Electrophysiological Screening to Assess Foot Drop Syndrome in Severe Acquired Brain Injury in Rehabilitative Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
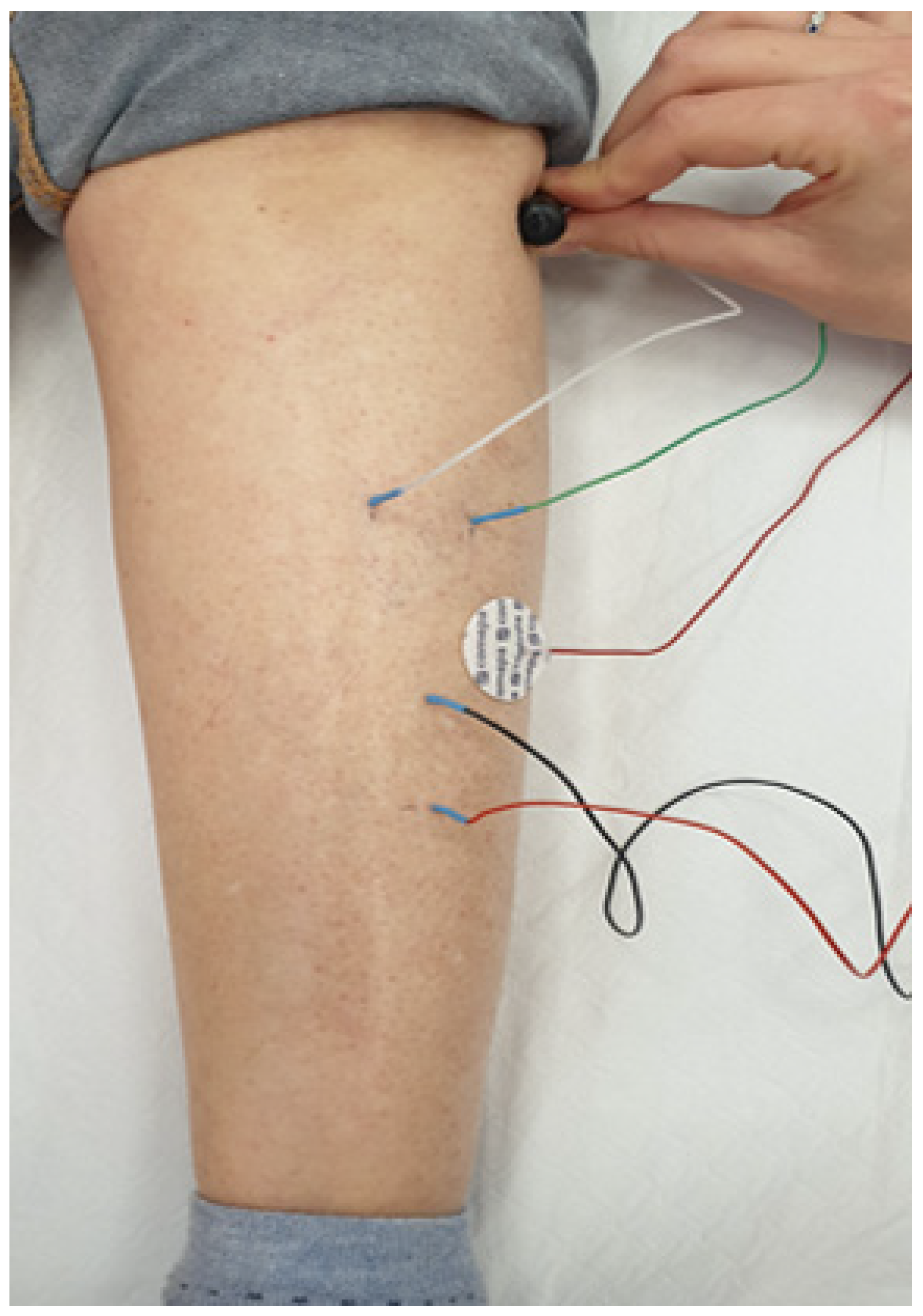
2.2. Study Procedures
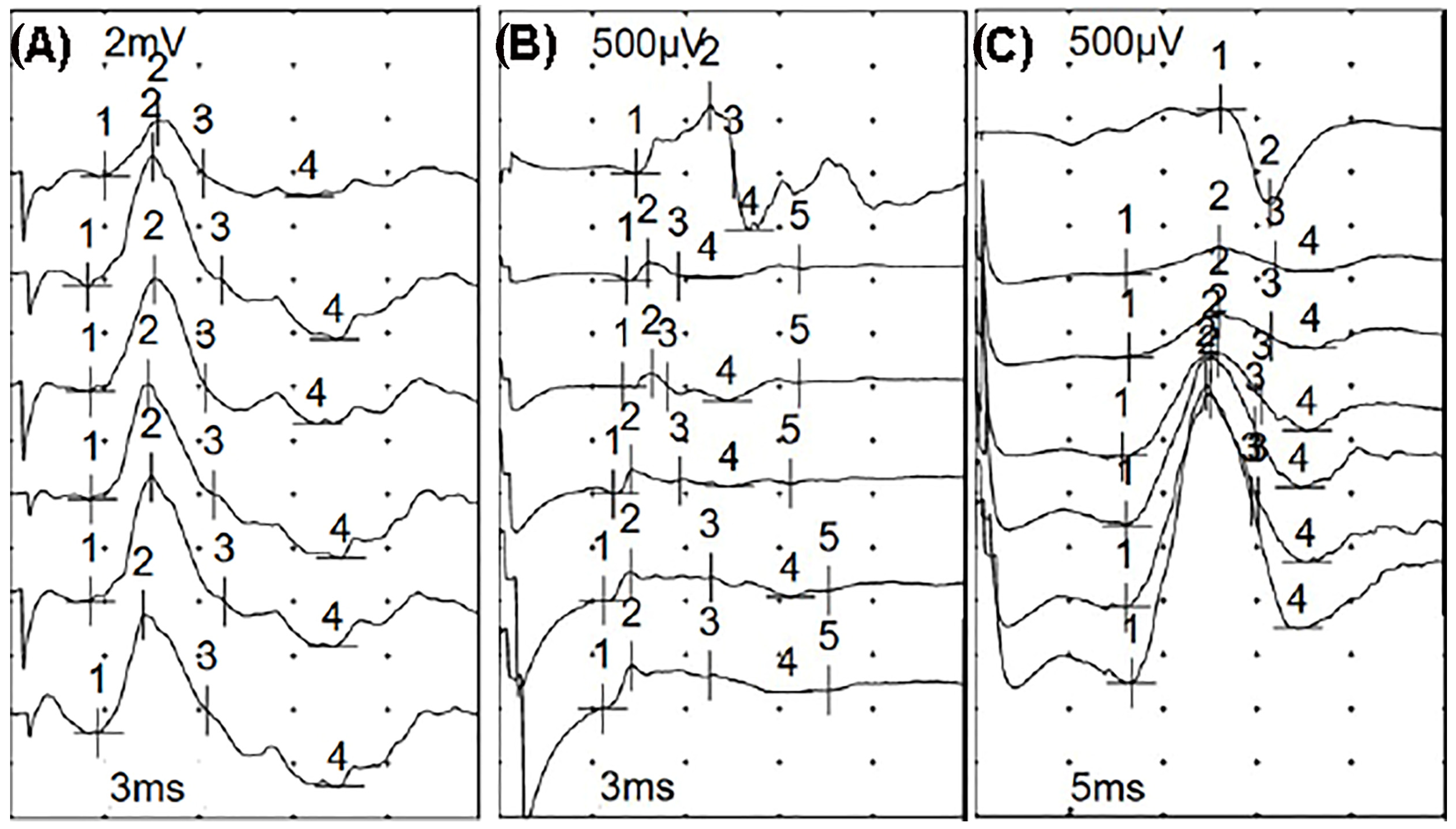
Electrophysiological Screening
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nath, R.K.; Somasundaram, C. Incidence, Etiology, and Risk Factors Associated with Foot Drop. Eplasty 2023, 23, e16. [Google Scholar] [PubMed]
- Stewart, J.D. Foot drop: Where, why and what to do? Pract. Neurol. 2008, 8, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.M.; Markel, M.; Thomas, B.G.; Sherman, W.F.; Thomas, B.H.; Kaye, A.D. An Update on Peroneal Nerve Entrapment and Neuropathy. Orthop. Rev. 2021, 13, 24937. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 4th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Hakiki, B.; Cecchi, F.; Pancani, S.; Romoli, A.M.; Draghi, F.; Scarpino, M.; Sterpu, R.; Mannini, A.; Macchi, C.; Grippo, A. Critical Illness Polyneuropathy and Myopathy and Clinical Detection of the Recovery of Consciousness in Severe Acquired Brain Injury Patients with Disorders of Consciousness after Rehabilitation. Diagnostics 2022, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Hakiki, B.; Draghi, F.; Scarpino, M.; Portaccio, E.; Romoli, A.; Mannini, A.; Atzori, T.; Lolli, F.; Macchi, C.; Grippo, A. Critical illness polyneuromyopathy: Functional impact after severe acquired brain injuries. Acta Neurol. Scand. 2020, 142, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Bolton, C.F. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol. 2011, 10, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Zifko, U.A.; Zipko, H.T.; Bolton, C.F. Clinical and electrophysiological findings in critical illness polyneuropathy. J. Neurol. Sci. 1998, 159, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Bertolini, G.; Guarneri, B.; Botteri, M.; Peli, E.; Andreoletti, S.; Bera, P.; Luciani, D.; Nardella, A.; Vittorielli, E.; et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: The Italian multi-centre CRIMYNE study. Crit. Care 2007, 11, R11. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Yang, M.; Macht, M.; Sottile, P.; Gray, L.; McNulty, M.; Quan, D. Screening for critical illness polyneuromyopathy with single nerve conduction studies. Intensive Care Med. 2014, 40, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Nattino, G.; Guarneri, B.; Fagoni, N.; Amantini, A.; Bertolini, G. GiVITI Study Investigators. Validation of the peroneal nerve test to diagnose critical illness polyneuropathy and myopathy in the intensive care unit: The multicentre Italian CRIMYNE-2 diagnostic accuracy study. F1000Research 2014, 3, 127. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.C.; Shapiro, B.E. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic- Ultrasound Correlations, 4th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Kelmenson, D.A.; Quan, D.; Moss, M. What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: A prospective cohort study. Crit. Care 2018, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.M.; Teener, J.W.; Raps, E.C.; Schotland, D.L.; Bird, S.J. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology 1996, 46, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Trojaborg, W.; Weimer, L.H.; Hays, A.P. Electrophysiologic studies in critical illness associated weakness: Myopathy or neuropathy—A reappraisal. Clin. Neurophysiol. 2001, 112, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Nordine, T.; Lefaucheur, J.P. Prédominance des atteintes myopathiques à l’origine des parésies acquises en réanimation. Intérêt de la technique de stimulation musculaire directe [The predominance of myopathy as a cause of intensive-care- unit-acquired paralysis: The diagnostic value of direct muscle stimulation]. Rev. Neurol. 2007, 163, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Nordine, T.; Rodriguez, P.; Brochard, L. Origin of ICU acquired paresis determined by direct muscle stimulation. J. Neurol. Neurosurg. Psychiatry 2006, 77, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.; DIRienzo, F.; Fontana, A.; Tolfa, M.; Bartolo, M.; Copetti, M. Functional outcome of critical illness polyneuropathy in patients affected by severe brain injury. Eur. J. Phys. Rehabil. Med. 2017, 53, 910–919. [Google Scholar] [CrossRef] [PubMed]

| CIPNM (n° 10) | No CIPNM (n° 10) | p-Level | |
|---|---|---|---|
| Age (years) | 63 [41–75] | 59.5 [18–74] | U = 43.5; p-level = 0.64 |
| Sex (F/M) | 6/4 | 5/5 | χ2 = 0.2; p-level = 0.66 |
| Traumatic etiology | 2 | 2 | n.s |
| Vascular etiology | 8 | 8 | n.s |
| Time post-onset (days) | 48 [19–76] | 29 [19–58] | U = 30.5; p-level = 0.15 |
| Feeding | |||
| PEG (% yes) at admission | 3 | 3 | n.s. |
| PEG (% yes) at 2 months | 2 | 2 | n.s. |
| NGT (% yes) at admission | 4 | 7 | χ2= 1.8; p-level = 0.15 |
| NGT (% yes) at 2 months | 2 | 2 | n.s. |
| Tracheostomy | |||
| % Yes at admission | 8 | 10 | χ2= 0.5; p-level = 0.48 |
| % Yes at 2 months | 8 | 1 | χ2 = 9.9; p-level = 0.001 |
| Clinical scores | |||
| CRS-R at admission | 15 [3–23] | 12 [4–23] | U = 43; p-level = 0.62 |
| CRS-R at 2 months | 18 [4–23] | 18 [10–23] | U = 47; p-level = 0.84 |
| Δ CRS-R at two months | 3 [0–10] | 6 [0–10] | U = 26; p-level = 0.07 |
| LCF at admission | 4 [2–5] | 3 [2–6] | U = 47.5; p-level = 0.87 |
| LCF at 2 months | 5 [3–7] | 5 [3–7] | U = 41; p-level = 0.5 |
| Δ LCF at two months | 1 [0–3] | 2 [0–3] | U 33.5; p-level = 0.21 |
| DRS at admission | 19 [17–26] | 21.5 [17–26] | U = 45; p-level = 0.73 |
| DRS at 2 months | 18 [15–24] | 18.5 [15–21] | U = 45; p-level = 0.71 |
| Δ DRS at two months | −2 [−1/−7] | −3.5 [−1/−7] | U 25.5; p-level= 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccione, F.; Cerasa, A.; Tonin, P.; Carozzo, S.; Calabrò, R.S.; Masiero, S.; Lucca, L.F. Electrophysiological Screening to Assess Foot Drop Syndrome in Severe Acquired Brain Injury in Rehabilitative Settings. Biomedicines 2024, 12, 878. https://doi.org/10.3390/biomedicines12040878
Piccione F, Cerasa A, Tonin P, Carozzo S, Calabrò RS, Masiero S, Lucca LF. Electrophysiological Screening to Assess Foot Drop Syndrome in Severe Acquired Brain Injury in Rehabilitative Settings. Biomedicines. 2024; 12(4):878. https://doi.org/10.3390/biomedicines12040878
Chicago/Turabian StylePiccione, Francesco, Antonio Cerasa, Paolo Tonin, Simone Carozzo, Rocco Salvatore Calabrò, Stefano Masiero, and Lucia Francesca Lucca. 2024. "Electrophysiological Screening to Assess Foot Drop Syndrome in Severe Acquired Brain Injury in Rehabilitative Settings" Biomedicines 12, no. 4: 878. https://doi.org/10.3390/biomedicines12040878
APA StylePiccione, F., Cerasa, A., Tonin, P., Carozzo, S., Calabrò, R. S., Masiero, S., & Lucca, L. F. (2024). Electrophysiological Screening to Assess Foot Drop Syndrome in Severe Acquired Brain Injury in Rehabilitative Settings. Biomedicines, 12(4), 878. https://doi.org/10.3390/biomedicines12040878









