Significant Genes Associated with Mortality and Disease Progression in Grade II and III Glioma
Abstract
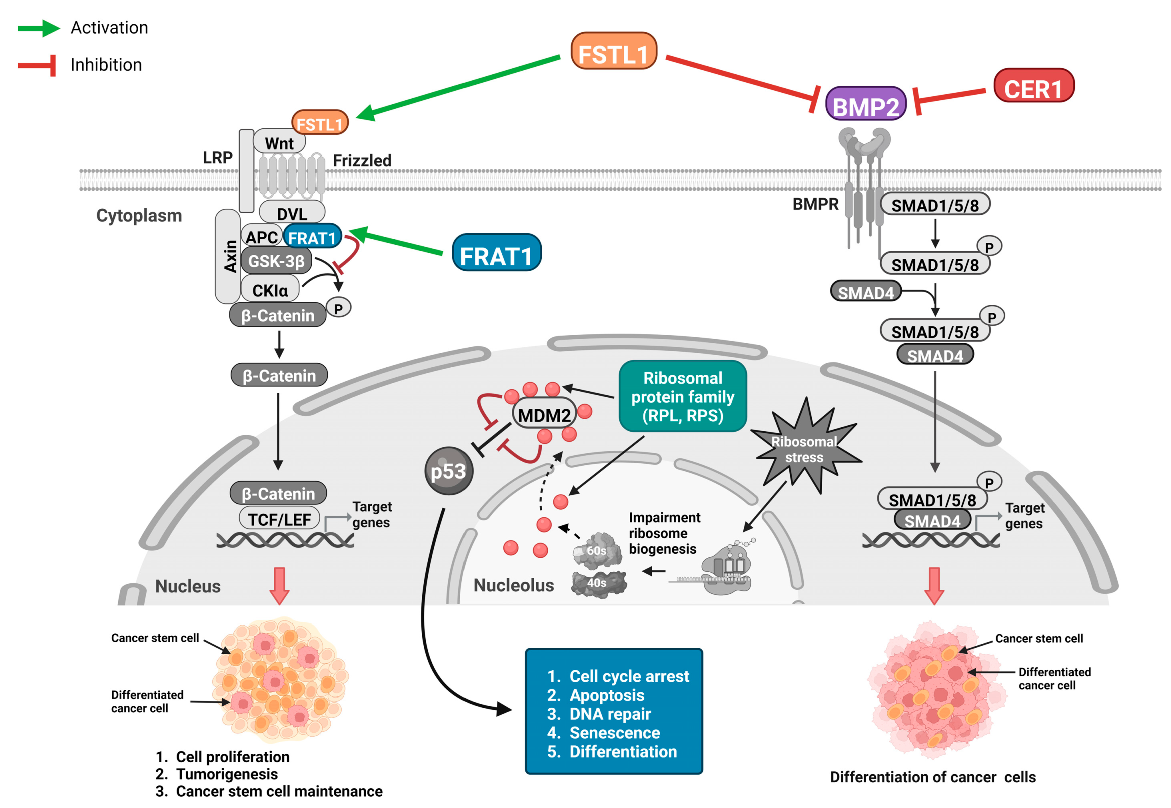
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Gene Sets Related to the Wnt/β-Catenin Pathway
2.3. Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Patients
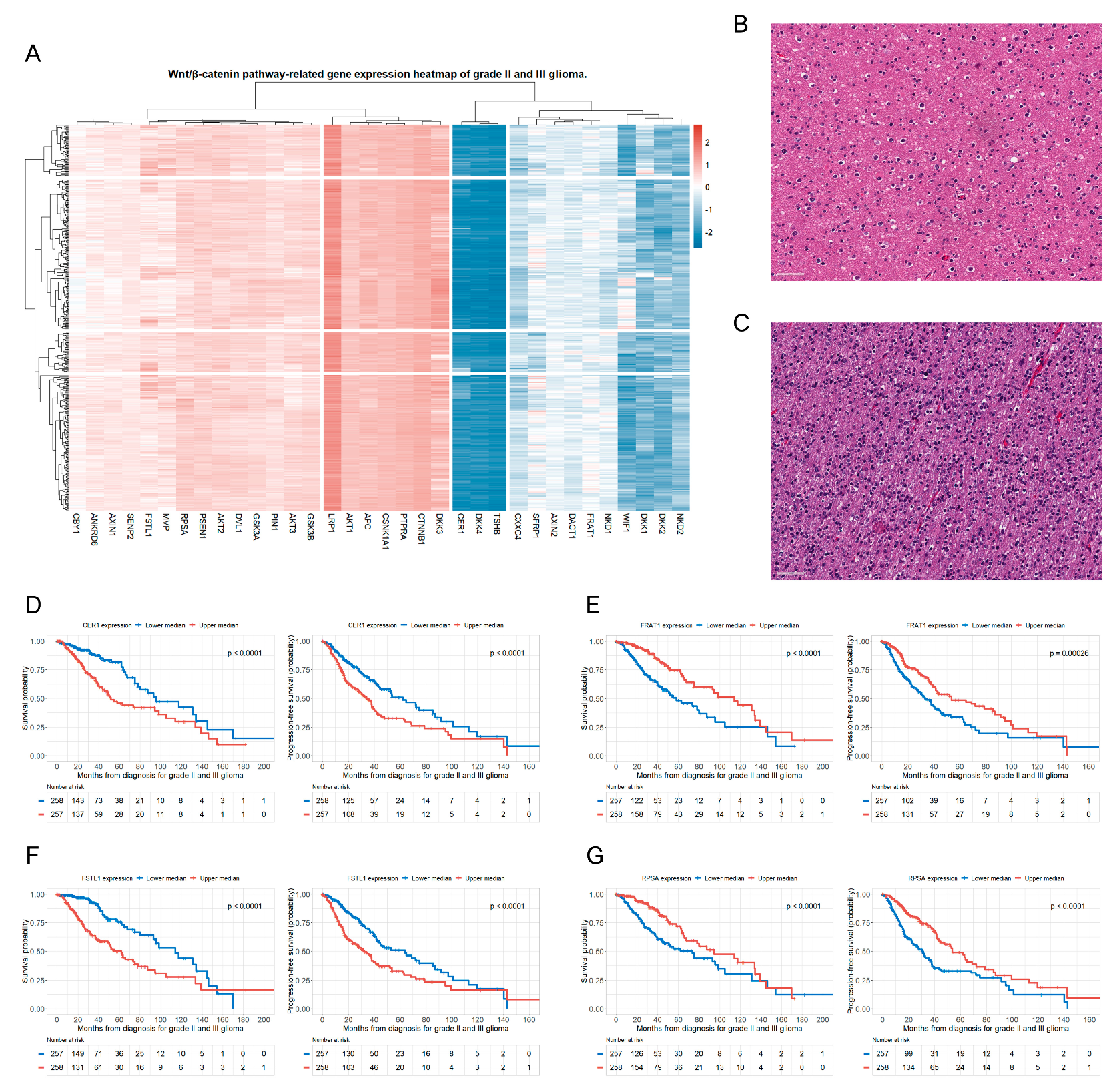
3.2. Genes of the Wnt/β-Catenin Pathway Associated with OS and PFS in Patients with Glioma
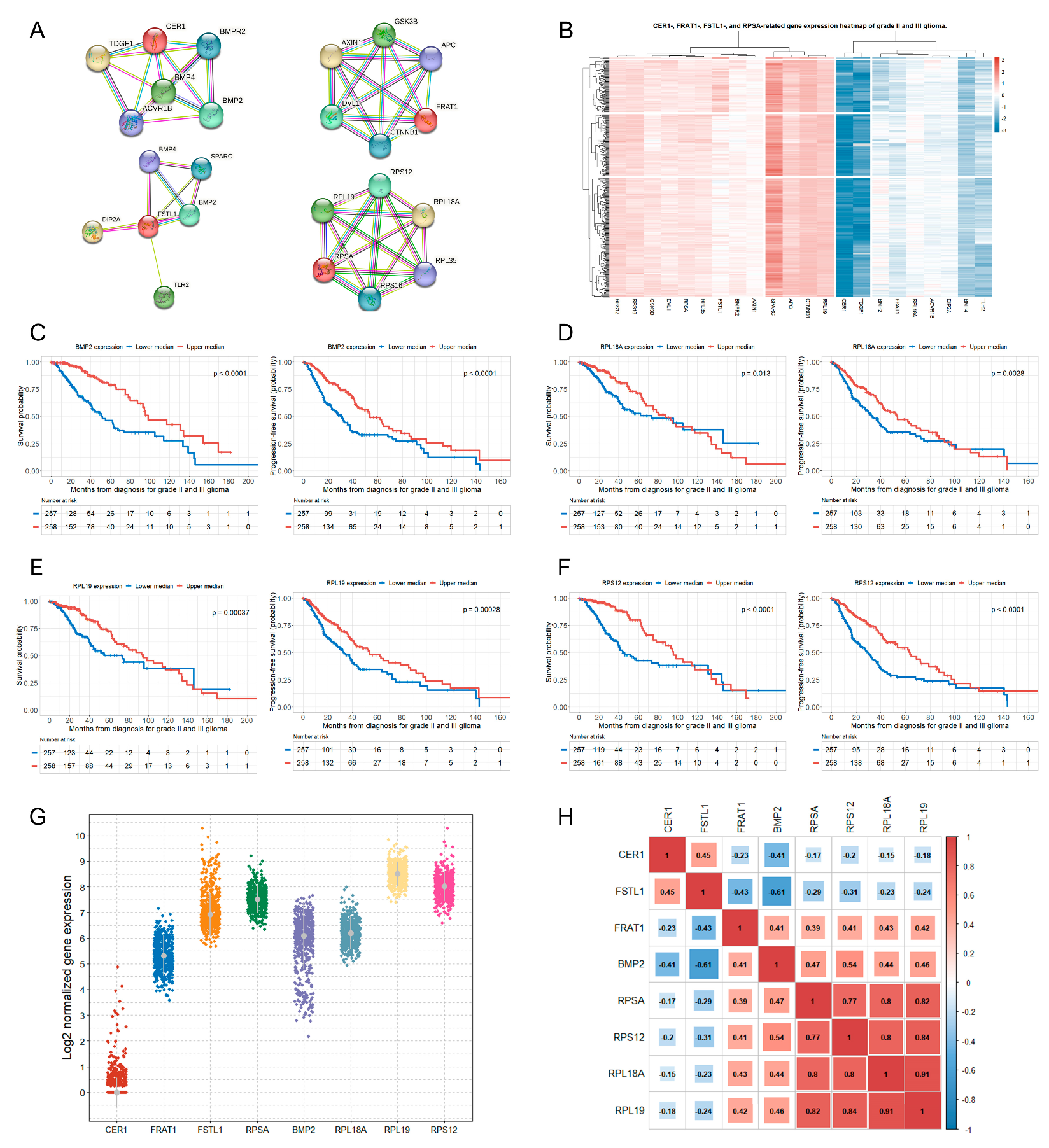
3.3. Genes Related to the Four Selected Significant Genes Associated with Both OS and PFS in Patients with Glioma
3.4. Correlations between the Selected Eight Significant Genes
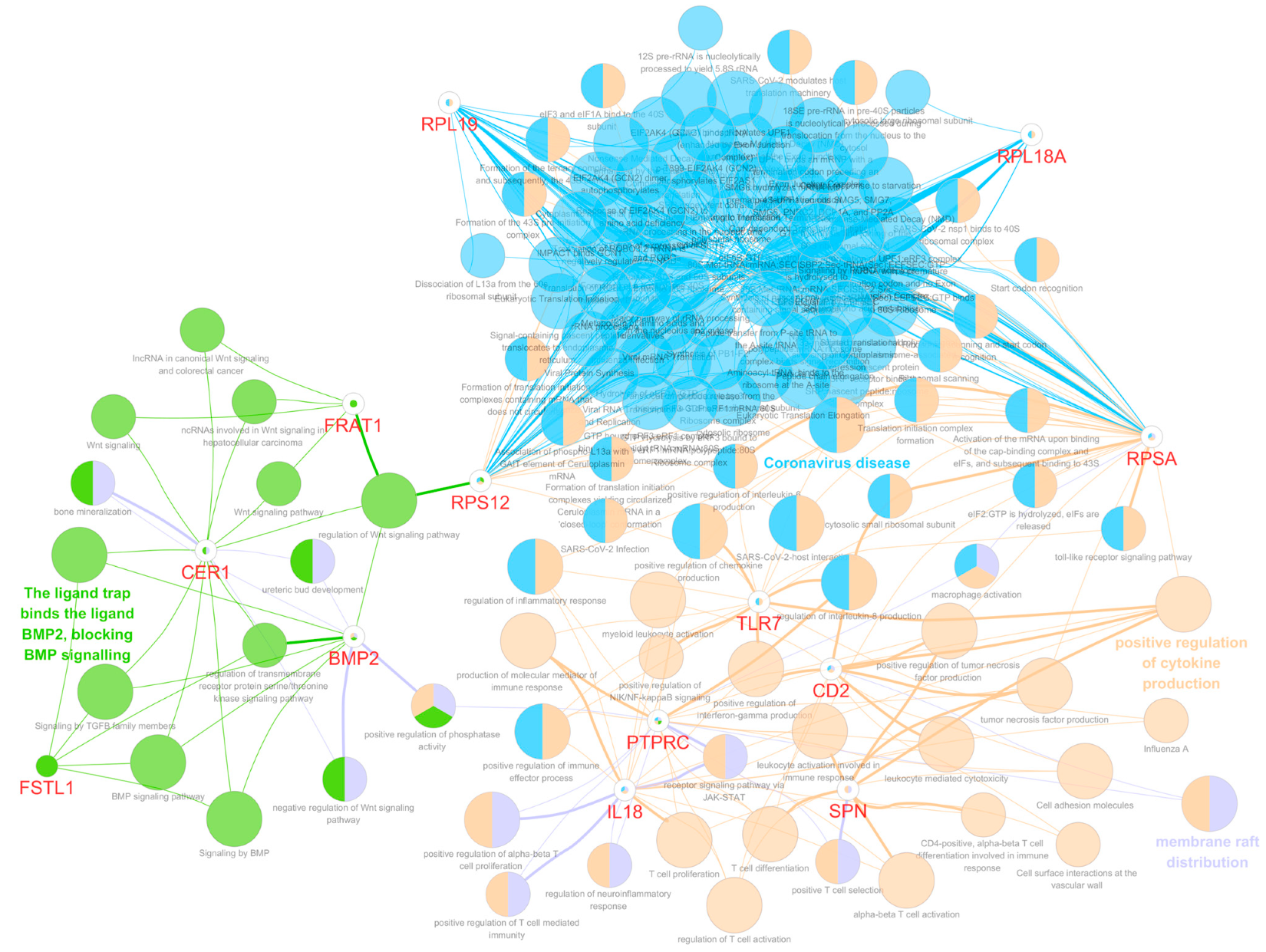
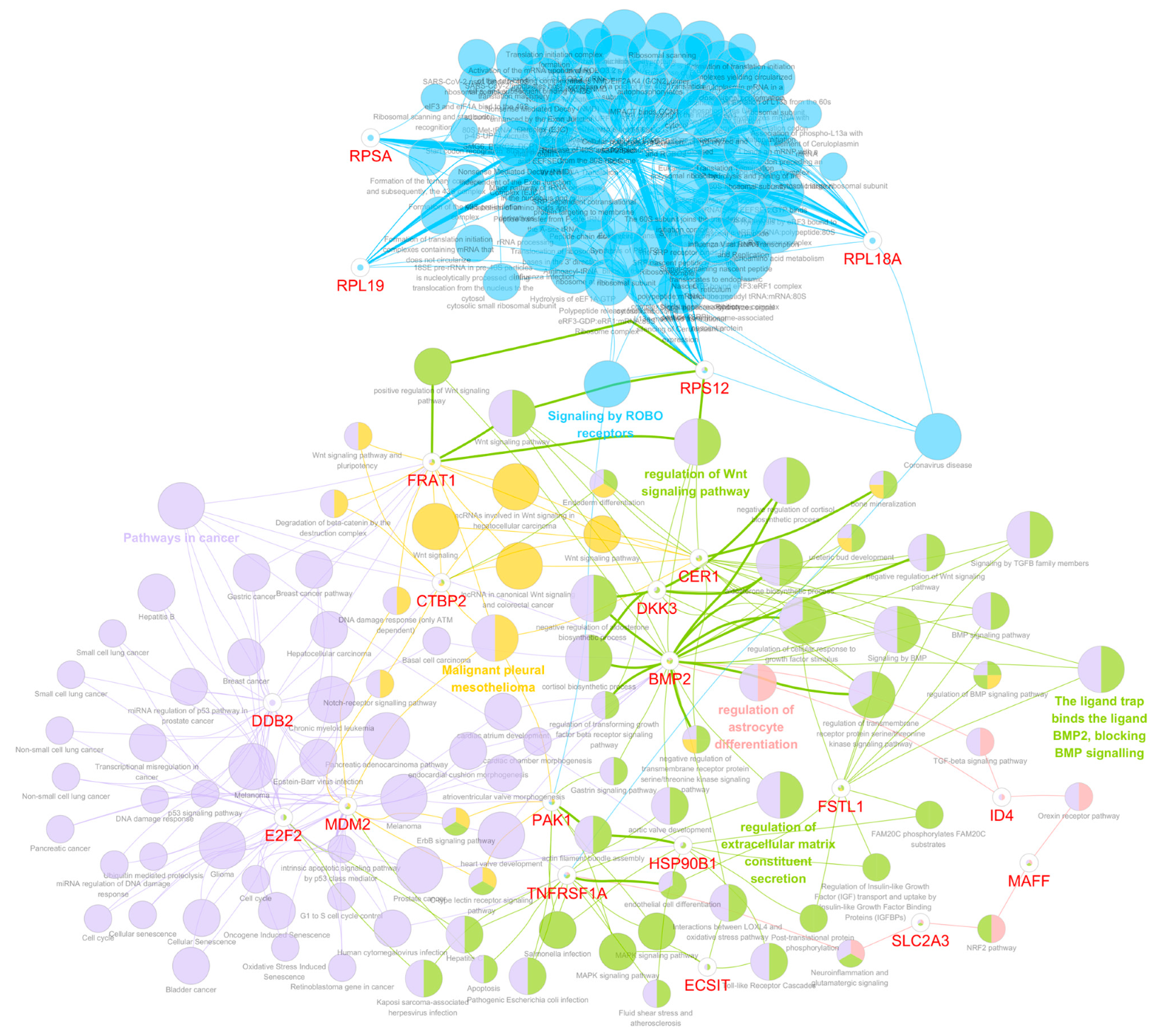
3.5. Functional Gene Ontology and Pathway Network Analyses
3.6. Functions of the Selected Eight Significant Genes on Mortality and Disease Progression in Glioma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesfin, F.B.; Al-Dhahir, M.A. Gliomas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer Stemness, Intratumoral Heterogeneity, and Immune Response across Cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef]
- Fodde, R.; Brabletz, T. Wnt/Beta-Catenin Signaling in Cancer Stemness and Malignant Behavior. Curr. Opin. Cell Biol. 2007, 19, 150–158. [Google Scholar] [CrossRef]
- Chen, Y.-H.; McGowan, L.D.; Cimino, P.J.; Dahiya, S.; Leonard, J.R.; Lee, D.Y.; Gutmann, D.H. Mouse Low-Grade Gliomas Contain Cancer Stem Cells with Unique Molecular and Functional Properties. Cell Rep. 2015, 10, 1899–1912. [Google Scholar] [CrossRef]
- Denysenko, T.; Annovazzi, L.; Cassoni, P.; Melcarne, A.; Mellai, M.; Schiffer, D. WNT/β-Catenin Signaling Pathway and Downstream Modulators in Low- and High-Grade Glioma. Cancer Genom. Proteom. 2016, 13, 31–45. [Google Scholar]
- Liu, C.; Tu, Y.; Sun, X.; Jiang, J.; Jin, X.; Bo, X.; Li, Z.; Bian, A.; Wang, X.; Liu, D.; et al. Wnt/Beta-Catenin Pathway in Human Glioma: Expression Pattern and Clinical/Prognostic Correlations. Clin. Exp. Med. 2011, 11, 105–112. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An Immeasurable Source of Knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Han, M.-H.; Min, K.-W.; Noh, Y.-K.; Kim, J.M.; Cheong, J.H.; Ryu, J.I.; Won, Y.D.; Koh, S.-H.; Myung, J.K.; Park, J.Y.; et al. High DKK3 Expression Related to Immunosuppression Was Associated with Poor Prognosis in Glioblastoma: Machine Learning Approach. Cancer Immunol. Immunother. 2022, 71, 3013–3027. [Google Scholar] [CrossRef]
- Jo, J.; van den Bent, M.J.; Nabors, B.; Wen, P.Y.; Schiff, D. Surveillance Imaging Frequency in Adult Patients with Lower-Grade (WHO Grade 2 and 3) Gliomas. Neuro Oncol. 2022, 24, 1035–1047. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Han, M.-H.; Min, K.-W.; Noh, Y.-K.; Kim, J.M.; Cheong, J.H.; Ryu, J.I.; Won, Y.D.; Koh, S.-H.; Park, Y.M. Identification of Genes from Ten Oncogenic Pathways Associated with Mortality and Disease Progression in Glioblastoma. Front. Oncol. 2022, 12, 965638. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Hwang, M.; Han, M.-H.; Park, H.-H.; Choi, H.; Lee, K.-Y.; Lee, Y.J.; Kim, J.M.; Cheong, J.H.; Ryu, J.I.; Min, K.-W.; et al. LGR5 and Downstream Intracellular Signaling Proteins Play Critical Roles in the Cell Proliferation of Neuroblastoma, Meningioma and Pituitary Adenoma. Exp. Neurobiol. 2019, 28, 628–641. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- He, Y.; Lin, Z.; Tan, S. Identification of Prognosis-Related Gene Features in Low-Grade Glioma Based on ssGSEA. Front Oncol 2022, 12, 1056623. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/Beta-Catenin Pathway in Cancer: Update on Effectors and Inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zheng, G.; Liu, Y.; Li, J.; Huang, H.; Xu, C.; Zeng, Y.; Zhang, X.; Qin, J.; et al. Follistatin-like 1 (FSTL1) Interacts with Wnt Ligands and Frizzled Receptors to Enhance Wnt/β-Catenin Signaling in Obstructed Kidneys in Vivo. J. Biol. Chem. 2022, 298, 102010. [Google Scholar] [CrossRef]
- Sun, X.-X.; DeVine, T.; Challagundla, K.B.; Dai, M.-S. Interplay between Ribosomal Protein S27a and MDM2 Protein in P53 Activation in Response to Ribosomal Stress. J. Biol. Chem. 2011, 286, 22730–22741. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; de Sampaio E Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the Vascular Fragility of the Blood Brain Barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef]
- Xu, B. Prediction and Analysis of Hub Genes between Glioblastoma and Low-Grade Glioma Using Bioinformatics Analysis. Medicine 2021, 100, e23513. [Google Scholar] [CrossRef]
- Han, M.-H.; Baek, J.M.; Min, K.-W.; Cheong, J.H.; Ryu, J.I.; Won, Y.D.; Kwon, M.J.; Koh, S.-H. DKK3 Expression Is Associated with Immunosuppression and Poor Prognosis in Glioblastoma, in Contrast to Lower-Grade Gliomas. BMC Neurol. 2023, 23, 183. [Google Scholar] [CrossRef]
- Das, S.; Srikanth, M.; Kessler, J.A. Cancer Stem Cells and Glioma. Nat. Rev. Neurol. 2008, 4, 427–435. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Cooper, M.K. Cancer Stem Cells in Glioma: Challenges and Opportunities. Transl. Cancer Res. 2013, 2, 429. [Google Scholar] [CrossRef]
- Lau, M.C.-C.; Ng, K.Y.; Wong, T.L.; Tong, M.; Lee, T.K.; Ming, X.-Y.; Law, S.; Lee, N.P.; Cheung, A.L.; Qin, Y.-R.; et al. FSTL1 Promotes Metastasis and Chemoresistance in Esophageal Squamous Cell Carcinoma through NFκB-BMP Signaling Cross-Talk. Cancer Res. 2017, 77, 5886–5899. [Google Scholar] [CrossRef]
- Xu, J.; Qi, X.; Gong, J.; Yu, M.; Zhang, F.; Sha, H.; Gao, X. Fstl1 Antagonizes BMP Signaling and Regulates Ureter Development. PLoS ONE 2012, 7, e32554. [Google Scholar] [CrossRef]
- Prakash, S.; Borreguero, L.J.J.; Sylva, M.; Flores Ruiz, L.; Rezai, F.; Gunst, Q.D.; de la Pompa, J.-L.; Ruijter, J.M.; van den Hoff, M.J.B. Deletion of Fstl1 (Follistatin-Like 1) From the Endocardial/Endothelial Lineage Causes Mitral Valve Disease. Arter. Thromb. Vasc. Biol. 2017, 37, e116–e130. [Google Scholar] [CrossRef]
- Panda, P.K.; Naik, P.P.; Praharaj, P.P.; Meher, B.R.; Gupta, P.K.; Verma, R.S.; Maiti, T.K.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; et al. Abrus Agglutinin Stimulates BMP-2-Dependent Differentiation through Autophagic Degradation of β-Catenin in Colon Cancer Stem Cells. Mol. Carcinog. 2018, 57, 664–677. [Google Scholar] [CrossRef]
- Caja, L.; Bellomo, C.; Moustakas, A. Transforming Growth Factor β and Bone Morphogenetic Protein Actions in Brain Tumors. FEBS Lett. 2015, 589, 1588–1597. [Google Scholar] [CrossRef]
- Loh, J.-J.; Li, T.-W.; Zhou, L.; Wong, T.-L.; Liu, X.; Ma, V.W.S.; Lo, C.-M.; Man, K.; Lee, T.K.; Ning, W.; et al. FSTL1 Secreted by Activated Fibroblasts Promotes Hepatocellular Carcinoma Metastasis and Stemness. Cancer Res. 2021, 81, 5692–5705. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Y.; Lou, C.; He, Y.; Zhang, Y.; Zhang, Q. FSTL1 Enhances Chemoresistance and Maintains Stemness in Breast Cancer Cells via Integrin Β3/Wnt Signaling under miR-137 Regulation. Cancer Biol. Ther. 2019, 20, 328–337. [Google Scholar] [CrossRef]
- Bach, D.-H.; Park, H.J.; Lee, S.K. The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol. Ther. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Voorneveld, P.W.; Kodach, L.L.; Jacobs, R.J.; van Noesel, C.J.M.; Peppelenbosch, M.P.; Korkmaz, K.S.; Molendijk, I.; Dekker, E.; Morreau, H.; van Pelt, G.W.; et al. The BMP Pathway Either Enhances or Inhibits the Wnt Pathway Depending on the SMAD4 and P53 Status in CRC. Br. J. Cancer 2015, 112, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-Z.; Miyake, S.; Akiyama, Y.; Yuasa, Y. BMP-2 Modulates the Proliferation and Differentiation of Normal and Cancerous Gastric Cells. Biochem. Biophys. Res. Commun. 2004, 316, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Yue, S.; Alfayez, M.; Kassem, M.; Liu, F.-F.; Aldahmash, A.; Alajez, N.M. Bone Morphogenetic Protein 2 (BMP2) Induces Growth Suppression and Enhances Chemosensitivity of Human Colon Cancer Cells. Cancer Cell Int. 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, D.; Liu, X.; He, S.; Yu, Z.; Wang, J. Inhibitory Effect of BMP-2 on the Proliferation of Breast Cancer Cells. Mol. Med. Rep. 2012, 6, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, Y.; Long, X.; Xiao, P.; Ren, X.; Yu, J. BMP Signaling and Its Paradoxical Effects in Tumorigenesis and Dissemination. Oncotarget 2016, 7, 78206–78218. [Google Scholar] [CrossRef]
- Perekatt, A.O.; Shah, P.P.; Cheung, S.; Jariwala, N.; Wu, A.; Gandhi, V.; Kumar, N.; Feng, Q.; Patel, N.; Chen, L.; et al. SMAD4 Suppresses WNT-Driven Dedifferentiation and Oncogenesis in the Differentiated Gut Epithelium. Cancer Res. 2018, 78, 4878–4890. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Saarela, U.; Railo, A.; Prunskaite-Hyyryläinen, R.; Skovorodkin, I.; Anthony, S.; Katsu, K.; Liu, Y.; Shan, J.; Salgueiro, A.M.; et al. A Secreted BMP Antagonist, Cer1, Fine Tunes the Spatial Organization of the Ureteric Bud Tree during Mouse Kidney Development. PLoS ONE 2011, 6, e27676. [Google Scholar] [CrossRef]
- Cai, W.; Albini, S.; Wei, K.; Willems, E.; Guzzo, R.M.; Tsuda, M.; Giordani, L.; Spiering, S.; Kurian, L.; Yeo, G.W.; et al. Coordinate Nodal and BMP Inhibition Directs Baf60c-Dependent Cardiomyocyte Commitment. Genes Dev. 2013, 27, 2332–2344. [Google Scholar] [CrossRef]
- Guo, G.; Liu, J.; Ren, Y.; Mao, X.; Hao, Y.; Zhong, C.; Chen, X.; Wang, X.; Wu, Y.; Lian, S.; et al. FRAT1 Enhances the Proliferation and Tumorigenesis of CD133+Nestin+ Glioma Stem Cells In Vitro and In Vivo. J. Cancer 2020, 11, 2421–2430. [Google Scholar] [CrossRef]
- Guo, G.; Mao, X.; Wang, P.; Liu, B.; Zhang, X.; Jiang, X.; Zhong, C.; Huo, J.; Jin, J.; Zhuo, Y. The Expression Profile of FRAT1 in Human Gliomas. Brain Res. 2010, 1320, 152–158. [Google Scholar] [CrossRef]
- Yang, B.; Liu, D.; Ren, Y.-Q.; Sun, Y.-Q.; Zhang, J.-P.; Wang, X.-G.; Wu, Y.-Q.; Wang, S.-L.; Guo, S.-H.; Guo, G. FRAT1 Promotes the Angiogenic Properties of Human Glioblastoma Cells via VEGFA. Mol. Med. Rep. 2022, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Kuai, D.; Cai, S.; Xue, N.; Liu, Y.; Hao, J.; Fan, Y.; Jin, J.; Mao, X.; Liu, B.; et al. Knockdown of FRAT1 Expression by RNA Interference Inhibits Human Glioblastoma Cell Growth, Migration and Invasion. PLoS ONE 2013, 8, e61206. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nawijn, M.; Franca-Koh, J.; Zevenhoven, J.; van der Gulden, H.; Jonkers, J.; Berns, A. Frat Is Dispensable for Canonical Wnt Signaling in Mammals. Genes Dev. 2005, 19, 425–430. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Berns, A. Re-Evaluating the Role of Frat in Wnt-Signal Transduction. Cell Cycle 2005, 4, 4065–4072. [Google Scholar] [CrossRef]
- Deisenroth, C.; Franklin, D.A.; Zhang, Y. The Evolution of the Ribosomal Protein-MDM2-P53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026138. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.S. Emerging Functions of Ribosomal Proteins in Gene-Specific Transcription and Translation. Biochem. Biophys. Res. Commun. 2009, 379, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Brady, C.A.; Johnson, T.M.; Lee, E.Y.; Park, E.J.; Scott, M.P.; Attardi, L.D. Full P53 Transcriptional Activation Potential Is Dispensable for Tumor Suppression in Diverse Lineages. Proc. Natl. Acad. Sci. USA 2011, 108, 17123–17128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deisenroth, C.; Zhang, Y. RP–MDM2–P53 Pathway: Linking Ribosomal Biogenesis and Tumor Surveillance. Trends Cancer 2016, 2, 191–204. [Google Scholar] [CrossRef]
- Zakharova, G.; Efimov, V.; Raevskiy, M.; Rumiantsev, P.; Gudkov, A.; Belogurova-Ovchinnikova, O.; Sorokin, M.; Buzdin, A. Reclassification of TCGA Diffuse Glioma Profiles Linked to Transcriptomic, Epigenetic, Genomic and Clinical Data, according to the 2021 WHO CNS Tumor Classification. Int. J. Mol. Sci. 2022, 24, 157. [Google Scholar] [CrossRef]

| Characteristics | Total |
|---|---|
| Number | 515 |
| Sex, female, n (%) | 230 (44.7) |
| Age at diagnosis of glioma, mean ± SD, y | 42.9 ± 13.4 |
| Time duration between glioma diagnosis and death (months), mean ± SD | 31.7 ± 31.5 |
| Time duration between glioma diagnosis and disease progression (months), mean ± SD | 25.8 ± 25.7 |
| WHO-grade glioma, n (%) | |
| Grade II | 250 (48.5) |
| Grade III | 265 (51.5) |
| Glioma histological type, n (%) | |
| Astrocytoma | 194 (37.7) |
| Oligodendroglioma | 191 (37.1) |
| Oligoastrocytoma | 130 (25.2) |
| IDH1 mutation, n (%) | |
| Yes | 91 (17.7) |
| No | 34 (6.6) |
| Missing data | 390 (75.7) |
| Karnofsky Performance Scale Index, median (IQR) | 80.0 (80.0–90.0) |
| Missing data, n (%) | 52 (10.1) |
| Radiation treatment, n (%) | |
| Yes | 296 (57.5) |
| No | 185 (35.9) |
| Missing data | 34 (6.6) |
| Tumor laterality, n (%) | |
| Left | 250 (48.5) |
| Right | 253 (49.1) |
| Missing data | 12 (2.3) |
| Overall Survival | Progression-Free Survival | |||||
|---|---|---|---|---|---|---|
| Log-Rank Test (Kaplan–Meier Analysis) | Multivariate Cox Regression Analysis * | Log-Rank Test (Kaplan–Meier Analysis) | Multivariate Cox Regression Analysis * | |||
| 1. Wnt/β-catenin signaling (34 genes were analyzed) (upper median vs. lower median) | p | HR (95% CI) | p | p | HR (95% CI) | p |
| AKT2 | <0.001 | 1.43 (0.93–2.21) | 0.106 | <0.001 | 1.54 (1.10–2.18) | 0.014 |
| CER1 | <0.001 | 2.56 (1.69–3.89) | <0.001 | <0.001 | 1.80 (1.32–2.46) | <0.001 |
| CXXC4 | 0.002 | 0.71 (0.47–1.06) | 0.095 | <0.001 | 0.67 (0.49–0.92) | 0.014 |
| DKK3 | 0.035 | 1.44 (0.97–2.15) | 0.075 | 0.031 | 1.32 (0.98–1.79) | 0.071 |
| FRAT1 | <0.001 | 0.45 (0.30–0.68) | <0.001 | <0.001 | 0.63 (0.47–0.86) | 0.004 |
| FSTL1 | <0.001 | 1.92 (1.24–2.98) | 0.003 | <0.001 | 1.68 (1.21–2.33) | 0.002 |
| NKD1 | 0.008 | 0.94 (0.62–1.41) | 0.752 | 0.031 | 0.90 (0.66–1.24) | 0.528 |
| PTPRA | 0.002 | 0.86 (0.58–1.29) | 0.472 | 0.001 | 0.73 (0.54–1.00) | 0.051 |
| RPSA | <0.001 | 0.56 (0.37–0.84) | 0.005 | <0.001 | 0.51 (0.37–0.69) | <0.001 |
| SENP2 | 0.009 | 0.67 (0.45–1.01) | 0.054 | 0.022 | 0.67 (0.49–0.92) | 0.013 |
| 2. CER1-related genes (5 genes were analyzed) (upper median vs. lower median) | ||||||
| ACVR1B | 0.038 | 0.71 (0.47–1.06) | 0.096 | 0.026 | 0.79 (0.58–1.08) | 0.139 |
| BMP2 | <0.001 | 0.39 (0.25–0.62) | <0.001 | <0.001 | 0.41 (0.30–0.58) | <0.001 |
| BMP4 | 0.005 | 0.66 (0.44–1.00) | 0.049 | 0.029 | 0.77 (0.56–1.04) | 0.091 |
| 3. FRAT1-related genes (5 genes were analyzed) (upper median vs. lower median) | ||||||
| None | … | … | … | … | … | … |
| 4. FSTL1-related genes (5 genes were analyzed) (upper median vs. lower median) | ||||||
| BMP2 | <0.001 | 0.39 (0.25–0.62) | <0.001 | <0.001 | 0.41 (0.30–0.58) | <0.001 |
| BMP4 | 0.005 | 0.66 (0.44–1.00) | 0.049 | 0.029 | 0.77 (0.56–1.04) | 0.091 |
| SPARC | 0.001 | 0.82 (0.53–1.26) | 0.360 | <0.001 | 0.71 (0.52–0.98) | 0.035 |
| TLR2 | <0.001 | 1.60 (0.99–2.56) | 0.053 | <0.001 | 1.58 (1.12–2.24) | 0.010 |
| 5. RPSA-related genes (5 genes were analyzed) (upper median vs. lower median) | ||||||
| RPL18A | 0.013 | 0.55 (0.37–0.83) | 0.004 | 0.003 | 0.60 (0.44–0.82) | 0.001 |
| RPL19 | <0.001 | 0.50 (0.33–0.75) | 0.001 | <0.001 | 0.59 (0.43–0.80) | 0.001 |
| RPS12 | <0.001 | 0.52 (0.34–0.80) | 0.003 | <0.001 | 0.53 (0.39–0.73) | <0.001 |
| Multivariate Cox Analyses of the Selected Four Significant Genes Related to Wnt/β-Catenin Signaling | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | ||||||||
| CER1 * | FRAT1 * | FSTL1 * | RPSA * | |||||
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Female (vs. male) | 0.81 (0.54–1.20) | 0.287 | 0.82 (0.55–1.22) | 0.335 | 0.93 (0.62–1.38) | 0.705 | 0.84 (0.56–1.26) | 0.399 |
| Age | 1.06 (1.05–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| WHO grade III (vs. grade II) | 2.16 (1.37–3.41) | 0.001 | 2.29 (1.44–3.62) | <0.001 | 1.94 (1.23–3.04) | 0.004 | 2.10 (1.34–3.29) | 0.001 |
| Histological type | ||||||||
| Oligodendroglioma (vs. astrocytoma) | 0.49 (0.30–0.78) | 0.003 | 0.53 (0.34–0.85) | 0.008 | 0.60 (0.37–0.97) | 0.038 | 0.55 (0.34–0.89) | 0.014 |
| Oligoastrocytoma (vs. astrocytoma) | 0.68 (0.40–1.15) | 0.151 | 0.67 (0.39–1.13) | 0.133 | 0.64 (0.38–1.07) | 0.090 | 0.66 (0.39–1.11) | 0.118 |
| Karnofsky Performance Scale Index | 0.99 (0.97–1.01) | 0.163 | 1.00 (0.98–1.01) | 0.518 | 0.99 (0.98–1.01) | 0.429 | 0.99 (0.98–1.01) | 0.415 |
| Radiation treatment | 1.10 (0.66–1.85) | 0.713 | 1.13 (0.68–1.88) | 0.645 | 1.03 (0.61–1.72) | 0.922 | 1.09 (0.65–1.82) | 0.741 |
| Tumor laterality, left (vs. right) | 0.99 (0.66–1.48) | 0.967 | 1.14 (0.77–1.70) | 0.512 | 1.21 (0.81–1.81) | 0.348 | 1.20 (0.80–1.78) | 0.381 |
| CER1, upper median (vs. lower median) | 2.56 (1.69–3.89) | <0.001 | N/A | N/A | N/A | |||
| FRAT1, upper median (vs. lower median) | N/A | 0.45 (0.30–0.68) | <0.001 | N/A | N/A | |||
| FSTL1, upper median (vs. lower median) | N/A | N/A | 1.92 (1.24–2.98) | 0.003 | N/A | |||
| RPSA, upper median (vs. lower median) | N/A | N/A | N/A | 0.56 (0.37–0.84) | 0.005 | |||
| Progression-free survival | ||||||||
| CER1 * | FRAT1 * | FSTL1 * | RPSA * | |||||
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Female (vs. male) | 1.01 (0.75–1.37) | 0.932 | 1.07 (0.79–1.44) | 0.657 | 1.13 (0.84–1.53) | 0.415 | 1.01 (0.74–1.36) | 0.961 |
| Age | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| WHO grade III (vs. grade II) | 1.35 (0.96–1.91) | 0.084 | 1.39 (0.99–1.96) | 0.058 | 1.30 (0.93–1.84) | 0.130 | 1.36 (0.97–1.90) | 0.075 |
| Histological type | ||||||||
| Oligodendroglioma (vs. astrocytoma) | 0.52 (0.36–0.75) | 0.001 | 0.53 (0.37–0.76) | 0.001 | 0.61 (0.42–0.88) | 0.009 | 0.53 (0.37–0.76) | 0.001 |
| Oligoastrocytoma (vs. astrocytoma) | 0.57 (0.38–0.86) | 0.007 | 0.57 (0.38–0.86) | 0.007 | 0.57 (0.38–0.85) | 0.006 | 0.54 (0.36–0.81) | 0.003 |
| Karnofsky Performance Scale Index | 0.99 (0.98–1.01) | 0.253 | 1.00 (0.99–1.01) | 0.704 | 1.00 (0.99–1.01) | 0.670 | 1.00 (0.99–1.01) | 0.619 |
| Radiation treatment | 0.76 (0.53–1.09) | 0.139 | 0.77 (0.54–1.11) | 0.163 | 0.73 (0.51–1.05) | 0.088 | 0.69 (0.48–0.99) | 0.044 |
| Tumor laterality, left (vs. right) | 1.12 (0.82–1.51) | 0.481 | 1.19 (0.88–1.62) | 0.255 | 1.20 (0.89–1.63) | 0.239 | 1.19 (0.88–1.61) | 0.262 |
| CER1, upper median (vs. lower median) | 1.80 (1.32–2.46) | <0.001 | N/A | N/A | N/A | |||
| FRAT1, upper median (vs. lower median) | N/A | 0.63 (0.47–0.86) | 0.004 | N/A | N/A | |||
| FSTL1, upper median (vs. lower median) | N/A | N/A | 1.68 (1.21–2.33) | 0.002 | N/A | |||
| RPSA, upper median (vs. lower median) | N/A | N/A | N/A | 0.51 (0.37–0.69) | <0.001 | |||
| Multivariate Cox analyses of the selected four significant genes related to CER1, FRAT1, FSTL1, and RPSA | ||||||||
| Overall survival | ||||||||
| BMP2 * | RPL18A * | RPL19 * | RPS12 * | |||||
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Female (vs. male) | 0.89 (0.60–1.32) | 0.560 | 0.87 (0.59–1.29) | 0.492 | 0.86 (0.58–1.28) | 0.459 | 0.85 (0.57–1.26) | 0.407 |
| Age | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.04–1.07) | <0.001 | 1.06 (1.04–1.07) | <0.001 |
| WHO grade III (vs. grade II) | 2.04 (1.30–3.20) | 0.002 | 2.19 (1.39–3.46) | 0.001 | 2.20 (1.40–3.47) | 0.001 | 2.10 (1.34–3.31) | 0.001 |
| Histological type | ||||||||
| Oligodendroglioma (vs. astrocytoma) | 0.73 (0.45–1.19) | 0.209 | 0.51 (0.32–0.82) | 0.005 | 0.52 (0.33–0.83) | 0.006 | 0.64 (0.39–1.03) | 0.064 |
| Oligoastrocytoma (vs. astrocytoma) | 0.76 (0.45–1.29) | 0.306 | 0.61 (0.36–1.03) | 0.065 | 0.60 (0.36–1.02) | 0.061 | 0.72 (0.42–1.22) | 0.223 |
| Karnofsky Performance Scale Index | 0.99 (0.98–1.01) | 0.358 | 0.99 (0.98–1.01) | 0.465 | 0.99 (0.98–1.01) | 0.429 | 1.00 (0.98–1.01) | 0.540 |
| Radiation treatment | 1.08 (0.64–1.80) | 0.782 | 1.16 (0.69–1.94) | 0.583 | 1.22 (0.73–2.06) | 0.449 | 1.08 (0.64–1.82) | 0.768 |
| Tumor laterality, left (vs. right) | 1.28 (0.86–1.91) | 0.228 | 1.17 (0.78–1.74) | 0.445 | 1.18 (0.80–1.76) | 0.407 | 1.16 (0.78–1.72) | 0.472 |
| BMP2, upper median (vs. lower median) | 0.39 (0.25–0.62) | <0.001 | N/A | N/A | N/A | |||
| RPL18A, upper median (vs. lower median) | N/A | 0.55 (0.37–0.83) | 0.004 | N/A | N/A | |||
| RPL19, upper median (vs. lower median) | N/A | N/A | 0.50 (0.33–0.75) | 0.001 | N/A | |||
| RPS12, upper median (vs. lower median) | N/A | N/A | N/A | 0.52 (0.34–0.80) | 0.003 | |||
| Progression-free survival | ||||||||
| BMP2 * | RPL18A * | RPL19 * | RPS12 * | |||||
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Female (vs. male) | 1.05 (0.78–1.41) | 0.767 | 1.02 (0.75–1.38) | 0.890 | 1.03 (0.76–1.39) | 0.875 | 1.00 (0.74–1.36) | 1.000 |
| Age | 1.03 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 |
| WHO grade III (vs. grade II) | 1.36 (0.96–1.91) | 0.082 | 1.41 (1.00–1.99) | 0.051 | 1.39 (0.99–1.95) | 0.059 | 1.36 (0.97–1.91) | 0.078 |
| Histological type | ||||||||
| Oligodendroglioma (vs. astrocytoma) | 0.71 (0.48–1.03) | 0.070 | 0.51 (0.36–0.74) | <0.001 | 0.52 (0.36–0.75) | <0.001 | 0.60 (0.42–0.87) | 0.007 |
| Oligoastrocytoma (vs. astrocytoma) | 0.61 (0.40–0.92) | 0.018 | 0.53 (0.35–0.79) | 0.002 | 0.54 (0.36–0.81) | 0.003 | 0.58 (0.39–0.88) | 0.010 |
| Karnofsky Performance Scale Index | 1.00 (0.98–1.01) | 0.511 | 1.00 (0.99–1.01) | 0.709 | 1.00 (0.99–1.01) | 0.627 | 1.00 (0.99–1.01) | 0.735 |
| Radiation treatment | 0.70 (0.49–1.01) | 0.057 | 0.78 (0.54–1.12) | 0.170 | 0.80 (0.55–1.15) | 0.219 | 0.72 (0.50–1.03) | 0.073 |
| Tumor laterality, left (vs. right) | 1.20 (0.89–1.62) | 0.241 | 1.19 (0.88–1.61) | 0.257 | 1.19 (0.88–1.61) | 0.259 | 1.17 (0.87–1.58) | 0.307 |
| BMP2, upper median (vs. lower median) | 0.41 (0.30–0.58) | <0.001 | N/A | N/A | N/A | |||
| RPL18A, upper median (vs. lower median) | N/A | 0.60 (0.44–0.82) | 0.001 | N/A | N/A | |||
| RPL19, upper median (vs. lower median) | N/A | N/A | 0.59 (0.43–0.80) | 0.001 | N/A | |||
| RPS12, upper median (vs. lower median) | N/A | N/A | N/A | 0.53 (0.39–0.73) | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.M.; Cheong, J.H.; Ryu, J.I.; Won, Y.D.; Min, K.-W.; Han, M.-H. Significant Genes Associated with Mortality and Disease Progression in Grade II and III Glioma. Biomedicines 2024, 12, 858. https://doi.org/10.3390/biomedicines12040858
Choi BM, Cheong JH, Ryu JI, Won YD, Min K-W, Han M-H. Significant Genes Associated with Mortality and Disease Progression in Grade II and III Glioma. Biomedicines. 2024; 12(4):858. https://doi.org/10.3390/biomedicines12040858
Chicago/Turabian StyleChoi, Bo Mi, Jin Hwan Cheong, Je Il Ryu, Yu Deok Won, Kyueng-Whan Min, and Myung-Hoon Han. 2024. "Significant Genes Associated with Mortality and Disease Progression in Grade II and III Glioma" Biomedicines 12, no. 4: 858. https://doi.org/10.3390/biomedicines12040858






