Non-Invasive Radiofrequency Diathermy Neuromodulation Added to Supervised Therapeutic Exercise in Patellofemoral Pain Syndrome: A Single Blind Randomized Controlled Trial with Six Months of Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. Participants
2.4. Randomization and Blinding
2.5. Outcomes
2.6. Interventions
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Study Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, N.J.; Barton, C.J.; Van Middelkoop, M.; Callaghan, M.J.; Rathleff, M.S.; Vicenzino, B.T.; Davis, I.S.; Powers, C.M.; Macri, E.M.; Hart, H.F.; et al. 2018 Consensus Statement on Exercise Therapy and Physical Interventions (Orthoses, Taping and Manual Therapy) to Treat Patellofemoral Pain: Recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br. J. Sports Med. 2018, 52, 1170–1178. [Google Scholar] [CrossRef]
- Collins, N.J.; Crossley, K.M.; Darnell, R.; Vicenzino, B. Predictors of Short and Long Term Outcome in Patellofemoral Pain Syndrome: A Prospective Longitudinal Study. BMC Musculoskelet. Disord. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.; Guiguet-Auclair, C.; Coste, N.; Boisseau, N.; Gerbaud, L.; Pereira, B.; Coudeyre, E. Limited Effect of a Self-Management Exercise Program Added to Spa Therapy for Increasing Physical Activity in Patients with Knee Osteoarthritis: A Quasi-Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2020, 63, 181–188. [Google Scholar] [CrossRef]
- Campbell, R.; Evans, M.; Tucker, M.; Quilty, B.; Dieppe, P.; Donovan, J.L. Why Don’t Patients Do Their Exercises? Understanding Non-Compliance with Physiotherapy in Patients with Osteoarthritis of the Knee. J. Epidemiol. Community Health 2001, 55, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; Aguilar-Ferrándiz, M.E.; Espejo-Antúnez, L. Monopolar Dielectric Diathermy by Emission of Radiofrequency in Patellofemoral Pain. A Single-Blind-Randomized Clinical Trial. Electromagn. Biol. Med. 2020, 39, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Doyle, E. Appraisal of Clinical Practice Guideline: Patellofemoral Pain: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability and Health From the Academy of Orthopaedic Physical Therapy of the American Physical Therapy. J. Physiother. 2020, 66, 134. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Herbland, A.; Watson, T. Continuous-Mode 448 KHz Capacitive Resistive Monopolar Radiofrequency Induces Greater Deep Blood Flow Changes Compared to Pulsed Mode Shortwave: A Crossover Study in Healthy Adults. Eur. J. Physiother. 2017, 19, 137–146. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Treatment Using 448 KHz Capacitive Resistive Monopolar Radiofrequency Improves Pain and Function in Patients with Osteoarthritis of the Knee Joint: A Randomised Controlled Trial. Physiotherapy 2019, 105, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Giombini, A.; Di Cesare, A.; Safran, M.A.; Ciatti, R.; Maffulli, N. Short-Term Effectiveness of Hyperthermia for Supraspinatus Tendinopathy in Athletes: A Short-Term Randomized Controlled Study. Am. J. Sports Med. 2006, 34, 1247–1254. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Radiofrequency-Based Treatment in Therapy-Related Clinical Practice–a Narrative Review. Part II: Chronic Conditions. Phys. Ther. Rev. 2015, 20, 241–254. [Google Scholar] [CrossRef][Green Version]
- Diego, I.M.A.; Fernández-Carnero, J.; Val, S.L.; Cano-de-la-Cuerda, R.; Calvo-Lobo, C.; Piédrola, R.M.; Oliva, L.C.L.; Rueda, F.M. Analgesic Effects of a Capacitive-Resistive Monopolar Radiofrequency in Patients with Myofascial Chronic Neck Pain: A Pilot Randomized Controlled Trial. Rev. Assoc. Med. Bras. 2019, 65, 156–164. [Google Scholar] [CrossRef]
- Bito, T.; Tashiro, Y.; Suzuki, Y.; Kajiwara, Y.; Zeidan, H.; Kawagoe, M.; Sonoda, T.; Nakayama, Y.; Yokota, Y.; Shimoura, K.; et al. Acute Effects of Capacitive and Resistive Electric Transfer (CRet) on the Achilles Tendon. Electromagn. Biol. Med. 2019, 38, 48–54. [Google Scholar] [CrossRef]
- García-Marín, M.; Rodríguez-Almagro, D.; Castellote-Caballero, Y.; Achalandabaso-Ochoa, A.; Lomas-Vega, R.; Ibáñez-Vera, A.J. Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. J. Clin. Med. 2021, 10, 1611. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; de la Cruz-Torres, B. Efficacy of Monopolar Dielectric Transmission Radio Frequency in Panniculus Adiposus and Cellulite Reduction Efficacy of Monopolar Dielectric Transmission Radio Frequency in Panniculus Adiposus and Cellulite Reduction. J. Cosmet. Laser Ther. 2017, 19, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kemp, J.; Roos, E.; Skou, S.; Dundules, K.; Pazzinatto, M.; Francis, M.; Lannin, N.; Wallis, J.; Crossley, K. Program Evaluation of GLA:D® Australia: Physiotherapist Training Outcomes and Effectiveness of Implementation for People with Knee Osteoarthritis. Osteoarthr. Cartil. Open 2021, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, P. Patellofemoral Pain Syndrome. In Common Pediatric Knee Injuries; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–86. [Google Scholar]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale Development, Measurement Properties, and Clinical Application. North American Orthopaedic Rehabilitation Research Network. Phys. Ther. 1999, 79, 371–383. [Google Scholar] [PubMed]
- Crossley, K.M.; Stefanik, J.; Selfe, J.; Collins, N.; Davids, I.; Powers, C.; McConnell, J.; Vicenzino, B.; Bazet-Jones, D.M.; Esculier, J.-F.; et al. 2016 Patellofemoral Pain Consensus Statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, Definitions, Clinical Examination, Natural History, Patellofemoral Osteoarthritis and Patient-Reported Outcome Measures. Br. J. Sports Med. 2016, 50, 833–834. [Google Scholar] [CrossRef]
- Albornoz Cabello, M.; Rebollo Roldán, J.; García Pérez, R. Personal Psychological Apprehension Scale (EAPP) in Physical Therapy|Escala de Aprensión Psicológica Personal (EAPP) En Fisioterapia. Rev. Iberoam. Fisioter. Kinesiol. 2005, 8, 77–87. [Google Scholar] [CrossRef]
- Rennie, S. Electrophysical Agents—Contraindications And Precautions: An Evidence-Based Approach to Clinical Decision Making In Physical Therapy. Physiother. Can. 2010, 62, 1–80. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Vasold, K.L.; Parks, A.C.; Phelan, D.M.L.; Pontifex, M.B.; Pivarnik, J.M. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test-Retest Reliability, Validity, and Minimum Detectable Change of Visual Analog, Numerical Rating, and Verbal Rating Scales for Measurement of Osteoarthritic Knee Pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gámez, J.; Pecos-Martín, D.; Kujala, U.M.; Martínez-Merinero, P.; Montañez-Aguilera, F.J.; Romero-Franco, N.; Gallego-Izquierdo, T. Validation and Cultural Adaptation of “Kujala Score” in Spanish. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2845–2853. [Google Scholar] [CrossRef]
- Çelik, D.; Çoban, Ö.; Kiliçoglu, Ö. Minimal Clinically Important Difference of Commonly Used Hip-, Knee-, Foot-, and Ankle-Specific Questionnaires: A Systematic Review. J. Clin. Epidemiol. 2019, 113, 44–57. [Google Scholar] [CrossRef]
- Brosseau, L.; Balmer, S.; Tousignant, M.; O’Sullivan, J.P.; Goudreault, C.; Goudreault, M.; Gringras, S. Intra- and Intertester Reliability and Criterion Validity of the Parallelogram and Universal Goniometers for Measuring Maximum Active Knee Flexion and Extension of Patients with Knee Restrictions. Arch. Phys. Med. Rehabil. 2001, 82, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; de Sire, A.; Fallea, C.; Albano, M.; Grossi, G.; Bettoni, E.; Di Paolo, S.; Agostini, F.; Bernetti, A.; Puntillo, F.; et al. Efficacy of Radiofrequency as Therapy and Diagnostic Support in the Management of Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Selfe, J. Exercises Supervised by Physiotherapists Improve Pain and Function in Patients with Patellofemoral Pain. J. Physiother. 2010, 56, 61. [Google Scholar] [CrossRef][Green Version]
- Giles, L.; Webster, K.E.; Mcclelland, J.; Cook, J.L. Quadriceps Strengthening with and without Blood Flow Restriction in the Treatment of Patellofemoral Pain: A Double-Blind Randomised Trial. Br. J. Sports Med. 2017, 51, 1688–1694. [Google Scholar] [CrossRef]
- De Oliveira Silva, D.; Rathleff, M.S.; Petersen, K.; De Azevedo, F.M.; Barton, C.J. Manifestations of Pain Sensitization Across Different Painful Knee Disorders: A Systematic Review Including Meta-Analysis and Metaregression. Pain Med. 2019, 20, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, R.A.; Lankhorst, N.E.; Van Linschoten, R.; Bierma-Zeinstra, S.M.A.; Van Middelkoop, M. Exercise for Treating Patellofemoral Pain Syndrome: An Abridged Version of Cochrane Systematic Review. Eur. J. Phys. Rehabil. Med. 2016, 52, 110–133. [Google Scholar] [PubMed]
- Logan, C.A.; Bhashyam, A.R.; Tisosky, A.J.; Haber, D.B.; Jorgensen, A.; Roy, A.; Provencher, M.T. Systematic Review of the Effect of Taping Techniques on Patellofemoral Pain Syndrome. Sports Health Multidiscip. Approach 2017, 9, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; Barrios-Quinta, C.J.; Lara-Palomo, I.C.; de los Ángeles Cardero-Durán, M.; Espejo-Antúnez, L. Effects of Radiofrequency Diathermy Plus Therapeutic Exercises on Pain and Functionality of Patients with Patellofemoral Pain Syndrome: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 2348. [Google Scholar] [CrossRef]
- Sinatti, P.; Sánchez Romero, E.A.; Martínez-Pozas, O.; Villafañe, J.H. Effects of Patient Education on Pain and Function and Its Impact on Conservative Treatment in Elderly Patients with Pain Related to Hip and Knee Osteoarthritis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6194. [Google Scholar] [CrossRef]

| Total Sample (n = 86) | RF Group (n = 43) | Control Group (n = 43) | p Value * | |
|---|---|---|---|---|
| Mean age (years) | 42 (4.27) | 42 (4.39) | 43 (4.1) | 0.159 |
| Height (cm) | 167 (10.48) | 166 (11.21) | 167 (9.80) | 0.683 |
| Weight (kg) | 78.3 (14.85) | 77.7 (14.01) | 78.9 (15.78) | 0.712 |
| Body Mass Index | 28.2 (4.93) | 28.3 (5.21) | 28.2 (4.69) | 0.929 |
| Fat mass (%) | 31.5 (10.30) | 31.6 (10.63) | 31.5 (10.08) | 0.968 |
| Metabolic age (years) | 51 (19.46) | 49 (20.71) | 53 (18.15) | 0.361 |
| BMR (KJ) | 6716 (1407.18) | 6640 (1282.65) | 6792 (1533.13) | 0.620 |
| LEFS (%) | 54 (18.27) | 57 (20.43) | 51 (15.38) | 0.104 |
| KUJALA (%) | 53 (18.18) | 55 (21.07) | 51 (14.81) | 0.390 |
| VAS (mm) | 58 (18.2) | 55 (20.6) | 61 (15.0) | 0.127 |
| Flexion (°) | 113 (12.37) | 115 (11.67) | 112 (13.06) | 0.363 |
| Extension (°) | 0.7 (1.95) | 0.9 (2.25) | 0.6 (1.62) | 0.412 |
| PPAS | 27 (8.01) | 26 (7.07) | 30 (8.11) | 0.052 |
| Group | Baseline | Immediate Post-Test | Follow-Up | Baseline/Immediate Post-Test Differences | Between-Group Mean Changes on the Immediate Post-Test | Immediate Post-Test/Follow-Up Differences | Between-Group Mean Changes at the Follow-Up | |
|---|---|---|---|---|---|---|---|---|
| LEFS (%) | RFD | 57 ± 20.43 | 74 ± 14.71 | 77 ± 15.38 | 17 (13–20) ** | 18 (11–23) †† | 3 (1–4) * | 16 (9–21) †† |
| Control | 51 ± 15.39 | 56 ± 12.83 | 61 ± 12.03 | 5 (4–7) ** | 5 (4–6) ** | |||
| KUJALA (%) | RFD | 55 ± 21.07 | 74 ± 13.61 | 78 ± 14.09 | 19 (14–23) ** | 17 (11–23) †† | 4 (2–6) * | 14 (9–20) †† |
| Control | 53 ± 14.9 | 57 ± 15.1 | 64 ± 12.6 | 4 (3–5) ** | 7 (4–8) ** | |||
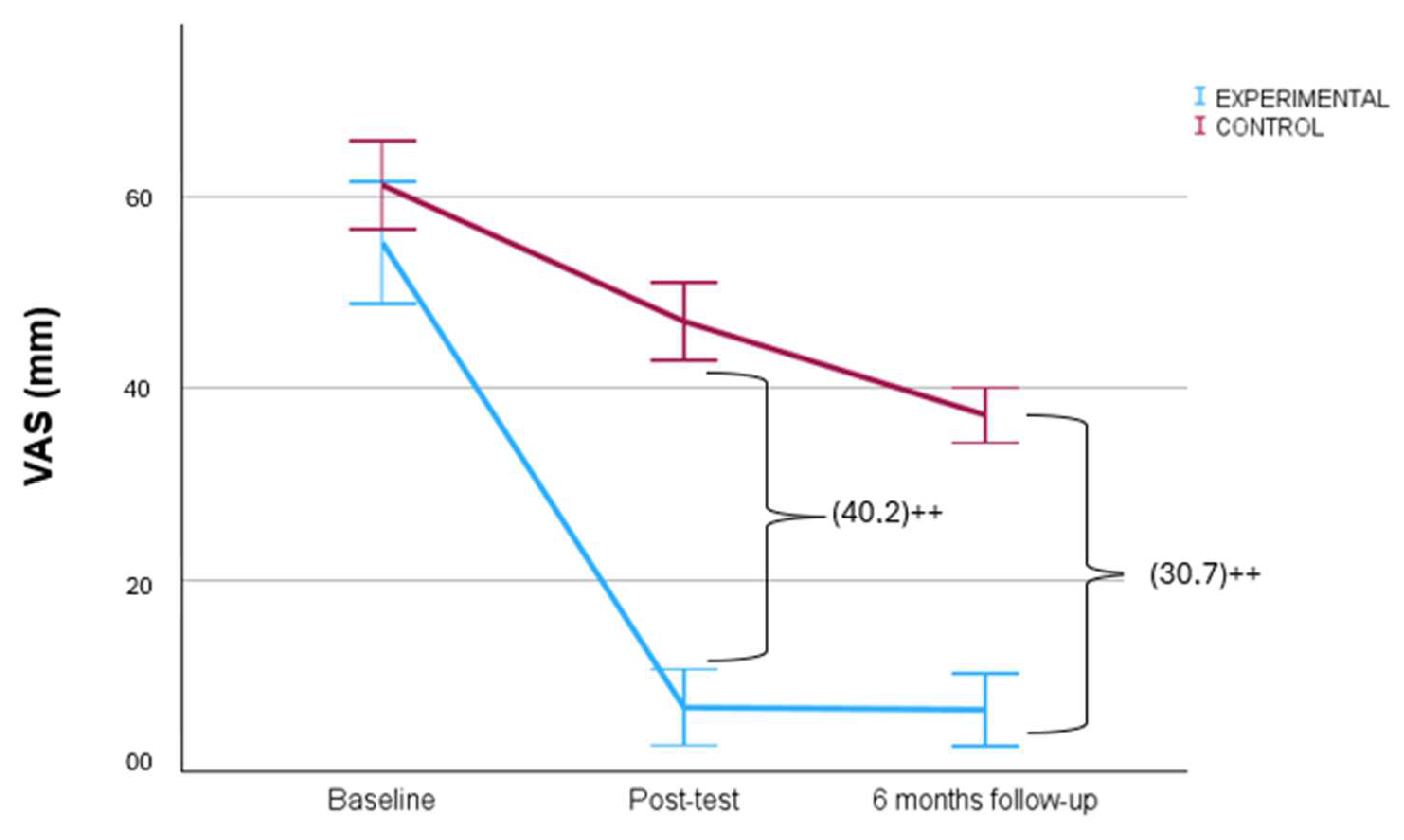
| VAS (mm) | RFD | 55.2 ± 20.6 | 6.7 ± 12.8 | 6.5 ± 12.3 | 48.4 (41.9–54.9) ** | 40.2 (45.8–34.6) †† | 0.2 (−1.9–2.4) | 30.7 (35.4–26.0) †† |
| Control | 61.1 ± 15.0 | 46.9 ± 13.2 | 37.2 ± 9.3 | 14.2 (11.5–16.8) ** | 9.7 (5.9–13.5) ** | |||
| Flexion (°) | RFD | 115 ± 11.6 | 133 ± 7.6 | 134 ± 5.6 | 18 (16–20) ** | 20 (15–24) †† | 1 (0.5–2.1) | 24 (20–28) †† |
| Control | 112 ± 13.0 | 115 ± 10.2 | 115 ± 7.5 | 3 (0.4–4.2) * | 0.7 (−2.7–1.4) | |||
| Extension (°) | RFD | 0.9 ± 2.2 | 0.1 ± 0.7 | 0 ± 0 | 0.8 (0.1–1.5) * | 0.6 (0.3–1.2) | 0.1 (−0.1–0.3) | 0.7 (0.3–1.3) † |
| Control | 0.6 ± 1.62 | 0.7 ± 2.08 | 0.7 ± 2.07 | 0.1 (−0.3–0.2) | 0 (−0.1–0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; Barrios-Quinta, C.J.; Espejo-Antúnez, L.; Lara-Palomo, I.C.; de los Ángeles Cardero-Durán, M. Non-Invasive Radiofrequency Diathermy Neuromodulation Added to Supervised Therapeutic Exercise in Patellofemoral Pain Syndrome: A Single Blind Randomized Controlled Trial with Six Months of Follow-Up. Biomedicines 2024, 12, 850. https://doi.org/10.3390/biomedicines12040850
Albornoz-Cabello M, Ibáñez-Vera AJ, Barrios-Quinta CJ, Espejo-Antúnez L, Lara-Palomo IC, de los Ángeles Cardero-Durán M. Non-Invasive Radiofrequency Diathermy Neuromodulation Added to Supervised Therapeutic Exercise in Patellofemoral Pain Syndrome: A Single Blind Randomized Controlled Trial with Six Months of Follow-Up. Biomedicines. 2024; 12(4):850. https://doi.org/10.3390/biomedicines12040850
Chicago/Turabian StyleAlbornoz-Cabello, Manuel, Alfonso Javier Ibáñez-Vera, Cristo Jesús Barrios-Quinta, Luis Espejo-Antúnez, Inmaculada Carmen Lara-Palomo, and María de los Ángeles Cardero-Durán. 2024. "Non-Invasive Radiofrequency Diathermy Neuromodulation Added to Supervised Therapeutic Exercise in Patellofemoral Pain Syndrome: A Single Blind Randomized Controlled Trial with Six Months of Follow-Up" Biomedicines 12, no. 4: 850. https://doi.org/10.3390/biomedicines12040850
APA StyleAlbornoz-Cabello, M., Ibáñez-Vera, A. J., Barrios-Quinta, C. J., Espejo-Antúnez, L., Lara-Palomo, I. C., & de los Ángeles Cardero-Durán, M. (2024). Non-Invasive Radiofrequency Diathermy Neuromodulation Added to Supervised Therapeutic Exercise in Patellofemoral Pain Syndrome: A Single Blind Randomized Controlled Trial with Six Months of Follow-Up. Biomedicines, 12(4), 850. https://doi.org/10.3390/biomedicines12040850








