Organoids Modeling Stroke in a Petri Dish
Abstract
1. Introduction
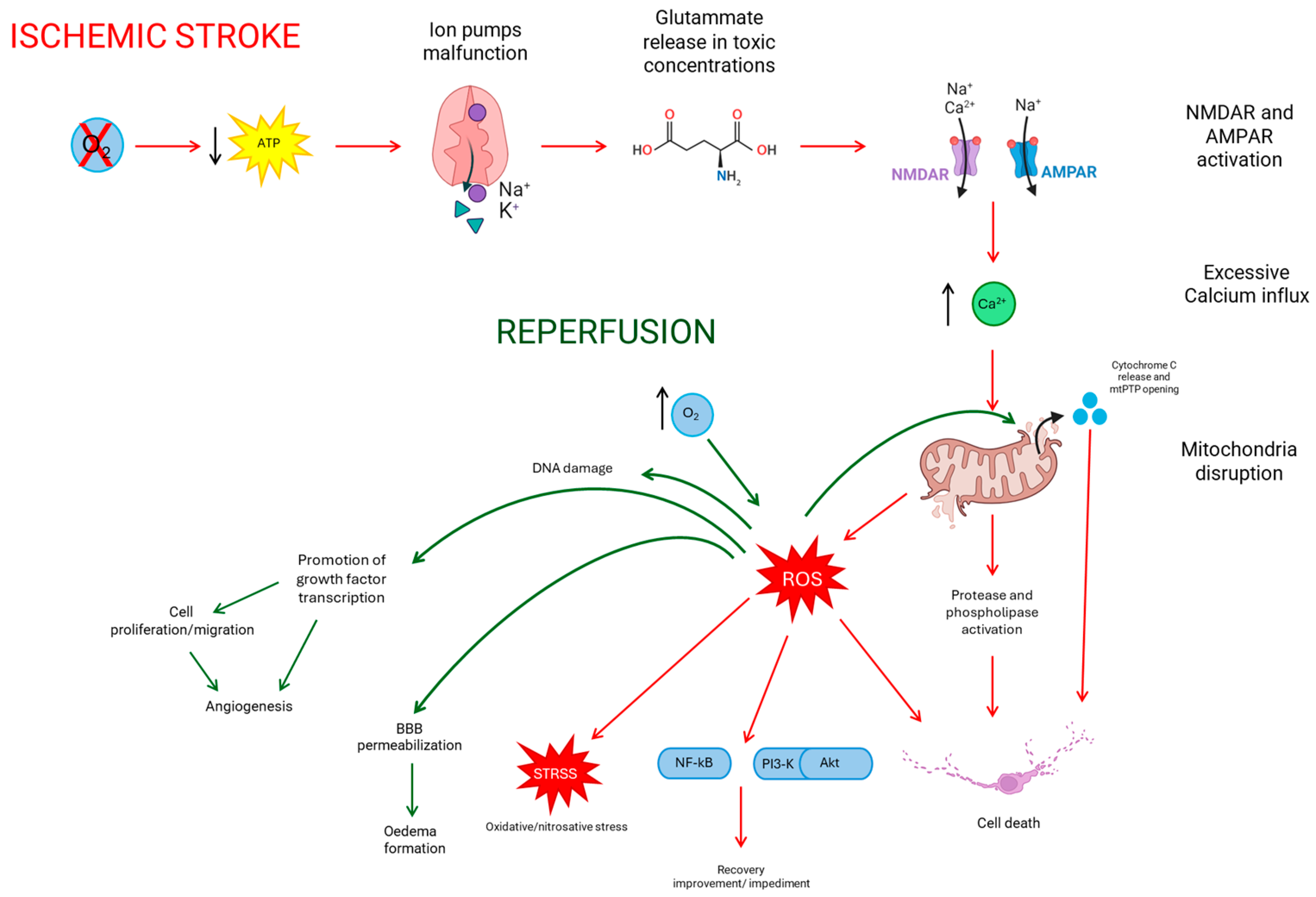
2. Ischemic Stroke
2.1. Treatments
2.2. Reperfusion
3. Brain Organoids
3.1. Organoid Culture and Modeling
3.2. Brain Organoids
3.3. Advantages and Limitation in the Use of Organoids
4. Brain Organoids as a Tool for Ischemic Stroke Studies
5. The Use of Brain Organoids as Therapeutics against Ischemic Stroke
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years from 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Takahashi, H.; Asahina, R.; Fujioka, M.; Matsui, T.K.; Kato, S.; Mori, E.; Hioki, H.; Yamamoto, T.; Kobayashi, K.; Tsuboi, A. Ras-like Gem GTPase Induced by Npas4 Promotes Activity-Dependent Neuronal Tolerance for Ischemic Stroke. Proc. Natl. Acad. Sci. USA 2021, 118, e2018850118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Wang, X.; Zhang, X.; Xu, T.; Miao, C. Humanized Cerebral Organoids-Based Ischemic Stroke Model for Discovering of Potential Anti-Stroke Agents. Acta Pharmacol. Sin. 2023, 44, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-N.; Wang, Z.; Xu, T.-Y.; Cheng, M.-H.; Li, W.-L.; Miao, C.-Y. Cerebral Organoids Repair Ischemic Stroke Brain Injury. Transl. Stroke Res. 2020, 11, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.J. Ischemic Stroke: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef]
- Gaston-Breton, R.; Maïza Letrou, A.; Hamoudi, R.; Stonestreet, B.S.; Mabondzo, A. Brain Organoids for Hypoxic-Ischemic Studies: From Bench to Bedside. Cell. Mol. Life Sci. 2023, 80, 318. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Q.; Zeng, L.-H.; Li, C.-T.; He, D.-H.; Zhao, H.-D.; Xu, Y.-N.; Jin, Z.-T.; Gao, C. Brain Organoids Are New Tool for Drug Screening of Neurological Diseases. Neural Regen. Res. 2023, 18, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, I.; Lancaster, M.A. Brain Organoids for the Study of Human Neurobiology at the Interface of In Vitro and In Vivo. Nat. Neurosci. 2020, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Amado, B.; Melo, L.; Pinto, R.; Lobo, A.; Barros, P.; Gomes, J.R. Ischemic Stroke, Lessons from the Past towards Effective Preclinical Models. Biomedicines 2022, 10, 2561. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.B.; Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Ischemic Stroke: Basic Pathophysiology and Neuroprotective Strategies. In Ischemic Stroke: Basic Pathophysiology and Neuroprotective Strategies; González, R.G., Hirsch, J.A., Lev, M.H., Schaefer, P.W., Schwamm, L.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-12750-2. [Google Scholar]
- George, P.M.; Steinberg, G.K. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron 2015, 87, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial Reactive Oxygen Species: A Double Edged Sword in Ischemia/Reperfusion vs Preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging Neuroprotective Strategies for the Treatment of Ischemic Stroke: An Overview of Clinical and Preclinical Studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.-L.; Guo, Z.-N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, e3804979. [Google Scholar] [CrossRef] [PubMed]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger Signals in Stroke and Their Role on Microglia Activation after Ischemia. Ther. Adv. Neurol. Disord. 2018, 11, 175628641877425. [Google Scholar] [CrossRef]
- Xia, Y.; Pu, H.; Leak, R.K.; Shi, Y.; Mu, H.; Hu, X.; Lu, Z.; Foley, L.M.; Hitchens, T.K.; Dixon, C.E.; et al. Tissue Plasminogen Activator Promotes White Matter Integrity and Functional Recovery in a Murine Model of Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2018, 115, E9230–E9238. [Google Scholar] [CrossRef]
- Bhaskar, S.; Stanwell, P.; Cordato, D.; Attia, J.; Levi, C. Reperfusion Therapy in Acute Ischemic Stroke: Dawn of a New Era? BMC Neurol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Rabinstein, A.A. Update on Treatment of Acute Ischemic Stroke. Contin. Lifelong Learn. Neurol. 2020, 26, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Miao, C.-Y. NAMPT as a Therapeutic Target against Stroke. Trends Pharmacol. Sci. 2015, 36, 891–905. [Google Scholar] [CrossRef]
- Shi, L.; Rocha, M.; Leak, R.K.; Zhao, J.; Bhatia, T.N.; Mu, H.; Wei, Z.; Yu, F.; Weiner, S.L.; Ma, F.; et al. A New Era for Stroke Therapy: Integrating Neurovascular Protection with Optimal Reperfusion. J. Cereb. Blood Flow Metab. 2018, 38, 2073–2091. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Abdalkader, M.; Siegler, J.E.; Yaghi, S.; Sarraj, A.; Campbell, B.C.V.; Yoo, A.J.; Zaidat, O.O.; Kaesmacher, J.; Pujara, D.; et al. Mechanical Thrombectomy for Large Ischemic Stroke. Neurology 2023, 101, e922–e932. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in Acute Stroke: Targeting Excitotoxicity, Oxidative and Nitrosative Stress, and Inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Chapter Six—Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 298, pp. 229–317. [Google Scholar]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-Reperfusion Injury in Stroke. Interv. Neurol. 2012, 1, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Olmez, I.; Ozyurt, H. Reactive Oxygen Species and Ischemic Cerebrovascular Disease. Neurochem. Int. 2012, 60, 208–212. [Google Scholar] [CrossRef]
- Bektas, H.; Wu, T.-C.; Kasam, M.; Harun, N.; Sitton, C.W.; Grotta, J.C.; Savitz, S.I. Increased Blood–Brain Barrier Permeability on Perfusion CT Might Predict Malignant Middle Cerebral Artery Infarction. Stroke 2010, 41, 2539–2544. [Google Scholar] [CrossRef]
- Sasai, Y. Next-Generation Regenerative Medicine: Organogenesis from Stem Cells in 3D Culture. Cell Stem Cell 2013, 12, 520–530. [Google Scholar] [CrossRef]
- Sasai, Y. Cytosystems Dynamics in Self-Organization of Tissue Architecture. Nature 2013, 493, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol.-Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, J.; Park, H.; Choi, H.; Kim, J. Modelling Neurodegenerative Diseases with 3D Brain Organoids. Biol. Rev. 2020, 95, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Rooney, L.A.; Daniszewski, M.; Gulluyan, L.; Liang, H.H.; Cook, A.L.; Hewitt, A.W.; Pébay, A. Culture Variabilities of Human iPSC-Derived Cerebral Organoids Are a Major Issue for the Modelling of Phenotypes Observed in Alzheimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mokhtari, R.; Pedrosa, E.; Kirschenbaum, M.; Bayrak, C.; Zheng, D.; Lachman, H.M. CRISPR/Cas9-Mediated Heterozygous Knockout of the Autism Gene CHD8 and Characterization of Its Transcriptional Networks in Cerebral Organoids Derived from iPS Cells. Mol. Autism 2017, 8, 11. [Google Scholar] [CrossRef]
- Schukking, M.; Miranda, H.C.; Trujillo, C.A.; Negraes, P.D.; Muotri, A.R. Direct Generation of Human Cortical Organoids from Primary Cells. Stem Cells Dev. 2018, 27, 1549–1556. [Google Scholar] [CrossRef]
- Ghaedi, M.; Niklason, L.E. Human Pluripotent Stem Cells (iPSC) Generation, Culture, and Differentiation to Lung Progenitor Cells; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1576, pp. 55–92. [Google Scholar] [CrossRef]
- Hartley, B.J.; Brennand, K.J. Neural Organoids for Disease Phenotyping, Drug Screening and Developmental Biology Studies. Neurochem. Int. 2017, 106, 85–93. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Kadoshima, T.; Sakaguchi, H.; Nakano, T.; Soen, M.; Ando, S.; Eiraku, M.; Sasai, Y. Self-Organization of Axial Polarity, inside-out Layer Pattern, and Species-Specific Progenitor Dynamics in Human ES Cell–Derived Neocortex. Proc. Natl. Acad. Sci. USA 2013, 110, 20284–20289. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused Cerebral Organoids Model Interactions between Brain Regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Müller, F. The Embryonic Human Brain: An Atlas of Developmental Stages, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-69462-5. [Google Scholar]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Tanaka, Y.; Cakir, B.; Xiang, Y.; Sullivan, G.J.; Park, I.-H. Synthetic Analyses of Single-Cell Transcriptomes from Multiple Brain Organoids and Fetal Brain. Cell Rep. 2020, 30, 1682–1689.e3. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G. Brain Organoids: Advances, Applications and Challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Qian, X.; Su, Y.; Adam, C.D.; Deutschmann, A.U.; Pather, S.R.; Goldberg, E.M.; Su, K.; Li, S.; Lu, L.; Jacob, F.; et al. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 2020, 26, 766–781.e9. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral Organoids at the Air–Liquid Interface Generate Diverse Nerve Tracts with Functional Output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.-D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.-P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Huang, W.-K.; Wong, S.Z.H.; Pather, S.R.; Nguyen, P.T.T.; Zhang, F.; Zhang, D.Y.; Zhang, Z.; Lu, L.; Fang, W.; Chen, L.; et al. Generation of Hypothalamic Arcuate Organoids from Human Induced Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 1657–1670.e10. [Google Scholar] [CrossRef]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS Barrier-Forming Organoids with Cerebrospinal Fluid Production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef]
- Winanto; Khong, Z.-J.; Hor, J.-H.; Ng, S.-Y. Spinal Cord Organoids Add an Extra Dimension to Traditional Motor Neuron Cultures. Neural Regen. Res. 2019, 14, 1515–1516. [Google Scholar] [CrossRef]
- Chhibber, T.; Bagchi, S.; Lahooti, B.; Verma, A.; Al-Ahmad, A.; Paul, M.K.; Pendyala, G.; Jayant, R.D. CNS Organoids: An Innovative Tool for Neurological Disease Modeling and Drug Neurotoxicity Screening. Drug Discov. Today 2020, 25, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.D.; Paşca, S.P. Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron 2018, 100, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Rouleau, N.; Parker, R.N.; Walsh, K.G.; Gehrke, L.; Kaplan, D.L. A 3D Human Brain–like Tissue Model of Herpes-Induced Alzheimer’s Disease. Sci. Adv. 2020, 6, eaay8828. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Brawner, A.T.; Li, S.; Liu, J.-J.; Kim, H.; Xue, H.; Pang, Z.P.; Kim, W.-Y.; Hart, R.P.; Liu, Y.; et al. OLIG2 Drives Abnormal Neurodevelopmental Phenotypes in Human iPSC-Based Organoid and Chimeric Mouse Models of Down Syndrome. Cell Stem Cell 2019, 24, 908–926.e8. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.-R.; et al. Zika Virus Infection Induces RNAi-Mediated Antiviral Immunity in Human Neural Progenitors and Brain Organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef]
- Bian, S.; Repic, M.; Guo, Z.; Kavirayani, A.; Burkard, T.; Bagley, J.A.; Krauditsch, C.; Knoblich, J.A. Genetically Engineered Cerebral Organoids Model Brain Tumor Formation. Nat. Methods 2018, 15, 631–639. [Google Scholar] [CrossRef]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.-Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional Cortical Neurons and Astrocytes from Human Pluripotent Stem Cells in 3D Culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef]
- Nestor, M.W.; Paull, D.; Jacob, S.; Sproul, A.A.; Alsaffar, A.; Campos, B.A.; Noggle, S.A. Differentiation of Serum-Free Embryoid Bodies from Human Induced Pluripotent Stem Cells into Networks. Stem Cell Res. 2013, 10, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal Models of Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling Amyloid Beta and Tau Pathology in Human Cerebral Organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Comi, G.P.; Corti, S. Advancing Drug Discovery for Neurological Disorders Using iPSC-Derived Neural Organoids. Int. J. Mol. Sci. 2021, 22, 2659. [Google Scholar] [CrossRef] [PubMed]
- Hartlaub, A.M.; McElroy, C.A.; Maitre, N.L.; Hester, M.E. Modeling Human Brain Circuitry Using Pluripotent Stem Cell Platforms. Front. Pediatr. 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A Novel Modality in Disease Modeling. Bio-Des. Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Y.; Qin, S.; Zhao, W.; Chu, Q.; Wu, K. Organoid Technology in Disease Modelling, Drug Development, Personalized Treatment and Regeneration Medicine. Exp. Hematol. Oncol. 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.S.; Hu, X.; Shan, Z.; Wu, Y.; Lei, L. Brain Organoid: A 3D Technology for Investigating Cellular Composition and Interactions in Human Neurological Development and Disease Models in Vitro. Stem Cell Res. Ther. 2021, 12, 430. [Google Scholar] [CrossRef]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human Organoids in Basic Research and Clinical Applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An In Vivo Model of Functional and Vascularized Human Brain Organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.P.; Hou, Z.; Propson, N.E.; Zhang, J.; Engstrom, C.J.; Costa, V.S.; Jiang, P.; Nguyen, B.K.; Bolin, J.M.; Daly, W.; et al. Human Pluripotent Stem Cell-Derived Neural Constructs for Predicting Neural Toxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 12516–12521. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided Self-Organization and Cortical Plate Formation in Human Brain Organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, L.; Kuang, Y.; Venkataramani, V.; Jin, F.; Hein, K.; Zafeiriou, M.P.; Lenz, C.; Moebius, W.; Kilic, E.; et al. Extracellular Vesicles Derived from Neural Progenitor Cells—A Preclinical Evaluation for Stroke Treatment in Mice. Transl. Stroke Res. 2021, 12, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, N.; Matsui, T.K.; Iguchi, N.; Kinugawa, K.; Morikawa, N.; Sakaguchi, Y.M.; Shiota, T.; Kobashigawa, S.; Nakanishi, M.; Matsubayashi, M.; et al. Gene Expression Profiles of Human Cerebral Organoids Identify PPAR Pathway and PKM2 as Key Markers for Oxygen-Glucose Deprivation and Reoxygenation. Front. Cell. Neurosci. 2021, 15, 605030. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.-H.; Kang, H.K.; Kook, M.G.; Choi, S.W.; Kang, K.-S. Modeling of Hypoxic Brain Injury through 3D Human Neural Organoids. Cells 2021, 10, 234. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Yang, D.; Huang, Z.-Q.; Lin, Y.-H.; Wu, H.-Y.; Chang, L.; Luo, C.-X.; Xu, Y.; Liu, Y.; Zhu, D.-Y. Cerebral Organoids Transplantation Repairs Infarcted Cortex and Restores Impaired Function after Stroke. Npj Regen. Med. 2023, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-Enabled Organoids: Construction, Analysis, and Application. Bioact. Mater. 2023, 31, 525–548. [Google Scholar] [CrossRef] [PubMed]

| Study | OGD Type | Techniques | Main Results |
|---|---|---|---|
| Testing pro/anti-apoptotic compounds (Z-VAD-FMK and navitoclax) and neuroprotective compounds (edaravone, butylphthalide, P7C3-A20, and ZL006) [7] | Oxygen/glucose deprivation for 8 h | Cytotoxicity LDH release, TUNEL assay Nissl’s staining, and Caspase 3 activity | All the compounds had the expected effect on organoids, suggesting brain organoids can be used for drug-screening experiments. |
| Testing possible neuroprotective/neurodegenerative properties of EV on organoids after OGD [82] | Oxygen/glucose deprivation for 8 h and 24 h of reoxygenation | Treatment with different concentrations of neural progenitor cells (NPC)-derived EVs | NPC-EVs reduced the cell death rate of OGD organoids suggesting a possible use of organoids to discover new therapy against stroke and to better understand the molecular mechanisms of stroke. |
| Looking for OGD/R markers on organoids [83] | Oxygen/glucose deprivation and reperfusion | Transcriptome analysis and gene networking | The expression level of PKM2 of brain organoids after OGD/R was elevated, suggesting PKM2 could be a possible hypoxic marker. |
| Modelling hypoxic brain injuries [84] | Oxygen/glucose deprivation, hypoxia, glucose deprivation | Analyzing organoids’ size, cell proliferation, and disruption. | OGD organoids were the most damaged and they did not benefit from reoxygenation phase. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgi, C.; Castelli, V.; d’Angelo, M.; Cimini, A. Organoids Modeling Stroke in a Petri Dish. Biomedicines 2024, 12, 877. https://doi.org/10.3390/biomedicines12040877
Giorgi C, Castelli V, d’Angelo M, Cimini A. Organoids Modeling Stroke in a Petri Dish. Biomedicines. 2024; 12(4):877. https://doi.org/10.3390/biomedicines12040877
Chicago/Turabian StyleGiorgi, Chiara, Vanessa Castelli, Michele d’Angelo, and Annamaria Cimini. 2024. "Organoids Modeling Stroke in a Petri Dish" Biomedicines 12, no. 4: 877. https://doi.org/10.3390/biomedicines12040877
APA StyleGiorgi, C., Castelli, V., d’Angelo, M., & Cimini, A. (2024). Organoids Modeling Stroke in a Petri Dish. Biomedicines, 12(4), 877. https://doi.org/10.3390/biomedicines12040877








