal (diarrhea, flatulence) Its poor bioavailability should be improved. |
| Catechins in Green Tea [85,86] | Low 250–1200 mg green tea extract | Inhibition of NF-κB, ↑ Apoptosis | Conflicting results regarding its efficacy, which are attributed to the antioxidant state of each individual. |
| Coenzyme Q10 [81,82] | Low 100–300 mg | ↑ PPARs alpha and gamma, ↑ AMPK, anti-inflammatory and antioxidant role | ↓ severity of NAFLD, ↓ GGT, AST and ALT. AEs: Conflicting results on muscle function. |
| Curcumin from Curcuma longa [81,82,87] | Low 100–1500 mg | Mechanism of action largely unknown, anti-inflammatory effects | ↓ hepatic steatosis, ↓ AST. AEs: mild, such as diarrhea, headache and rash. |
| Omega 3 Fatty Acids [81,82] | Moderate 2–4 g EPA and DHA | ↓ VLDL, ↓ enzymes for the synthesis of TG, ↑ beta oxidation of fatty acids, ↓ proinflammatory cytokines, such as IL-6 and TNF-α, ↓ activation of NF-κB, ↓ IR | ↓ hepatic steatosis ↓ TGs. AEs: mild gastrointestinal |
| Prebiotics, such as oligofructose, inulin and psyllium [81,82] | Very Low | ↓ IR ↓ Inflammation via the modulation of gut microbiota | ↓ liver steatosis Generally safe. |
| Probiotics, such as Lactobacillus and Bifidobacteria and next-generation probiotics, such as Akkermansiamuciniphila and Faecalibacteriumprausnitzii [88,89] | Very Low 1–100 billion | ↓ IR ↓ Inflammation via the modulation of gut microbiota | ↓ liver steatosis Generally safe. |
| Resveratrol [81,82,87] | Very Low 100–300 mg | Activation of AMPK/SIRT1 axis, ↓ ROS, Induces Autophagy | Conflicting results regarding its efficacy. AEs: if dose >2500 mg |
| Silymarin from milk thistle [90,91] | Moderate 100–500 mg | Scavenger of ROS, ↓ activation of NF-κB, ↓ Procollagen III and TGF-β, ↑ PPAR action | ↓ liver steatosis and fibrosis AEs: non-significant Its poor bioavailability must be improved. |
| Vitamin D [81] | Very Low 1000–5000 UI | Immuno-modulatory and anti-inflammatory effects, effect on TLRs, which are implicated in the pathogenesis of NAFLD | Generally safe. |
| Vitamin E [81] | Very Low 100–1200 UI | Scavenger of ROS | Conflicting results about its safety; should be further investigated at high doses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.; Vallianou, N.G.; Geladari, E.; Panoilia, M.P.; Daskou, A.; Stratigou, T.; Karampela, I.; Tsilingiris, D.; Dalamaga, M. NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics. Biomedicines 2024, 12, 826. https://doi.org/10.3390/biomedicines12040826
Kounatidis D, Vallianou NG, Geladari E, Panoilia MP, Daskou A, Stratigou T, Karampela I, Tsilingiris D, Dalamaga M. NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics. Biomedicines. 2024; 12(4):826. https://doi.org/10.3390/biomedicines12040826
Chicago/Turabian StyleKounatidis, Dimitris, Natalia G. Vallianou, Eleni Geladari, Maria Paraskevi Panoilia, Anna Daskou, Theodora Stratigou, Irene Karampela, Dimitrios Tsilingiris, and Maria Dalamaga. 2024. "NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics" Biomedicines 12, no. 4: 826. https://doi.org/10.3390/biomedicines12040826
APA StyleKounatidis, D., Vallianou, N. G., Geladari, E., Panoilia, M. P., Daskou, A., Stratigou, T., Karampela, I., Tsilingiris, D., & Dalamaga, M. (2024). NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics. Biomedicines, 12(4), 826. https://doi.org/10.3390/biomedicines12040826









