Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
2.3. Study Approval
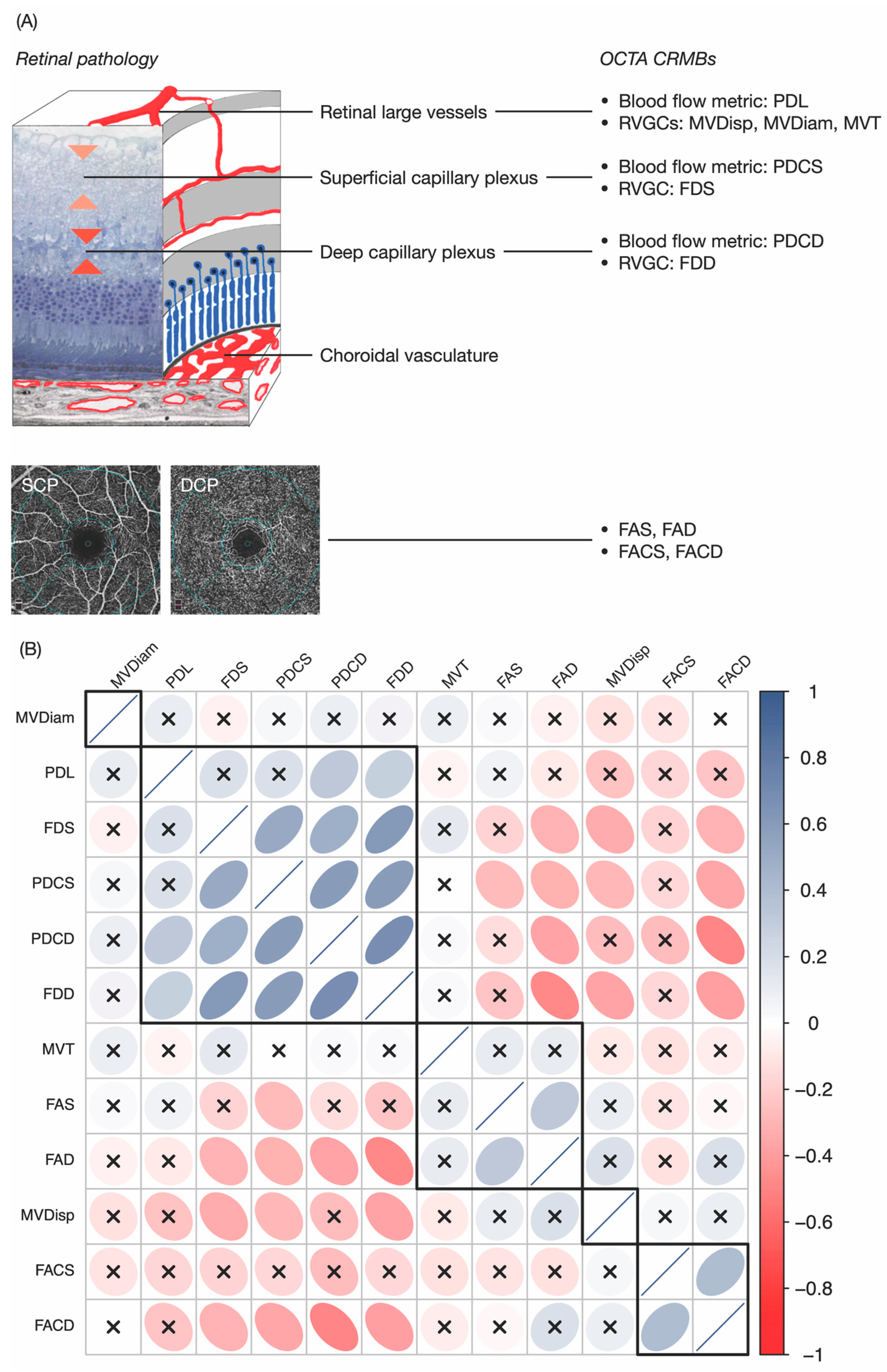
3. Results and Discussion
3.1. OCTA CRMBs Generation
3.2. OCTA CRMBs Characterize Retinal Pathology
3.3. OCTA CRMBs Correlate with Retinal Function
3.4. OCTA CRMBs as Risk Factors of RVO
3.5. OCTA CRMBs Significantly Correlate with Visual Acuity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clémence-Fau, M.; Schwan, R.; Angioi-Duprez, K.; Laprévote, V.; Schwitzer, T. Retinal structural changes in mood disorders: The optical coherence tomography to better understand physiopathology? Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110080. [Google Scholar] [CrossRef]
- Allon, R.; Aronov, M.; Belkin, M.; Maor, E.; Shechter, M.; Fabian, I.D. Retinal Microvascular Signs as Screening and Prognostic Factors for Cardiac Disease: A Systematic Review of Current Evidence. Am. J. Med. 2021, 134, 36–47.e7. [Google Scholar] [CrossRef] [PubMed]
- Biffi, E.; Turple, Z.; Chung, J.; Biffi, A. Retinal biomarkers of Cerebral Small Vessel Disease: A systematic review. PLoS ONE 2022, 17, e0266974. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.G.M.; Rodrigues Neto, T.D.S.; da Silva Neto, E.D.; Preti, R.C.; Monteiro, M.L.R.; Zacharias, L.C. A standardized method to quantitatively analyze optical coherence tomography angiography images of the macular and peripapillary vessels. Int. J. Retina Vitreous 2022, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Bobba, V.; Figueiredo, N.; Sevgi, D.D.; Lu, C.; Braman, N.; Alilou, M.; Sharma, S.; Srivastava, S.K.; Madabhushi, A.; et al. Radiomics-based assessment of ultra-widefield leakage patterns and vessel network architecture in the PERMEATE study: Insights into treatment durability. Br. J. Ophthalmol. 2021, 105, 1155–1160. [Google Scholar] [CrossRef]
- Tan, W.; Yao, X.; Le, T.T.; Tan, B.; Schmetterer, L.; Chua, J. The New Era of Retinal Imaging in Hypertensive Patients. Asia. Pac. J. Ophthalmol. (Phila.) 2022, 11, 149–159. [Google Scholar] [CrossRef]
- Kalra, G.; Zarranz-Ventura, J.; Chahal, R.; Bernal-Morales, C.; Lupidi, M.; Chhablani, J. Optical coherence tomography (OCT) angiolytics: A review of OCT angiography quantitative biomarkers. Surv. Ophthalmol. 2022, 67, 1118–1134. [Google Scholar] [CrossRef]
- Tao, W.; Kwapong, W.R.; Xie, J.; Wang, Z.; Guo, X.; Liu, J.; Ye, C.; Wu, B.; Zhao, Y.; Liu, M. Retinal microvasculature and imaging markers of brain frailty in normal aging adults. Front. Aging Neurosci. 2022, 14, 945964. [Google Scholar] [CrossRef]
- Cuenca, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Kutsyr, O.; Albertos-Arranz, H.; Fernández-Sánchez, L.; Martínez-Gil, N.; Noailles, A.; López-Garrido, J.A.; López-Gálvez, M.; et al. Interpretation of OCT and OCTA images from a histological approach: Clinical and experimental implications. Prog. Retin. Eye Res. 2020, 77, 100828. [Google Scholar] [CrossRef]
- Yang, D.; Ran, A.R.; Nguyen, T.X.; Lin, T.P.H.; Chen, H.; Lai, T.Y.Y.; Tham, C.C.; Cheung, C.Y. Deep Learning in Optical Coherence Tomography Angiography: Current Progress, Challenges, and Future Directions. Diagnostics 2023, 13, 326. [Google Scholar] [CrossRef]
- Lemmens, S.; Devulder, A.; Van Keer, K.; Bierkens, J.; De Boever, P.; Stalmans, I. Systematic Review on Fractal Dimension of the Retinal Vasculature in Neurodegeneration and Stroke: Assessment of a Potential Biomarker. Front. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, M.; Sun, L.; Wang, Y.; Chen, Y. Evaluation of microvascular network with optical coherence tomography angiography (OCTA) in branch retinal vein occlusion (BRVO). BMC Ophthalmol. 2020, 20, 154. [Google Scholar] [CrossRef]
- Reagan, A.M.; Gu, X.; Paudel, S.; Ashpole, N.M.; Zalles, M.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A.; Otalora, L.; Freeman, W.M.; et al. Age-related focal loss of contractile vascular smooth muscle cells in retinal arterioles is accelerated by caveolin-1 deficiency. Neurobiol. Aging 2018, 71, 1–12. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kılıç, K.; Villafranca-Baughman, D.; Can, A.; Di Polo, A.; Dalkara, T. Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 2019, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Lee, K.E.; Danforth, L.; Tsai, M.Y.; Gangnon, R.E.; Meuer, S.E.; Wong, T.Y.; Cheung, C.Y.; Klein, B.E.K. The Relationship of Retinal Vessel Geometric Characteristics to the Incidence and Progression of Diabetic Retinopathy. Ophthalmology 2018, 125, 1784–1792. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.R.; Sun, G.; Zhang, L.; Zhu, M.; Tian, T.; Guo, C.; Mazhar, S.; Yang, J.K.; Li, Y. Diagnosing Diabetic Retinopathy in OCTA Images Based on Multilevel Information Fusion Using a Deep Learning Framework. Comput. Math. Methods Med. 2022, 2022, 4316507. [Google Scholar] [CrossRef]
- Nicholson, L.; Talks, S.J.; Amoaku, W.; Talks, K.; Sivaprasad, S. Retinal vein occlusion (RVO) guideline: Executive summary. Eye 2022, 36, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Kapik, B.; Hu, A.; Harris, A.; Ip, M.S.; Blodi, B. Anatomic Biomarkers of Macular Edema Associated with Retinal Vein Occlusion. Ophthalmol. Retina 2022, 6, 1206–1220. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Hussain, R.M.; Taraborelli, D.; Pollack, J.S.; Williams, D.F. Longer-Term Anti-VEGF Therapy Outcomes in Neovascular Age-Related Macular Degeneration, Diabetic Macular Edema, and Vein Occlusion-Related Macular Edema: Clinical Outcomes in 130 247 Eyes. Ophthalmol. Retina 2022, 6, 796–806. [Google Scholar] [CrossRef]
- Nagasato, D.; Muraoka, Y.; Tanabe, M.; Nishigori, N.; Osaka, R.; Mitamura, Y.; Tabuchi, H.; Kadomoto, S.; Murakami, T.; Ooto, S.; et al. Foveal Thickness Fluctuation in Anti-VEGF Treatment for Branch Retinal Vein Occlusion: A Long-term Study. Ophthalmol. Retina 2022, 6, 567–574. [Google Scholar] [CrossRef]
- Choi, K.E.; Yun, C.; Cha, J.; Kim, S.W. OCT angiography features associated with macular edema recurrence after intravitreal bevacizumab treatment in branch retinal vein occlusion. Sci. Rep. 2019, 9, 14153. [Google Scholar] [CrossRef] [PubMed]
- Adhi, M.; Filho, M.A.; Louzada, R.N.; Kuehlewein, L.; de Carlo, T.E.; Baumal, C.R.; Witkin, A.J.; Sadda, S.R.; Sarraf, D.; Reichel, E.; et al. Retinal Capillary Network and Foveal Avascular Zone in Eyes with Vein Occlusion and Fellow Eyes Analyzed with Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT486–OCT494. [Google Scholar] [CrossRef] [PubMed]
- Koulisis, N.; Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Burkemper, B.; Olmos de Koo, L.C.; Moshfeghi, A.A.; Ameri, H.; Puliafito, C.A.; Isozaki, V.L.; et al. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PLoS ONE 2017, 12, e0176404. [Google Scholar] [CrossRef] [PubMed]
- Youm, D.J.; Ha, M.M.; Chang, Y.; Song, S.J. Retinal vessel caliber and risk factors for branch retinal vein occlusion. Curr. Eye Res. 2012, 37, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Saladino, A.; Amato, A.; Bandello, F.; Battaglia Parodi, M. The impact of different thresholds on optical coherence tomography angiography images binarization and quantitative metrics. Sci. Rep. 2021, 11, 14758. [Google Scholar] [CrossRef] [PubMed]
- Stino, H.; de Llano Pato, E.; Steiner, I.; Mahnert, N.; Pawloff, M.; Hasun, M.; Weidinger, F.; Schmidt-Erfurth, U.; Pollreisz, A. Macular Microvascular Perfusion Status in Hypertensive Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 5493. [Google Scholar] [CrossRef] [PubMed]
- Al-Nofal, M.; de Boer, I.; Agirman, S.; Wilms, A.E.; Zamanipoor Najafabadi, A.H.; Terwindt, G.M.; Notting, I.C. Optical coherence tomography angiography biomarkers of microvascular alterations in RVCL-S. Front. Neurol. 2020, 13, 989536. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Cefalo, C.M.A.; Ferreri, C.; Sansone, A.; Vitale, M.; Serantoni, C.; Abeltino, A.; Mezza, T.; Ferraro, P.M.; De Spirito, M.; et al. Erythrocyte membrane fluidity: A novel biomarker of residual cardiovascular risk in type 2 diabetes. Eur. J. Clin. Investig. 2024, 54, e14121. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yi, Q.; Wu, Y.; Zheng, Y.; Liu, Y.; Macerollo, A.; Fu, H.; Xu, Y.; Zhang, J.; Behera, A.; et al. Deep segmentation of OCTA for evaluation and association of changes of retinal microvasculature with Alzheimer’s disease and mild cognitive impairment. Br. J. Ophthalmol. 2024, 108, 432–439. [Google Scholar] [CrossRef]
- Seknazi, D.; Coscas, F.; Sellam, A.; Rouimi, F.; Coscas, G.; Souied, E.H.; Glacet-Bernard, A. Optical coherence tomography angiography in retinal vein occlusion: Correlations between macular vascular density, visual acuity, and peripheral nonperfusion area on uorescein angiography. Retina 2018, 38, 1562. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Hough, P.V. Method and Means for Recognizing Complex Patterns. U.S. Patent 3,069,654, 18 December 1962. [Google Scholar]
- Harris, C.G.; Stephens, M. A combined corner and edge detector. Alvey Vis. Conf. 1988, 15, 147–152. [Google Scholar]
- Pointer, M.R. A comparison of the CIE 1976 colour spaces. Color Res. Appl. 1981, 6, 108–118. [Google Scholar] [CrossRef]
- Sato, Y.; Nakajima, S.; Atsumi, H.; Koller, T.; Gerig, G.; Yoshida, S.; Kikinis, R. 3D multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. In CVRMed-MRCAS’97; Springer: Berlin/Heidelberg, Germany, 1997; pp. 213–222. [Google Scholar]
- Aghamohamadian-Sharbaf, M.; Pourreza, H.R.; Banaee, T. A novel curvature-based algorithm for automatic grading of retinal blood vessel tortuosity. IEEE J. Biomed. Health Inform. 2015, 20, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Romano, F.; Albertini, G.; Aragona, E.; Bandello, F.; Parodi, M.B. Vascular patterns in retinitis pigmentosa on swept-source optical coherence tomography angiography. J. Clin. Med. 2019, 8, 1425. [Google Scholar] [CrossRef]
- Conrath, J.; Valat, O.; Giorgi, R.; Adel, M.; Raccah, D.; Meyer, F.; Ridings, B. Semi-automated detection of the foveal avascular zone in fluorescein angiograms in diabetes mellitus. Clin. Exp. Ophthalmol. 2006, 34, 119–123. [Google Scholar] [CrossRef]



| RVO | Control | p-Value | Adjusted p-Value | |
|---|---|---|---|---|
| Number of Patients and Eyes | 43 (43 eyes) | 30 (30 eyes) | - | - |
| Sex (men/women) | 19/24 | 17/13 | 0.2940 | 0.3360 |
| Study eye (right/left) | 22/21 | 15/15 | 0.9221 | 0.9221 |
| Age, years | 55.42 ± 12.95 | 44.74 ± 15.37 | 0.0029 | 0.0077 |
| Subtype (BRVO/CRVO) | 25/18 | - | - | - |
| Subtype (ischemic/non-ischemic) | 11/32 | - | - | - |
| Diabetes mellitus, yes/no | 2/41 | 0/30 | 0.2310 | 0.3080 |
| Hypertension, yes/no | 15/28 | 0/30 | 0.0003 | 0.0012 |
| Hyperlipidaemia, yes/no | 6/37 | 0/30 | 0.0327 | 0.0523 |
| Atherosclerosis, yes/no | 11/32 | 1/29 | 0.0117 | 0.0234 |
| Duration from the initial visit, months | 2.00 ± 2.06 | - | - | - |
| BCVA at initial visit | 0.43 ± 0.29 | 0.93±0.10 | <0.001 | <0.001 |
| BCVA at inclusion | 0.73 ± 0.28 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Li, Y.; Xie, L.; Chiu, K.; Hao, X.; Xu, J.; Luo, J.; Sham, P.-C. Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation. Biomedicines 2024, 12, 868. https://doi.org/10.3390/biomedicines12040868
Lu B, Li Y, Xie L, Chiu K, Hao X, Xu J, Luo J, Sham P-C. Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation. Biomedicines. 2024; 12(4):868. https://doi.org/10.3390/biomedicines12040868
Chicago/Turabian StyleLu, Bingwen, Yiming Li, Like Xie, Kin Chiu, Xiaofeng Hao, Jing Xu, Jie Luo, and Pak-Chung Sham. 2024. "Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation" Biomedicines 12, no. 4: 868. https://doi.org/10.3390/biomedicines12040868
APA StyleLu, B., Li, Y., Xie, L., Chiu, K., Hao, X., Xu, J., Luo, J., & Sham, P.-C. (2024). Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation. Biomedicines, 12(4), 868. https://doi.org/10.3390/biomedicines12040868








