Hypoglossal Nerve Neuropathies—Analysis of Causes and Anatomical Background
Abstract
1. Introduction
1.1. Anatomy
1.2. Anatomical Variants
1.3. Clinical Basics
1.4. Materials and Methods
2. Vessel-Induced Neuropathies
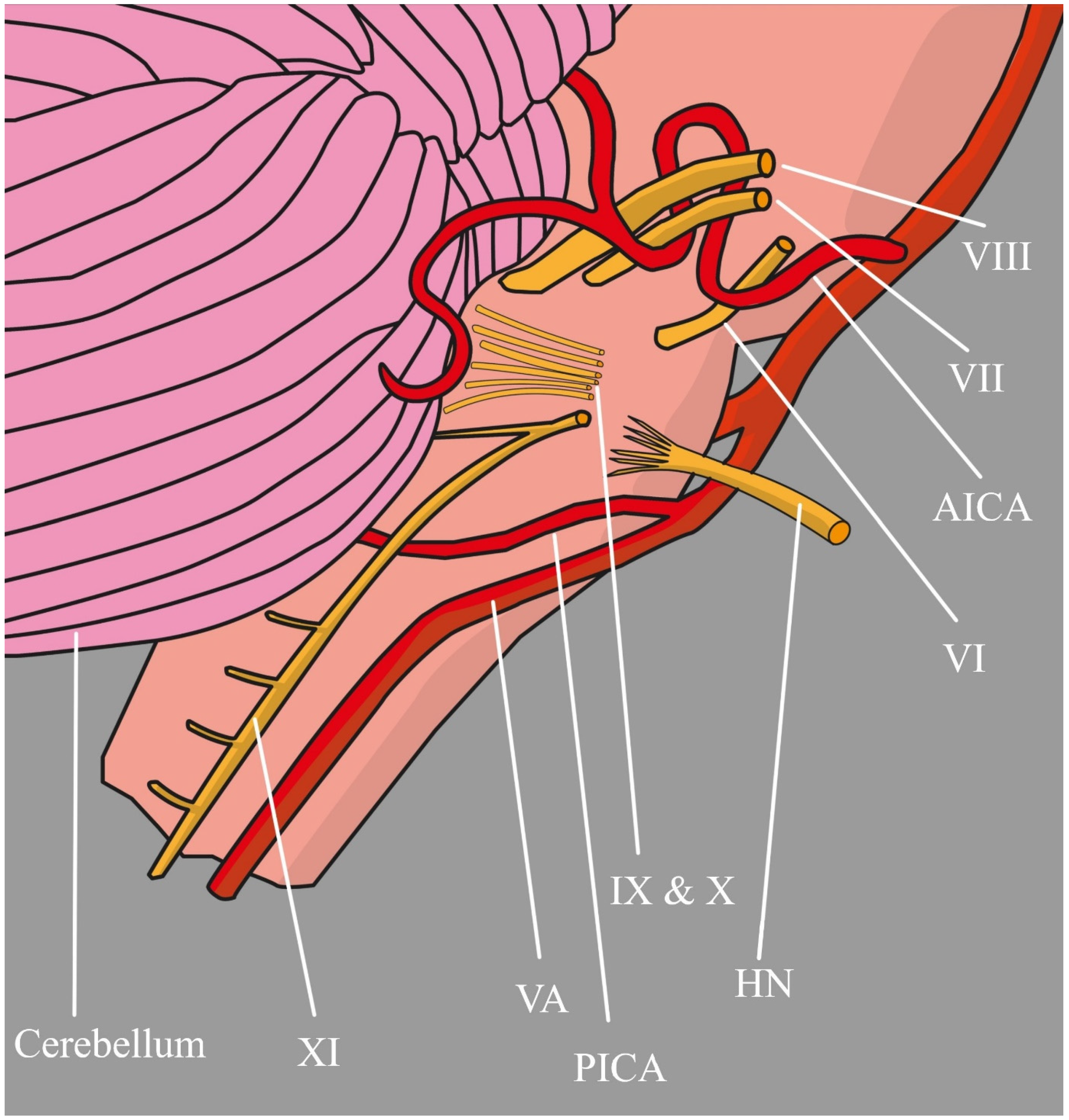
2.1. Vertebral Artery
2.2. Carotid Arteries
2.3. Dissection
2.4. Persistent Vessels
2.5. Dural Arteriovenous Fistula
2.6. Posterior Inferior Cerebellar Artery
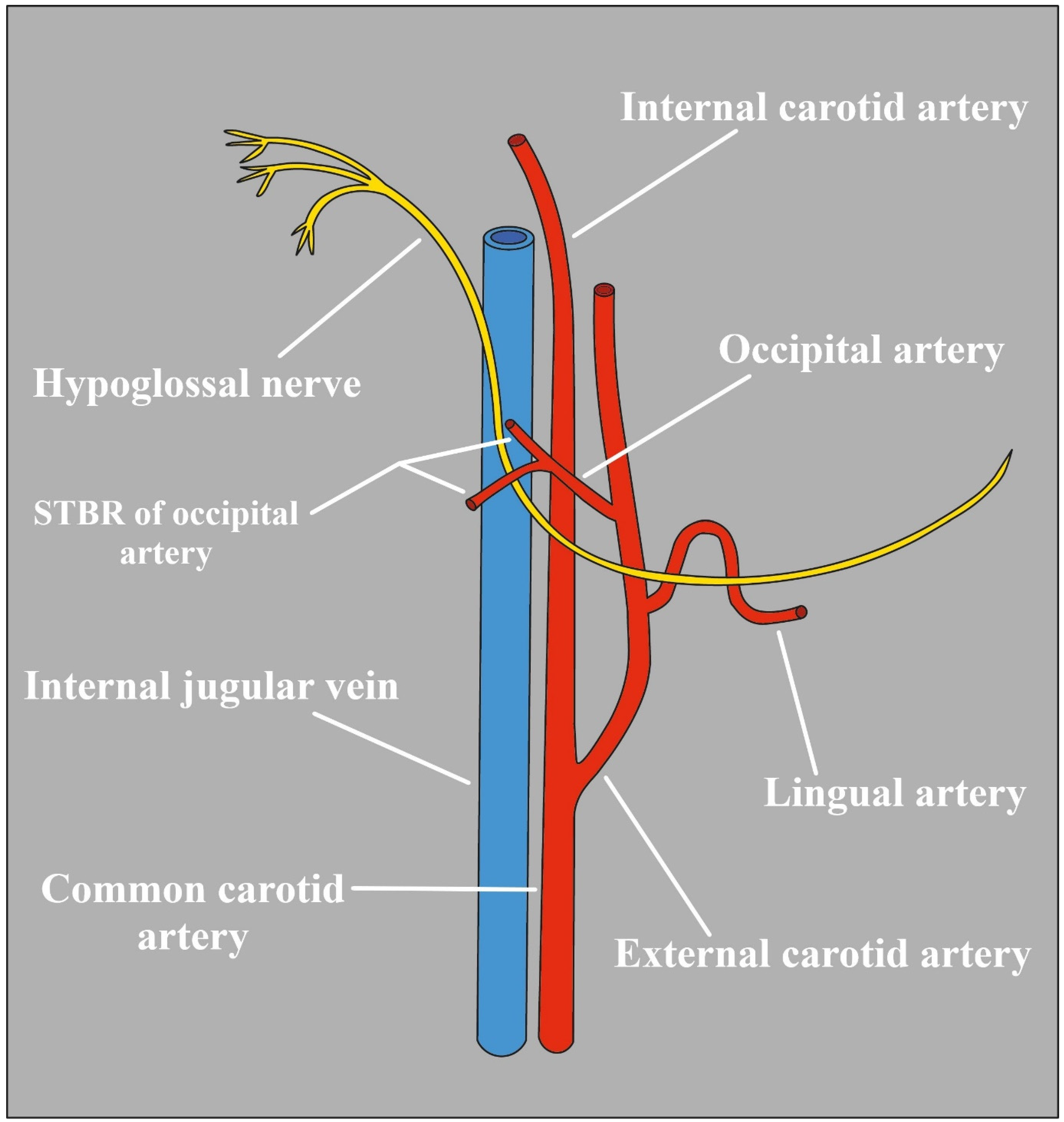
2.7. Occipital Artery
3. Iatrogenic Factors
3.1. Carotid Endarterectomy
3.2. Other Surgeries
3.3. Airway Management
3.3.1. Laryngeal Mask Airway
3.3.2. Intubation
3.4. Tapia’s Syndrome
3.5. Radiotherapy
4. Pathologic Masses
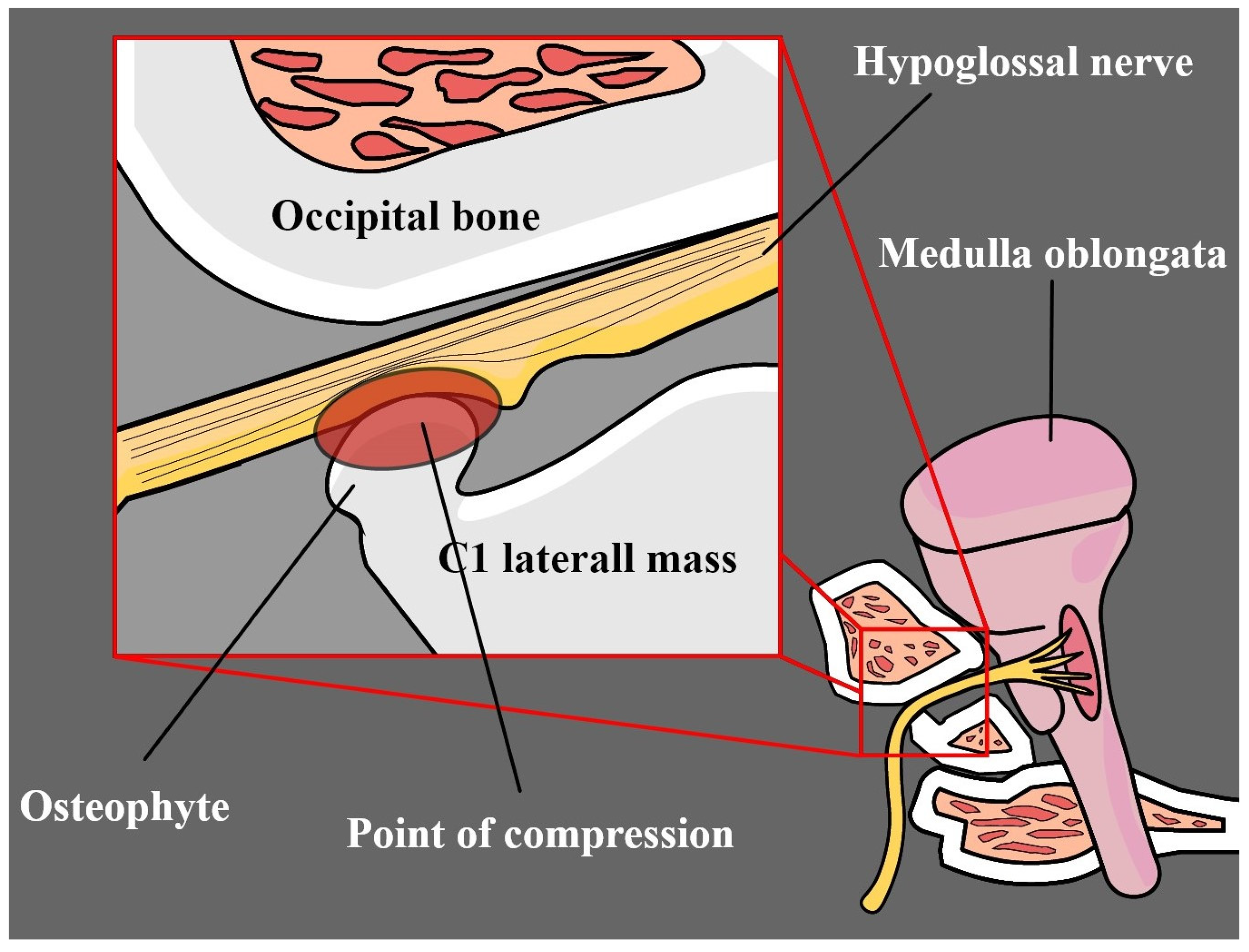
4.1. Osteophytes
4.2. Cysts
4.3. Tumors
4.4. Collet–Sicard Syndrome
4.5. Godtfredsen Syndrome
5. Trauma
Occipital Condyle
6. Systemic Diseases, Infections, and Inflammations
6.1. Rheumatoid Arthritis
6.2. Tuberculosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HN | hypoglossal nerve |
| VA | vertebral artery |
| ICA | internal carotid artery |
| ECA | external carotid artery |
| IJG | internal jugular vein |
| C | cervical spinal nerve/cervical vertebra |
| OA | occipital artery |
| PICA | posterior inferior cerebellar artery |
| DAVF | dural arteriovenous fistula |
| LMA | laryngeal mask airway |
| TP | Tapia’s syndrome |
| CS | Collet–Sicard syndrome |
| OCF | occipital condyle fracture |
References
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 0702077070. [Google Scholar]
- Thompson, E.O.; Smoker, W.R. Hypoglossal Nerve Palsy: A Segmental Approach. RadioGraphics 1994, 14, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Barkhaus, P. Cranial Nerve XII: The Hypoglossal Nerve. Semin. Neurol. 2009, 29, 045–052. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 1118430352. [Google Scholar]
- Fernando, D.A.; Lord, R.S.A.; Ozmen, J. The Blood Supply of the Hypoglossal Nerve and Its Relevance to Carotid Endarterectomy. Cardiovasc. Surg. 1999, 7, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gibo, H.; Marinković, S.; Nikodijević, I.; Štimec, B.; Erden, A. The Blood Supply of the Hypoglossal Nerve: The Microsurgical Anatomy of Its Cisternal Segment. Surg. Neurol. 1997, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Griessenauer, C.J.; Foreman, P.; Loukas, M.; Fisher, W.S.; Rizk, E.; Shoja, M.M.; Tubbs, R.S. Arterial Supply of the Lower Cranial Nerves: A Comprehensive Review. Clin. Anat. 2014, 27, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Iaconetta, G.; Solari, D.; Villa, A.; Castaldo, C.; Gerardi, R.M.; Califano, G.; Montagnani, S.; Cappabianca, P. The Hypoglossal Nerve: Anatomical Study of Its Entire Course. World Neurosurg. 2018, 109, e486–e492. [Google Scholar] [CrossRef] [PubMed]
- Aruede, G.; Brar, J.; Pepper, T.; Andi, K.; Hyde, N. Unilateral Aberrant Anatomy of the Hypoglossal Nerve. Surg. Radiol. Anat. 2021, 43, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, M.L.; Waltonen, J.D. Atypical Location of the Hypoglossal Nerve and Its Implications: A Case Report. Surg. Radiol. Anat. 2016, 38, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Wysiadecki, G.; Walocha, J.A.; Tubbs, R.S.; Iwanaga, J. Duplication of the Hypoglossal Nerve Branch to the Thyrohyoid Muscle: A Case Report. Kurume Med. J. 2023, 68, MS6834002. [Google Scholar] [CrossRef]
- Brennan, P.A.; Alam, P.; Ammar, M.; Tsiroyannis, C.; Zagkou, E.; Standring, S. Sternocleidomastoid Innervation from an Aberrant Nerve Arising from the Hypoglossal Nerve: A Prospective Study of 160 Neck Dissections. Surg. Radiol. Anat. 2017, 39, 205–209. [Google Scholar] [CrossRef]
- Stino, A.M.; Smith, B.E.; Temkit, M.; Reddy, S.N. Hypoglossal Nerve Palsy: 245 Cases. Muscle Nerve 2016, 54, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Gorolay, V.V.; Tran, N.-A.; Tade, R.; Baugnon, K.; Aiken, A.; Wu, X. The Ptotic Tongue—Imaging Appearance and Pathology Localization along the Course of the Hypoglossal Nerve. Neuroradiology 2023, 65, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.R. Twelfth-Nerve Palsy. Analysis of 100 Cases. Arch. Neurol. 1996, 53, 561. [Google Scholar] [CrossRef] [PubMed]
- Sayan, A.; Abeysinghe, A.H.M.K.; Brennan, P.A.; Ilankovan, V. Persistent Idiopathic Unilateral Hypoglassal Nerve Palsy: A Case Report. Br. J. Oral Maxillofac. Surg. 2014, 52, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Bagán-Sebastián, J.V.; Milián-Masanet, M.A.; Peñarrocha-Diago, M.; de Miguel, E.L. Persistent Idiopathic Unilateral Hypoglossal Nerve Palsy. J. Oral Maxillofac. Surg. 1998, 56, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Combarros, O.; de Arcaya, A.A.; Berciano, J. Isolated Unilateral Hypoglossal Nerve Palsy: Nine Cases. J. Neurol. 1998, 245, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Cho, K.L.; Lee, H.J.; Choi, S.H.; Lee, K.Y.; Kim, S.K.; Lee, J.H. A Case of Idiopathic Isolated Hypoglossal Nerve Palsy in a Korean Child. Korean J. Pediatr. 2011, 54, 515. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.W.S.; Fardy, M.J.; Crean, S.J. V Persistent Idiopathic Unilateral Isolated Hypoglossal Nerve Palsy: A Case Report. Br. Dent. J. 2004, 196, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Bademci, G.; Batay, F.; Yaşargil, M. “Triple Cross” of the Hypoglossal Nerve and Its Microsurgical Impact to Entrapment Disorders. Min—Minim. Invasive Neurosurg. 2006, 49, 234–237. [Google Scholar] [CrossRef]
- Graham, R.M.; Thomson, E.F.; Baldwin, A.J. Isolated Hypoglossal Nerve Palsy Due to a Vascular Anomaly. Int. J. Oral Maxillofac. Surg. 2007, 36, 759–761. [Google Scholar] [CrossRef]
- Morini, A.; Rozza, L.; Buganza, M.; Tranquillini, E.; Orrico, D.; Manera, V. Isolated Hypoglossal Nerve Palsy Due to an Anomalous Vertebral Artery Course: Report of Two Cases. Ital. J. Neurol. Sci. 1998, 19, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Toldo, I.; Manara, R.; Sartori, S.; Suppiej, A.; Drigo, P. Unilateral Hypoglossal Nerve Palsy Due to Neurovascular Conflict in a Child. Brain Dev. 2009, 31, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, P.; Raucq, E.; Fransen, P. Isolated Hypoglossal Nerve Paralysis and Hypoglossal–Vertebral Entrapment Syndrome. Acta Neurol. Belg. 2017, 117, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Vasović, L.; Trandafilović, M.; Jovanović, I.; Ugrenović, S.; Vlajković, S. Vertebral and/or Basilar Dolichoectasia in Human Adult Cadavers. Acta Neurochir. 2012, 154, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.H.; Kim, J.M.; Yang, M.S.; Kim, C.H. Resolution of Isolated Unilateral Hypoglossal Nerve Palsy Following Microvascular Decompression of the Intracranial Vertebral Artery. J. Korean Neurosurg. Soc. 2011, 49, 167. [Google Scholar] [CrossRef] [PubMed]
- Jannetta, P.J. Neurovascular Compression in Cranial Nerve and Systemic Disease. Ann. Surg. 1980, 192, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Rollnik, J.D.; Sindern, E.; Mosler, F.; im Spring, B.; Malin, J.P. Isolated Peripheral Hypoglossal Palsy Caused by a Kinking of the Left Vertebral Artery. Eur. Neurol. 1996, 36, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, S.; Lo Bartolo, M.L.; Nicoletti, A.; Reggio, E.; Lo Fermo, S.; Restivo, D.A.; Domina, E.; Reggio, A. Isolated, Unilateral, Reversible Palsy of the Hypoglossal Nerve. Eur. J. Neurol. 2000, 7, 347–349. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, K.; Takekawa, H.; Hirata, K. Isolated Hypoglossal Nerve Palsy Caused by Neurovascular Compression. Intern. Med. 2011, 50, 2701–2702. [Google Scholar] [CrossRef][Green Version]
- Nathan, H.; Levy, J. The Course and Relations of the Hypoglossal Nerve and the Occipital Artery. Am. J. Otolaryngol. 1982, 3, 128–132. [Google Scholar] [CrossRef]
- Kariuki, B.N.; Butt, F.; Mandela, P.; Odula, P. Surgical Anatomy of the Cervical Part of the Hypoglossal Nerve. Craniomaxillofac. Trauma Reconstr. 2018, 11, 021–027. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Kohyama, Y.; Takase, K. Peripheral Hypoglossal Nerve Palsy Caused by Lateral Position of the External Carotid Artery and an Abnormally High Position of Bifurcation of the External and Internal Carotid Arteries—A Case Report. Stroke 1984, 15, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Kohyama, Y.; Ueda, S. Surgical Management of Peripheral Hypoglossal Nerve Palsy Caused by Abnormal External Carotid Artery. No Shinkei Geka. 1983, 11, 1313–1318. [Google Scholar]
- Dokdok, M.; Göçmen, S.; Kahraman, S.; Kütükçü, Y. Isolated Unilateral Hypoglossal Nerve Palsy Caused by Internal Carotid Artery Loop. Cureus 2021, 13, e14819. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.J.; Weisman, R.A.; Savino, P.J.; Schatz, N.J. Aneurysm of the Internal Carotid Artery at the Base of the Skull: An Unusual Cause of Cranial Neuropathies. Otolaryngol. Neck Surg. 1980, 88, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Welling, R.E.; Taha, A.; Goel, T.; Cranley, J.; Krause, R.; Hafner, C.; Tew, J. Extracranial Carotid Artery Aneurysms. Surgery 1983, 93, 319–323. [Google Scholar] [PubMed]
- Straube, A.; Linn, J. Unilateral Headache Attacks and Ipsilateral Atrophy of the Tongue Due to Neurovascular Compression of the Hypoglossal Nerve. Cephalalgia 2008, 28, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Cano-Duran, A.J.; Sanchez Reyes, J.M.; Corbalan Sevilla, M.T.; Yucumá, D. Carotid Petrous Segment Aneurysm Presenting as Hypoglossal Nerve Palsy. Neuroradiology 2021, 63, 447–450. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Davis, S.; Tress, B.M.; Ebeling, P. Spontaneous Internal Carotid Artery Dissection Presenting as Hypoglossal Nerve Palsy. Stroke 1988, 19, 1151–1155. [Google Scholar] [CrossRef]
- Bouthillier, A.; van Loveren, H.R.; Keller, J.T. Segments of the Internal Carotid Artery: A New Classification. Neurosurgery 1996, 38, 425–433. [Google Scholar] [CrossRef]
- Veith, C.K.; Tedesco, J.A.; Landis, G.S. Bilateral Extracranial Internal Carotid Artery Aneurysms: Case Report and Review of Literature. Vascular 2016, 24, 549–551. [Google Scholar] [CrossRef]
- El-Sabrout, R.; Cooley, D.A. Extracranial Carotid Artery Aneurysms: Texas Heart Institute Experience. J. Vasc. Surg. 2000, 31, 702–712. [Google Scholar] [CrossRef]
- Patel, R.R.; Adam, R.; Maldjian, C.; Lincoln, C.M.; Yuen, A.; Arneja, A. Cervical Carotid Artery Dissection: Current Review of Diagnosis and Treatment. Cardiol. Rev. 2012, 20, 145–152. [Google Scholar] [CrossRef]
- Rodallec, M.H.; Marteau, V.; Gerber, S.; Desmottes, L.; Zins, M. Craniocervical Arterial Dissection: Spectrum of Imaging Findings and Differential Diagnosis. RadioGraphics 2008, 28, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- López, O.; Piñana, C.; Gramegna, L.L.; Rodríguez, J.; Hernández, D.; Tomasello, A. Endovascular Management of Internal Carotid Artery Dissection with Associated Aneurysm Using a Multilayer Flow Modulator. J. Vasc. Surg. Cases Innov. Technol. 2020, 6, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kesserwani, H. Isolated Palsy of the Cisternal Segment of the Hypoglossal Nerve Due to Arterial Dissection of the V4 Segment of the Vertebral Artery: A Case Report With a Side Note on Nerve Trunk Ischemia. Cureus 2020, 12, e9930. [Google Scholar] [CrossRef] [PubMed]
- Crissey, M.M.; Bernstein, E.F. Delayed Presentation of Carotid Intimal Tear Following Blunt Craniocervical Trauma. Surgery 1974, 75, 543–549. [Google Scholar]
- Malhotra, K.; Goyal, N.; Tsivgoulis, G. Internal Carotid Artery Occlusion: Pathophysiology, Diagnosis, and Management. Curr. Atheroscler. Rep. 2017, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Dissections of Brain-Supplying Arteries. Nat. Clin. Pract. Neurol. 2008, 4, 34–42. [Google Scholar] [CrossRef]
- Garnier, P.; Demasles, S.; Januel, A.-C.; Michel, D. Débord Intracrânien Des Dissections de l’artère Vertébrale Extracrânienne. Rev. Neurol. 2004, 160, 679–684. [Google Scholar] [CrossRef]
- McKeon, A.; Murphy, S.; McNamara, B.; Ryder, D.Q.; Galvin, R.J. Isolated Hypoglossal Nerve Palsy Due to Compression by a Dissecting Vertebral Artery. Eur. Neurol. 2005, 53, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Mahadevappa, K.; Chacko, T.; Nair, A.K. Isolated Unilateral Hypoglossal Nerve Palsy Due to Vertebral Artery Dissection. Clin. Med. Res. 2012, 10, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Mizumaki, Y.; Endo, S.; Yamatani, K.; Takaku, A.; Tsukamoto, E. Hypoglossal Nerve Paresis Caused by Spontaneous Dissection of Kinked Internal Carotid Artery—Case Report. Neurol. Med. Chir. 1998, 38, 165–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maiese, A.; Frati, P.; Manetti, A.C.; De Matteis, A.; Di Paolo, M.; La Russa, R.; Turillazzi, E.; Frati, A.; Fineschi, V. Traumatic Internal Carotid Artery Injuries: Do We Need a Screening Strategy? Literature Review, Case Report, and Forensic Evaluation. Curr. Neuropharmacol. 2022, 20, 1752–1773. [Google Scholar] [CrossRef] [PubMed]
- Pica, R.A.; Rockwell, B.H.; Raji, M.R.; Dastur, K.J.; Berkey, K.E. Traumatic Internal Carotid Artery Dissection Presenting as Delayed Hemilingual Paresis. AJNR. Am. J. Neuroradiol. 1996, 17, 86–88. [Google Scholar] [PubMed]
- Sturzenegger, M.; Huber, P. Cranial Nerve Palsies in Spontaneous Carotid Artery Dissection. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Argiolas, G.M.; Sumer, S.; Siotto, P.; Raz, E.; Sanfilippo, R.; Montisci, R.; Piga, M.; Wintermark, M. Association between Internal Carotid Artery Dissection and Arterial Tortuosity. Neuroradiology 2015, 57, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Murai, Y.; Shirokane, K.; Kitamura, T.; Tateyama, K.; Matano, F.; Mizunari, T.; Morita, A. Petrous Internal Carotid Artery Aneurysm: A Systematic Review. J. Nippon Med. Sch. 2020, 87, 172–183. [Google Scholar] [CrossRef]
- Rao, A.S.; Makaroun, M.S.; Marone, L.K.; Cho, J.S.; Rhee, R.; Chaer, R.A. Long-Term Outcomes of Internal Carotid Artery Dissection. J. Vasc. Surg. 2011, 54, 370–375. [Google Scholar] [CrossRef]
- Provenzale, J.M. MRI and MRA for Evaluation of Dissection of Craniocerebral Arteries: Lessons from the Medical Literature. Emerg. Radiol. 2009, 16, 185–193. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Sarikaya, B. Comparison of Test Performance Characteristics of MRI, MR Angiography, and CT Angiography in the Diagnosis of Carotid and Vertebral Artery Dissection: A Review of the Medical Literature. Am. J. Roentgenol. 2009, 193, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Z.; Koike, G.; Seguchi, K.; Shindo, K.; Sugita, K. Unilateral Tongue Atrophy Due to an Enlarged Emissary Vein in the Hypoglossal Canal. Surg. Neurol. 1996, 45, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Meila, D.; Wetter, A.; Brassel, F.; Nacimiento, W. Intermittent Hypoglossal Nerve Palsy Caused by a Calcified Persistent Hypoglossal Artery: An Uncommon Neurovascular Compression Syndrome. J. Neurol. Sci. 2012, 323, 248–249. [Google Scholar] [CrossRef]
- Hikichi, H.; Ueno, T.; Iwamura, M.; Nishijima, H.; Arai, A.; Suzuki, C.; Midorikawa, H.; Nunomura, J.; Tomiyama, M. Hypoglossal Nerve Palsy Due to Compression by a Persistent Primitive Hypoglossal Artery: Case Report. J. Stroke Cerebrovasc. Dis. 2020, 29, 104459. [Google Scholar] [CrossRef]
- Wang, M.; Gu, J.; Lan, P.; Wan, S.; Zhou, Y.; Zheng, X.; Zhan, R. A Persistent Primitive Hypoglossal Artery As the Sole Supply to the Brain Associated with a Basilar Bifurcation Aneurysm. Front. Neurol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Al-Memar, A.; Thrush, D. Unilateral Hypoglossal Nerve Palsy Due to Aneurysm of the Stump of Persistent Hypoglossal Artery. J. Neurol. Neurosurg. Psychiatry 1998, 64, 405. [Google Scholar] [CrossRef][Green Version]
- Shapiro, R. Enlargement of the Hypoglossal Canal in the Presence of a Persistent Hypoglossal Artery. Radiology 1979, 133, 395–396. [Google Scholar] [CrossRef]
- Hamzoian, H.; Harris, B.; Ditamo, M.; Chaudhary, S. Peculiar Neurological Examination Secondary to Persistent Primitive Hypoglossal Artery. Cureus 2023, 15, e42249. [Google Scholar] [CrossRef]
- Gandhi, D.; Chen, J.; Pearl, M.; Huang, J.; Gemmete, J.J.; Kathuria, S. Intracranial Dural Arteriovenous Fistulas: Classification, Imaging Findings, and Treatment. Am. J. Neuroradiol. 2012, 33, 1007–1013. [Google Scholar] [CrossRef]
- Spittau, B.; Millán, D.S.; El-Sherifi, S.; Hader, C.; Singh, T.P.; Motschall, E.; Vach, W.; Urbach, H.; Meckel, S. Dural Arteriovenous Fistulas of the Hypoglossal Canal: Systematic Review on Imaging Anatomy, Clinical Findings, and Endovascular Management. J. Neurosurg. 2015, 122, 883–903. [Google Scholar] [CrossRef]
- Chan, N.H.H.L. Hypoglossal Dural Arteriovenous Fistula: A Rare Cause of Unilateral Hypoglossal Nerve Palsy. BJR|Case Rep. 2017, 3, 20160144. [Google Scholar] [CrossRef]
- Pei, W.; Huai-Zhang, S.; Shan-Cai, X.; Cheng, G.; Di, Z. Isolated Hypoglossal Nerve Palsy Due to Endovascular Treatment of a Dural Arteriovenous Fistula with Onyx-18. Interv. Neuroradiol. 2010, 16, 286–289. [Google Scholar] [CrossRef]
- Macchi, V.; Porzionato, A.; Parenti, A.; De Caro, R. The Course of the Posterior Inferior Cerebellar Artery May Be Related to Its Level of Origin. Surg. Radiol. Anat. 2004, 26, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ekuma, M.E.; Goto, T.; Hanaoka, Y.; Kanaya, K.; Horiuchi, T.; Hongo, K.; Ohaegbulam, S.C. Unilateral Isolated Hypoglossal Nerve Palsy Due to Pathologically Adherent PICA Fusiform Aneurysm—A Case Report. Surg. Neurol. Int. 2017, 8, 114. [Google Scholar] [CrossRef]
- Rhoton, A.L. The Cerebellar Arteries. Neurosurgery 2000, 47, 29–68. [Google Scholar] [CrossRef]
- Bissacco, D.; Domanin, M.; Romagnoli, S.; Martelli, E.; Civelli, V.; Gabrielli, L. Spontaneous Rupture of Multiple Occipital Artery Aneurysms in a Patient With Neurofibromatosis Type 1. Vasc. Endovasc. Surg. 2018, 52, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Illuminati, G.; Cannistrà, M.; Pizzardi, G.; Pasqua, R.; Frezzotti, F.; Calio’, F.G. True Aneurysm of the Proximal Occipital Artery: Case Report. Int. J. Surg. Case Rep. 2018, 43, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Scotti, G.; Melançon, D.; Olivier, A. Hypoglossal Paralysis Due to Compression by a Tortuous Internal Carotid Artery in the Neck. Neuroradiology 1978, 14, 263–265. [Google Scholar] [CrossRef]
- Kakisis, J.D.; Antonopoulos, C.N.; Mantas, G.; Moulakakis, K.G.; Sfyroeras, G.; Geroulakos, G. Cranial Nerve Injury After Carotid Endarterectomy: Incidence, Risk Factors, and Time Trends. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 320–335. [Google Scholar] [CrossRef]
- Cevik, O.M.; Usseli, M.I.; Babur, M.; Unal, C.; Eksi, M.S.; Guduk, M.; Ovalioglu, T.C.; Aksoy, M.E.; Pamir, M.N.; Bozkurt, B. The Carotid Endarterectomy Cadaveric Investigation for Cranial Nerve Injuries: Anatomical Study. Brain Sci. 2021, 11, 211. [Google Scholar] [CrossRef]
- Kojima, A.; Saga, I.; Ishikawa, M. Intraoperative Hypoglossal Nerve Mapping During Carotid Endarterectomy: Technical Note. World Neurosurg. 2018, 113, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ghedia, R.; Hughes, J.; Clarke, P. Hypoglossal Nerve Identification during Head and Neck Surgery. Clin. Otolaryngol. 2016, 41, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Shaha, A.R.; Medina, J.E.; Califano, J.A.; Wolf, G.T.; Ferlito, A.; Som, P.M.; Day, T.A. Consensus Statement on the Classification and Terminology of Neck Dissection. Arch. Otolaryngol. Neck Surg. 2008, 134, 536. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, L.D.; Basford, J.R.; Strommen, J.A.; Andersen, R.J. Hypoglossal Nerve Palsy from Cervical Spine Involvement in Rheumatoid Arthritis: 3 Case Reports. Arch. Phys. Med. Rehabil. 2002, 83, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, D.H.; Chun, S.M.; Choi, Y.-H. Irreversible Hypoglossal Nerve Injury and Concomitant Trigeminal System Dysfunction After Anterior Surgery to the Cervical Spine: Case Report and Literature Review. World Neurosurg. 2020, 136, 187–192. [Google Scholar] [CrossRef]
- Yasuda, T.; Togawa, D.; Hasegawa, T.; Yamato, Y.; Kobayashi, S.; Arima, H.; Matsuyama, Y. Hypoglossal Nerve Palsy as a Complication of an Anterior Approach for Cervical Spine Surgery. Asian Spine J. 2015, 9, 295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bu Bshait, M.; Alyami, H.; Al-Osail, E.; Al Arfaj, H. Ipsilateral Hypoglossal Nerve Palsy Following Left Hemithyroidectomy: Case Report and Review of Literature. Int. J. Surg. Case Rep. 2018, 51, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, J.; Abu Dahari, K.A.S.; Kaur, N.; Azman, M. Iatrogenic Hypoglossal Nerve Palsy, a Rare Complication Post Suspension Laryngoscopy. J. Taibah Univ. Med. Sci. 2022, 17, 623–625. [Google Scholar] [CrossRef]
- de Carvalho, A.S.; Dedivitis, R.A.; de Castro, M.A.F.; Nardi, C.E.M. Submandibular Gland Excision. Rev. Col. Bras. Cir. 2015, 42, 14–17. [Google Scholar] [CrossRef]
- Ahn, S.-W.; Kang, K.H. Hypoglossal Nerve Palsy Following the Robotic Thyroidectomy for the Papillary Thyroid Carcinoma: A Case Report. Int. J. Surg. Case Rep. 2015, 14, 133–135. [Google Scholar] [CrossRef][Green Version]
- Prim, M.P.; Diego, J.I.; Verdaguer, J.M.; Sastre, N.; Rabanal, I. Neurological Complications Following Functional Neck Dissection. Eur. Arch. Oto-Rhino-Laryngol. 2006, 263, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.D.; Schiavone, L.; La Mendola, F.M.C.; Timpanaro, T.; Cucuzza, M.E.; Greco, F.; Smilari, P.; Fiumara, A.; Praticò, A.D. Hypoglossal Nerve Paralysis in a Child after a Dental Procedure. Neurol. Neurochir. Pol. 2018, 52, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.C.; Barnes, C.; Spiekerman, C.F.; Bollag, L.A. Hypoglossal Nerve Palsy After Airway Management for General Anesthesia: An Analysis of 69 Patients. Anesth. Analg. 2015, 120, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Brain, A.I.J. Course of the Hypoglossal Nerve in Relation to the Position of the Laryngeal Mask Airway. Anaesthesia 1995, 50, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Lindsay, W.A. Bilateral Hypoglossal Nerve Injury Following the Use of the Laryngeal Mask Airway. Anaesthesia 2002, 57, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Trümpelmann, P.; Cook, T. Unilateral Hypoglossal Nerve Injury Following the Use of a ProSealTM Laryngeal Mask. Anaesthesia 2005, 60, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Takahoko, K.; Iwasaki, H.; Sasakawa, T.; Suzuki, A.; Matsumoto, H.; Iwasaki, H. Unilateral Hypoglossal Nerve Palsy after Use of the Laryngeal Mask Airway Supreme. Case Rep. Anesthesiol. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Sommer, M.; Schuldt, M.; Runge, U.; Gielen-Wijffels, S.; Marcus, M.A.E. Bilateral Hypoglossal Nerve Injury Following the Use of the Laryngeal Mask without the Use of Nitrous Oxide. Acta Anaesthesiol. Scand. 2004, 48, 377–378. [Google Scholar] [CrossRef]
- Lo, T.S. Unilateral Hypoglossal Nerve Palsy Following the Use of the Laryngeal Mask Airway. Can. J. Neurol. Sci. 2006, 33, 320–321. [Google Scholar] [CrossRef][Green Version]
- Trujillo, L.; Anghelescu, D.; Bikhazi, G. Unilateral Hypoglossal Nerve Injury Caused by a Laryngeal Mask Airway in an Infant. Pediatr. Anesth. 2011, 21, 708–709. [Google Scholar] [CrossRef]
- Nagai, K.; Sakuramoto, C.; Goto, F. Unilateral Hypoglossal Nerve Paralysis Following the Use of the Laryngeal Mask Airway. Anaesthesia 2007, 49, 603–604. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Street, M.K. Twelfth Cranial Nerve Paralysis Following Use of a Laryngeal Mask Airway. Anaesthesia 1994, 49, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, N.; Eliathamby, T.G.; Timms, M.S. Paralysis of the Hypoglossal and Pharyngeal Branches of the Vagus Nerve after Use of a LMA and ETT. Br. J. Anaesth. 2001, 87, 322. [Google Scholar] [PubMed]
- Hung, N.-K.; Lee, C.-H.; Chan, S.-M.; Yeh, C.-C.; Cherng, C.-H.; Wong, C.-S.; Wu, C.-T. Transient Unilateral Hypoglossal Nerve Palsy After Orotracheal Intubation for General Anesthesia. Acta Anaesthesiol. Taiwanica 2009, 47, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.; Besir, A.; Cekic, B.; Kosucu, M.; Geze, S. Transient Unilateral Combined Paresis of the Hypoglossal Nerve and Lingual Nerve Following Intubation Anesthesia. Braz. J. Anesthesiol. (Engl. Ed.) 2014, 64, 124–127. [Google Scholar] [CrossRef]
- Brimacombe, J.; Clarke, G.; Keller, C. Lingual Nerve Injury Associated with the ProSeal Laryngeal Mask Airway: A Case Report and Review of the Literature. Br. J. Anaesth. 2005, 95, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Tesei, F.; Poveda, L.M.; Strali, W.; Tosi, L.; Magnani, G.; Farneti, G. Unilateral Laryngeal and Hypoglossal Paralysis (Tapia’s Syndrome) Following Rhinoplasty in General Anaesthesia: Case Report and Review of the Literature. Acta Otorhinolaryngol. Ital. 2006, 26, 219–221. [Google Scholar] [PubMed]
- Leuzinger, K.; Misra, L. Unilateral Hypoglossal Nerve Palsy in a Patient with a Difficult Airway Requiring Prolonged Intubation. Case Rep. Anesthesiol. 2021, 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.; Bachmann, G.; Stennert, E. Hypoglossal Nerve Palsy as a Complication of Transoral Intubation for General Anesthesia. Anesthesiology 1997, 86, 1007. [Google Scholar] [CrossRef]
- Decavel, P.; Nahmias, O.; Petit, C.; Tatu, L. Lower Cranial Nerve Palsies in the COVID-19 Pandemic: A 10-Case Series of Intensive Care Unit Patients. Eur. Neurol. 2022, 85, 136–139. [Google Scholar] [CrossRef]
- Venkatesh, B.; Walker, D. Hypoglossal Neuropraxia Following Endotracheal Intubation. Anaesth. Intensive Care 1997, 25, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Weissman, O.; Weissman, O.; Farber, N.; Berger, E.; Grabov Nardini, G.; Zilinsky, I.; Winkler, E.; Haik, J. Hypoglossal Nerve Paralysis in a Burn Patient Following Mechanical Ventilation. Ann. Burns Fire Disasters 2013, 26, 86–89. [Google Scholar] [PubMed]
- Finsterer, J.; Hess, B. Unilateral Compression Neuropathy of the Hypoglossal Nerve Due to Head Suspension Orthosis in Mitochondriopathy. Int. J. Neurosci. 2004, 114, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Mega, C.; Ricci, A.; Giannone, S.; Facchini, F.; Gasbarrini, A. Tapia’s Syndrome as an Uncommon Complication after Cervical Spine Surgery with Tracheostomy: A Case Report and Literature Review. Spine Deform. 2020, 8, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Cariati, P.; Cabello, A.; Galvez, P.P.; Sanchez Lopez, D.; Garcia Medina, B. Tapia’s Syndrome: Pathogenetic Mechanisms, Diagnostic Management, and Proper Treatment: A Case Series. J. Med. Case Rep. 2016, 10, 23. [Google Scholar] [CrossRef]
- Bilbao, I.; Dopazo, C.; Caralt, M.; Castells, L.; Pando, E.; Gantxegi, A.; Charco, R. Isolated Bilateral Tapia’s Syndrome after Liver Transplantation: A Case Report and Review of the Literature. World J. Hepatol. 2016, 8, 1637. [Google Scholar] [CrossRef] [PubMed]
- Steehler, A.J.; Rothman, R.; Sadhar, B.; Saran, M.; Lipman, S.P.; Lipman, R.I. Tapia’s Syndrome After Cardiac Surgery: A Case Report and Review of Literature. Ear Nose Throat J. 2022, 014556132211138. [Google Scholar] [CrossRef] [PubMed]
- Decavel, P.; Petit, C.; Tatu, L. Tapia Syndrome at the Time of the COVID-19 Pandemic. Neurology 2020, 95, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Neo, S.; Lee, K.E. Collet-Sicard Syndrome: A Rare but Important Presentation of Internal Jugular Vein Thrombosis. Pract. Neurol. 2017, 17, 63–65. [Google Scholar] [CrossRef]
- Schoenberg, B.S.; Massey, E.W. Tapia’s Syndrome The Erratic Evolution of an Eponym. Arch. Neurol. 1979, 36, 257–260. [Google Scholar] [CrossRef]
- Chow, J.C.H.; Cheung, K.-M.; Au, K.-H.; Zee, B.C.Y.; Lee, J.; Ngan, R.K.C.; Lee, A.W.M.; Yiu, H.H.Y.; Li, K.W.S.; Leung, A.K.C.; et al. Radiation-Induced Hypoglossal Nerve Palsy after Definitive Radiotherapy for Nasopharyngeal Carcinoma: Clinical Predictors and Dose–Toxicity Relationship. Radiother. Oncol. 2019, 138, 93–98. [Google Scholar] [CrossRef]
- Au, K.H.; Ngan, R.K.C.; Ng, A.W.Y.; Poon, D.M.C.; Ng, W.T.; Yuen, K.T.; Lee, V.H.F.; Tung, S.Y.; Chan, A.T.C.; Sze, H.C.K.; et al. Treatment Outcomes of Nasopharyngeal Carcinoma in Modern Era after Intensity Modulated Radiotherapy (IMRT) in Hong Kong: A Report of 3328 Patients (HKNPCSG 1301 Study). Oral Oncol. 2018, 77, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, K.A.; Yuk, M.; Hubbard, R.; Gunn, G.B.; Fuller, C.D.; Lai, S.Y.; Lin, H.; Garden, A.S.; Rosenthal, D.I.; Hanna, E.Y.; et al. Delayed Lower Cranial Neuropathy after Oropharyngeal Intensity-Modulated Radiotherapy: A Cohort Analysis and Literature Review. Head Neck 2017, 39, 1516–1523. [Google Scholar] [CrossRef]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation Dose–Volume Effects of Optic Nerves and Chiasm. Int. J. Radiat. Oncol. 2010, 76, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J.-L.; Pradat, P.-F. Radiation-Induced Neuropathy in Cancer Survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Yamakami, I.; Higuchi, Y.; Fukutake, T. Isolated Hypoglossal Nerve Palsy Due to an Osteophyte with Atlantoaxial Dislocation. NMC Case Rep. J. 2020, 7, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Patron, V.; Roudaut, P.-Y.; Lerat, J.; Vivent, M.; Bessède, J.-P.; Aubry, K. Isolated Hypoglossal Palsy Due to Cervical Osteophyte. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Patro, S.N.; Torres, C.; Riascos, R. An Unusual Case of Isolated Hypoglossal Nerve Palsy Secondary to Osteophytic Projection from the Atlanto-Occipital Joint. Neuroradiol. J. 2014, 27, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Gassie, K.; Grewal, S.; Chen, S.G. Atlantooccipital Synovial Cyst with Isolated Hypoglossal Nerve Palsy: Case Report of Nonfusion Surgical Approach and Review of Literature. World Neurosurg. 2019, 126, 434–438. [Google Scholar] [CrossRef]
- Mendes-Araújo, L.; Rangel, C.; Domingues, R.C.; Gasparetto, E.L. Atlantoaxial Synovial Cyst Causing Isolated Unilateral Hypoglossal Nerve Paralysis. Br. J. Radiol. 2010, 83, e35–e38. [Google Scholar] [CrossRef]
- Baldauf, J.; Junghans, D.; Schroeder, H.W.S. Endoscope-Assisted Microsurgical Resection of an Intraneural Ganglion Cyst of the Hypoglossal Nerve. J. Neurosurg. 2005, 103, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Bilgin-Freiert, A.; Fugleholm, K.; Poulsgaard, L. Case Report: Intraneural Intracanalicular Ganglion Cyst of the Hypoglossal Nerve Treated by Extradural Transcondylar Approach. J. Neurol. Surg. Rep. 2015, 76, e180–e182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mujic, A.; Hunn, A.; Liddell, J.; Taylor, B.; Havlat, M.; Beasley, T. Isolated Unilateral Hypoglossal Nerve Paralysis Caused by an Atlanto-Occipital Joint Synovial Cyst. J. Clin. Neurosci. 2003, 10, 492–495. [Google Scholar] [CrossRef]
- Harbaugh, K.S.; Tiel, R.L.; Kline, D.G. Ganglion Cyst Involvement of Peripheral Nerves. J. Neurosurg. 1997, 87, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.; Mujic, A.; Hunn, A. An Intraneural Ganglion Cyst Causing Unilateral Hypoglossal Nerve Palsy. J. Clin. Neurosci. 2011, 18, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Wilton-Clark, M.S.; MacIsaac, R.; Camara-Lemarroy, C.R. Hypoglossal Canal Cyst Causing Unilateral XII Nerve Palsy. Can. J. Neurol. Sci. 2021, 48, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Grossi, P.M.; Filomena, C.A.; Friedman, A.H.; Fukushima, T. Unilateral Hypoglossal Nerve Palsy Caused by an Intraneural Ganglion Cyst. J. Neurosurg. 2010, 113, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.-K.; Bal, C.; Neidert, M.C.; Bozinov, O. The Transcondylar Approach to Access Symptomatic Arachnoid Cysts of the Hypoglossal Canal. Oper. Neurosurg. 2018, 14, E23–E25. [Google Scholar] [CrossRef] [PubMed]
- Elhammady, M.S.; Farhat, H.; Aziz-Sultan, M.A.; Morcos, J.J. Isolated Unilateral Hypoglossal Nerve Palsy Secondary to an Atlantooccipital Joint Juxtafacet Synovial Cyst. J. Neurosurg. Spine 2009, 10, 234–239. [Google Scholar] [CrossRef]
- Tarantino, R.; Marruzzo, D.; Colistra, D.; Mancarella, C.; Delfini, R. Twelfth Nerve Paresis Induced by an Unusual Posterior Fossa Arachnoid Cyst: Case Report and Literature Review. Br. J. Neurosurg. 2014, 28, 528–530. [Google Scholar] [CrossRef]
- De Ridder, D.; Alessi, G.; Lemmerling, M.; Fransen, H.; De Waele, L. Hemilingual Spasm: A New Neurosurgical Entity? J. Neurosurg. 2002, 97, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, K.; Jaraj, D.; Marlow, T.; Jensen, C.; Skoog, I.; Wikkelsø, C. Prevalence and Symptoms of Intracranial Arachnoid Cysts: A Population-Based Study. J. Neurol. 2016, 263, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Makino, A.; Hinokuma, K. An Arachnoid Cyst Involving Only the Hypoglossal Nerve: Case Report and Review of the Literature. Br. J. Neurosurg. 1999, 13, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Flemmer, M.C. The Nerve of Legal Entrapment. Oxf. Med. Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Heda, S.; Karthik, D.K.; Rao, E.S.; Deshpande, A. Hypoglossal Canal Schwannoma Causing Isolated Left 12th Cranial Nerve Palsy. BMJ Case Rep. 2018, 2018, bcr-2018-225544. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.; Turner, C.; DeVine, J.; Gerlinger, T. Jefferson Fracture Resulting in Collet-Sicard Syndrome. Spine 2000, 25, 395. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Moon, B.G. Developmental Abnormalities of the Craniocervical Junction Resulting in Collet-Sicard Syndrome. Spine J. 2016, 16, e635–e639. [Google Scholar] [CrossRef]
- Handley, T.P.B.; Miah, M.S.; Majumdar, S.; Hussain, S.S.M. Collet-Sicard Syndrome from Thrombosis of the Sigmoid-Jugular Complex: A Case Report and Review of the Literature. Int. J. Otolaryngol. 2010, 2010, 203587. [Google Scholar] [CrossRef]
- Smith, R.; Tassone, P.; Saada, J. Collet-Sicard Syndrome as a Result of Unilateral Carotid Artery Dissection. Case Rep. 2013, 2013, bcr2013200358. [Google Scholar] [CrossRef]
- Erben, Y.; Ghare, M.I.; Patel, A.; Mojibian, H.; Matouk, C. Collet-Sicard Syndrome Secondary to Internal Carotid Artery Pseudoaneurysm. J. Vasc. Surg. 2018, 67, 1596–1597. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Tomandl, B.; Duhm, C.; Stefan, H.; Neundörfer, B. Collet-Sicard Syndrome Due to Coiling and Dissection of the Internal Carotid Artery. Cerebrovasc. Dis. 2000, 10, 487–488. [Google Scholar] [CrossRef]
- Keane, J.R. Combined VIth and XIIth Cranial Nerve Palsies: A Clival Syndrome. Neurology 2000, 54, 1540–1541. [Google Scholar] [CrossRef] [PubMed]
- Wai, Y.Z.; Chong, Y.Y.; Dusa, N.M.; Lai, Y.P.; Lim, L.T. Godtfredsen Syndrome—Recurrent Clival Chondrosarcoma with 6 Years Follow up: A Case Report and Literature Review. BMC Neurol. 2022, 22, 134. [Google Scholar] [CrossRef]
- Ozdemir, B.; Kanat, A.; Batcik, S.; Batcik, O.E.; Celiker, M.; Kayayurt, K. Unilateral Isolated Hypoglossal Nerve Palsy Caused by Gunshot Injury. J. Craniofac. Surg. 2018, 29, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Kacker, A.; Komisar, A.; Kakani, R.S.; Reich, E.; Rothman, L. Tongue Paralysis Following Head Trauma. J. Laryngol. Otol. 1995, 109, 770–771. [Google Scholar] [CrossRef]
- Mousele, C.; Georgiopoulos, M.; Constantoyannis, C. Syringobulbia: A Delayed Complication Following Spinal Cord Injury—Case Report. J. Spinal Cord Med. 2019, 42, 260–264. [Google Scholar] [CrossRef]
- Carroll, Á.M.; Brackenridge, P. Post-Traumatic Syringomyelia: A Review of the Cases Presenting in a Regional Spinal Injuries Unit in the North East of England over a 5-Year Period. Spine 2005, 30, 1206–1210. [Google Scholar] [CrossRef]
- Rué, M.; Jecko, V.; Dautheribes, M.; Vignes, J.-R. Delayed Hypoglossal Nerve Palsy Following Unnoticed Occipital Condyle Fracture. Neurochirurgie 2013, 59, 221–223. [Google Scholar] [CrossRef]
- Maddox, J.J.; Rodriguez-Feo, J.A.; Maddox, G.E.; Gullung, G.; McGwin, G.; Theiss, S.M. Nonoperative Treatment of Occipital Condyle Fractures. Spine 2012, 37, E964–E968. [Google Scholar] [CrossRef]
- Caroli, E.; Rocchi, G.; Orlando, E.R.; Delfini, R. Occipital Condyle Fractures: Report of Five Cases and Literature Review. Eur. Spine J. 2005, 14, 487–492. [Google Scholar] [CrossRef]
- Anderson, P.A.; Montesano, P.X. Morphology and Treatment of Occipital Condyle Fractures. Spine 1988, 13, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, S.; Masood, Z.; Krueger, B.; Marciano, R.; Chen, D.; Houseman, C.; Insinga, S. Long-Term Resolution of Delayed Onset Hypoglossal Nerve Palsy Following Occipital Condyle Fracture: Case Report and Review of the Literature. J. Craniovertebr. Junction Spine 2017, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Lim, O.K.; Park, K.D.; Lee, J.K. Occipital Condyle Fracture With Isolated Unilateral Hypoglossal Nerve Palsy. Ann. Rehabil. Med. 2014, 38, 689. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuli, S.; Tator, C.H.; Fehlings, M.G.; Mackay, M. Occipital Condyle Fractures. Neurosurgery 1997, 41, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Stricker, T.; Steinlin, M.; Willi, U.V.; Nadal, D. Hypoglossal Nerve Palsy Associated with Deep Cervical Lymphadenopathy. Neurology 1998, 50, 1926–1927. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Jayawant, S.; Stoodley, N. Isolated Hypoglossal Nerve Palsy in a 14-Year-Old Girl. Eur. J. Paediatr. Neurol. 2002, 6, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ohri, N. Unilateral Hypoglossal Nerve Palsy Due to Infected Molar: A Rare Entity. J. Investig. Clin. Dent. 2011, 2, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.A.; Pazevic, J.P. Hypoglossal Nerve Palsy after Tooth Extraction. J. Oral Maxillofac. Surg. 1988, 46, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, T.; Ohta, R.; Yamane, F.; Abe, S.; Sano, C. Hypoglossal Nerve Palsy Following COVID-19 Vaccination in a Young Adult Complicated by Various Medicines. Cureus 2022, 14, e29212. [Google Scholar] [CrossRef]
- Panholzer, J.; Kellermair, L.; Eggers, C. Hypoglossal Nerve Palsy after SARS-CoV-2 Vaccination—Report of Two Cases. BMC Neurol. 2022, 22, 416. [Google Scholar] [CrossRef]
- Parano, E.; Giuffrida, S.; Restivo, D.; Saponara, R.; Greco, F.; Trifiletti, R. Reversible Palsy of the Hypoglossal Nerve Complicating Infectious Mononucleosis in a Young Child. Neuropediatrics 1998, 29, 46–47. [Google Scholar] [CrossRef]
- Boban, M.; Brinar, V.V.; Habek, M.; Radoš, M. Isolated Hypoglossal Nerve Palsy: A Diagnostic Challenge. Eur. Neurol. 2007, 58, 177–181. [Google Scholar] [CrossRef]
- Dziedzic, T.; Wojciechowski, J.; Nowak, A.; Marchel, A. Hypertrophic Pachymeningitis. Child’s Nerv. Syst. 2015, 31, 1025–1031. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Dahdaleh, N.S. Cervical Spine Manifestations of Rheumatoid Arthritis: A Review. Neurosurg. Rev. 2021, 44, 1957–1965. [Google Scholar] [CrossRef]
- Macedo, T.F.; Gow, P.J.; Heap, S.W.; Wallis, W.E. Bilateral Hypoglossal Nerve Palsy Due to Vertical Subluxation of the Odontoid Process in Rheumatoid Arthritis. Rheumatology 1988, 27, 317–320. [Google Scholar] [CrossRef]
- Konishi, A.; Higo, R.; Nito, T.; Masumoto, T.; Seichi, A. A Case Report of Unilateral Hypoglossal Neuroparalysis Resulting from Horizontal Subluxation in the Atlanto-Occipital Joint Due to Rheumatoid Arthritis. ORL 2003, 65, 306–309. [Google Scholar] [CrossRef]
- Menezes, A.H.; VanGilder, J.C.; Clark, C.R.; El-Khoury, G. Odontoid Upward Migration in Rheumatoid Arthritis. An Analysis of 45 Patients with “Cranial Settling”. J. Neurosurg. 1985, 63, 500–509. [Google Scholar] [CrossRef]
- Mathew, R.; Mumford, C.J.; Daroszewska, A. Unilateral Glossopharyngeal and Hypoglossal Nerve Palsies Due to Compression by a Rheumatoid Pannus. Rheumatology 2010, 49, 1996–1997. [Google Scholar] [CrossRef]
- Mehrotra, A.; Das, K.K.; Nair, A.P.; Kumar, R.; Srivastava, A.K.; Sahu, R.N.; Kumar, R. Pediatric Cranio-Vertebral Junction Tuberculosis: Management and Outcome. Child’s Nerv. Syst. 2013, 29, 809–814. [Google Scholar] [CrossRef]
- Ebadi, H.; Fathi, D. Unilateral Hypoglossal Nerve Palsy: As the Only Presentation of Tuberculosis. Acta Med. Iran. 2012, 50, 717–720. [Google Scholar]
- Pandey, R.; Bhayana, H.; Dhammi, I.K.; Jain, A.K. Hypoglossal Nerve Palsy as a Rare Complication of C1–C2 Pott’s Spine. Indian J. Orthop. 2019, 53, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Das, A. Craniovertebral Tuberculosis in Children: Experience of 23 Cases and Proposal for a New Classification. Child’s Nerv. Syst. 2015, 31, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.; Verma, N.; Mohindroo, S.; Azad, R.K.; Mohindroo, N. Isolated Tubercular Hypoglossal Nerve Paralysis. Trop. Doct. 2017, 47, 255–260. [Google Scholar] [CrossRef] [PubMed]
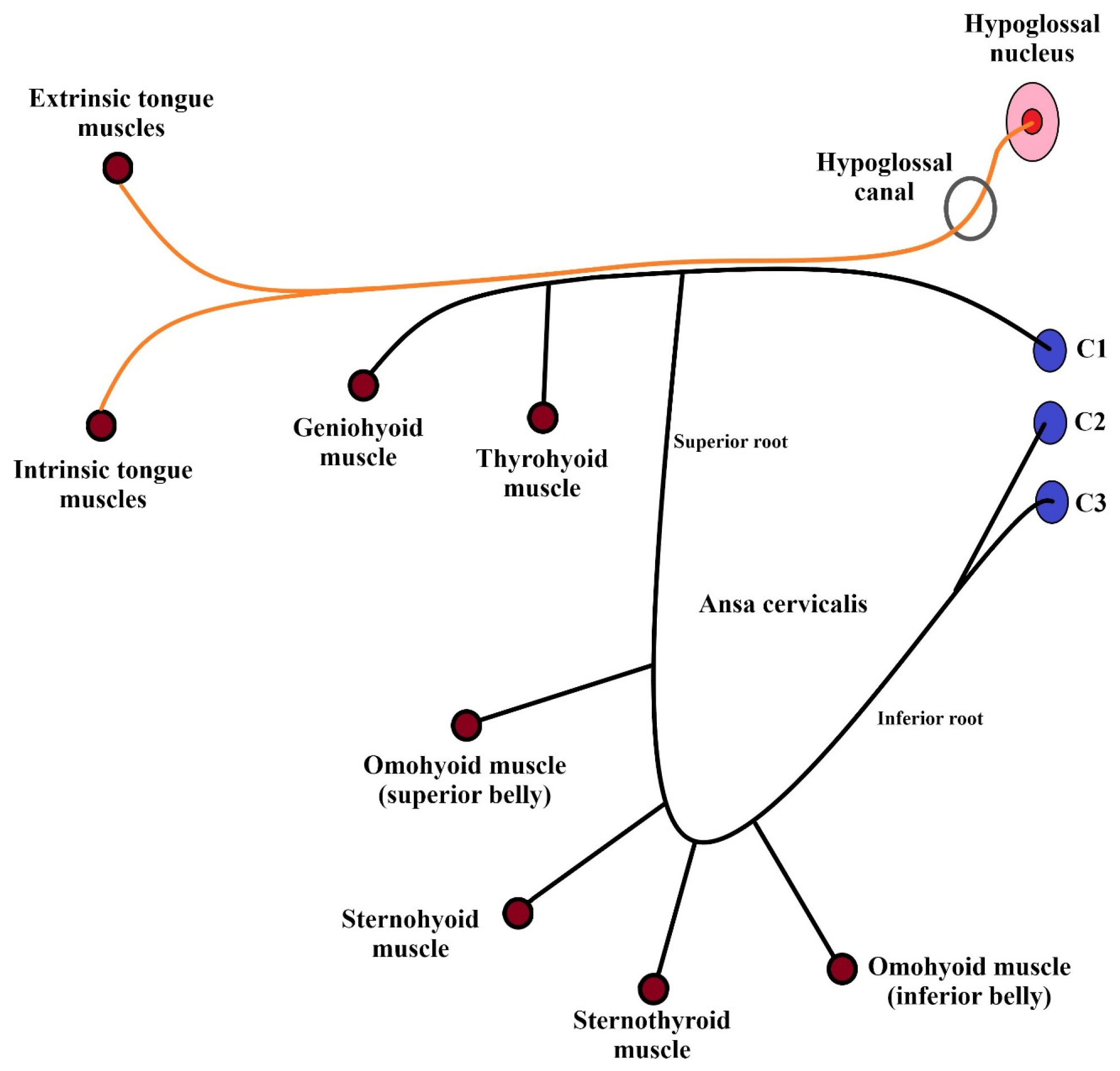
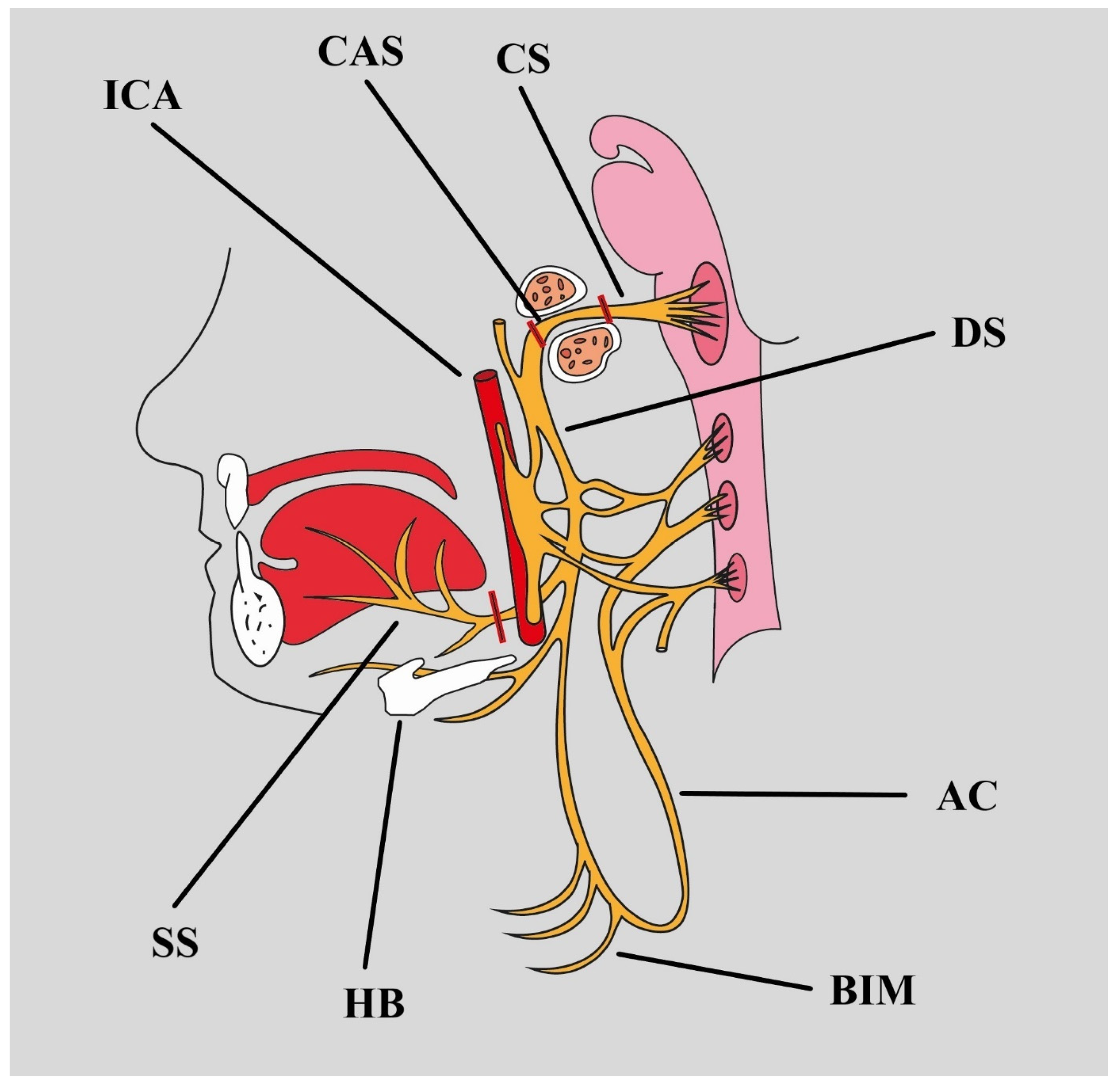
| Branches of the Hypoglossal Nerve | |||||
|---|---|---|---|---|---|
| Meningeal | Descending | Thyrohyoid | Geniohyoid | Muscular | |
| Supplied structures |
|
|
|
| Intrinsic tongue muscles:
|
| Point of origin | C1/C2 nerves | C1/C3 nerves | C1 nerve | C1 nerve | Medulla oblongata |
| Segments of the Hypoglossal Nerve | |||||
|---|---|---|---|---|---|
| Sublingual | |||||
| Cisternal | Canalar/Skull Base | Descending/ Carotid Space | Horizontal | Ascending | |
| Adjacent Structures |
| Hypoglossal canal:
|
|
|
|
| Distal boundaries | Dural pores in the posterior fossa | Exit of the neurocranium in the nasopharyngeal carotid space | Anterior margin of the sternocleidomastoid muscle | Edge of mylohyoid muscle, at the inferior surface of the tongue | Two to five terminal branches to the muscle bellies |
| Groups of Etiologies | Cause of Palsy |
|---|---|
| Vessels | Pathological changes or abnormal morphology of:
|
| Iatrogenic factors | Airway management |
| Radiotherapy | |
| Intraoperative injuries | |
| Pathologic masses | Tumors |
| Cysts | |
| Osteophytes | |
| Trauma | Direct damage |
| Occipital condyle fractures | |
| Atlantoaxial subluxation (also secondary to the rheumatoid arthritis or tuberculosis) | |
| Immunologic factors | Autoimmunologic diseases |
| Infections | |
| Vaccinations | |
| Oral cavity inflammations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgiel, A.; Zielinska, N.; Głowacka, M.; Olewnik, Ł. Hypoglossal Nerve Neuropathies—Analysis of Causes and Anatomical Background. Biomedicines 2024, 12, 864. https://doi.org/10.3390/biomedicines12040864
Węgiel A, Zielinska N, Głowacka M, Olewnik Ł. Hypoglossal Nerve Neuropathies—Analysis of Causes and Anatomical Background. Biomedicines. 2024; 12(4):864. https://doi.org/10.3390/biomedicines12040864
Chicago/Turabian StyleWęgiel, Andrzej, Nicol Zielinska, Mariola Głowacka, and Łukasz Olewnik. 2024. "Hypoglossal Nerve Neuropathies—Analysis of Causes and Anatomical Background" Biomedicines 12, no. 4: 864. https://doi.org/10.3390/biomedicines12040864
APA StyleWęgiel, A., Zielinska, N., Głowacka, M., & Olewnik, Ł. (2024). Hypoglossal Nerve Neuropathies—Analysis of Causes and Anatomical Background. Biomedicines, 12(4), 864. https://doi.org/10.3390/biomedicines12040864





