Abstract
Aim. The sulfhydryl (thiols) group of glutathione plays an important role in the neutralization of foreign organic compounds and the reduction in peroxides. The purpose of the study is to evaluate the concentration of sulfhydryl groups in the gingival tissue of healthy individuals and those with gingivitis or periodontitis, and to examine the differences between these groups. Material and methods. To assess the concentration of sulfhydryl groups (thiols) in the gingival tissue of healthy individuals and those with gingivitis or periodontitis, we used spectrophotometric analysis using dithionitrobenzoate (DTNB) as a reagent to measure the accessible sulfhydryl groups present in gingival tissue proteins. The sample was divided into three distinct groups: individuals with periodontal health, gingivitis, and periodontitis, and different indices were used to assess the periodontal status of the participants. Next, a statistical analysis was conducted to compare the concentrations of sulfhydryl groups among the different groups of patients. Conclusions. The results of this study showed significantly decreased levels of sulfhydryl (thiols) groups in gingival tissue from patients with gingivitis and periodontitis, compared with healthy people (control group). These results confirm the role of sulfhydryl (thiols) groups in defense against free radicals. They share a significant role in detoxification, signal transduction, apoptosis, and various other functions at the molecular level.
1. Introduction
Periodontal disease represents a broad spectrum of disorders involving periodontal tissues [1]. The two main forms, gingivitis and periodontitis, are often caused by the accumulation of biofilm in dental plaque [2,3]. Gingivitis is an inflammatory condition limited to the gums, but can, in predisposed subjects, evolve towards the more severe and destructive periodontitis [4,5]. The epithelium of the ulcerated periodontal pocket can constitute a direct route for the entry of periodontal pathogens, predominantly Gram-negative anaerobes (Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, Prevotella intermedia), which colonize the subgingival area and pass into the systemic circulation, potentially affecting other organs [6,7,8,9]. The organism triggers a host response through the establishment of an inflammatory reaction, and responds to biofilm accumulation in dental plaque through the establishment of an inflammatory reaction [10,11,12,13,14,15,16]. Dental biofilm is a complex microbial community that forms on the surface of teeth and can be composed of bacteria, fungi, and other microorganisms. This biofilm, along with other risk factors such as unhealthy lifestyles, genetic predisposition, and environmental factors, plays a key role in the development and progression of periodontitis [10,17,18,19]. Over the years, our understanding of this process has grown steadily, although at times there have been conflicting results in the scientific evidence. For example, it has been observed that ecological stress can affect the composition of the oral microbiota, promoting the growth of pathogenic [20,21]. These bacteria can proliferate and cause damage to gingival tissues, triggering an inflammatory response.
Neutrophils play a crucial role in the body’s inflammatory response. They are white blood cells that are rapidly recruited to damaged tissues to fight harmful pathogens [22,23,24]. When inflammation occurs, neutrophils are activated and migrate to the site of infection or tissue damage. This process, known as neutrophilia, is a hallmark of acute inflammation and is an important mechanism of the body’s defense against infection.
In inflamed periodontal tissues, neutrophils play a key role in eliminating pathogenic bacteria and promoting healing of damaged tissues. They release enzymes and chemicals that destroy bacteria and contribute to the removal of damaged tissues. However, excessive neutrophil activation and chronic inflammation can lead to damage to surrounding tissues and contribute to the progression of periodontal disease.
In addition, it is important to consider the role of inflammatory mediators produced by neutrophils and other immune cells in the context of periodontitis. These mediators, such as proinflammatory cytokines and tumor necrosis factors, may contribute to the progression of inflammation and destruction of periodontal tissues. Therefore, understanding the role of neutrophils and inflammatory mediators in the pathogenesis of periodontitis is critical for the development of new therapeutic strategies aimed at controlling inflammation and promoting gingival tissue healing [25]. While the body normally produces ROS during cellular processes, excessive ROS production due to factors like stress or pollution can lead to oxidative stress, causing cellular damage [10,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Neutrophils release inflammatory mediators and reactive oxygen species (ROS), which are highly reactive molecules containing oxygen [42,43,44,45,46,47,48,49,50]. ROS, including free radicals, are unstable and constantly seek to stabilize themselves by interacting with nearby molecules to overcome the body’s natural defenses and lead to cellular damage, causing oxidative stress (Figure 1).
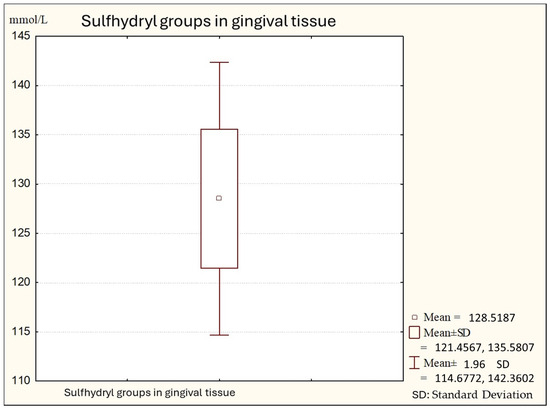
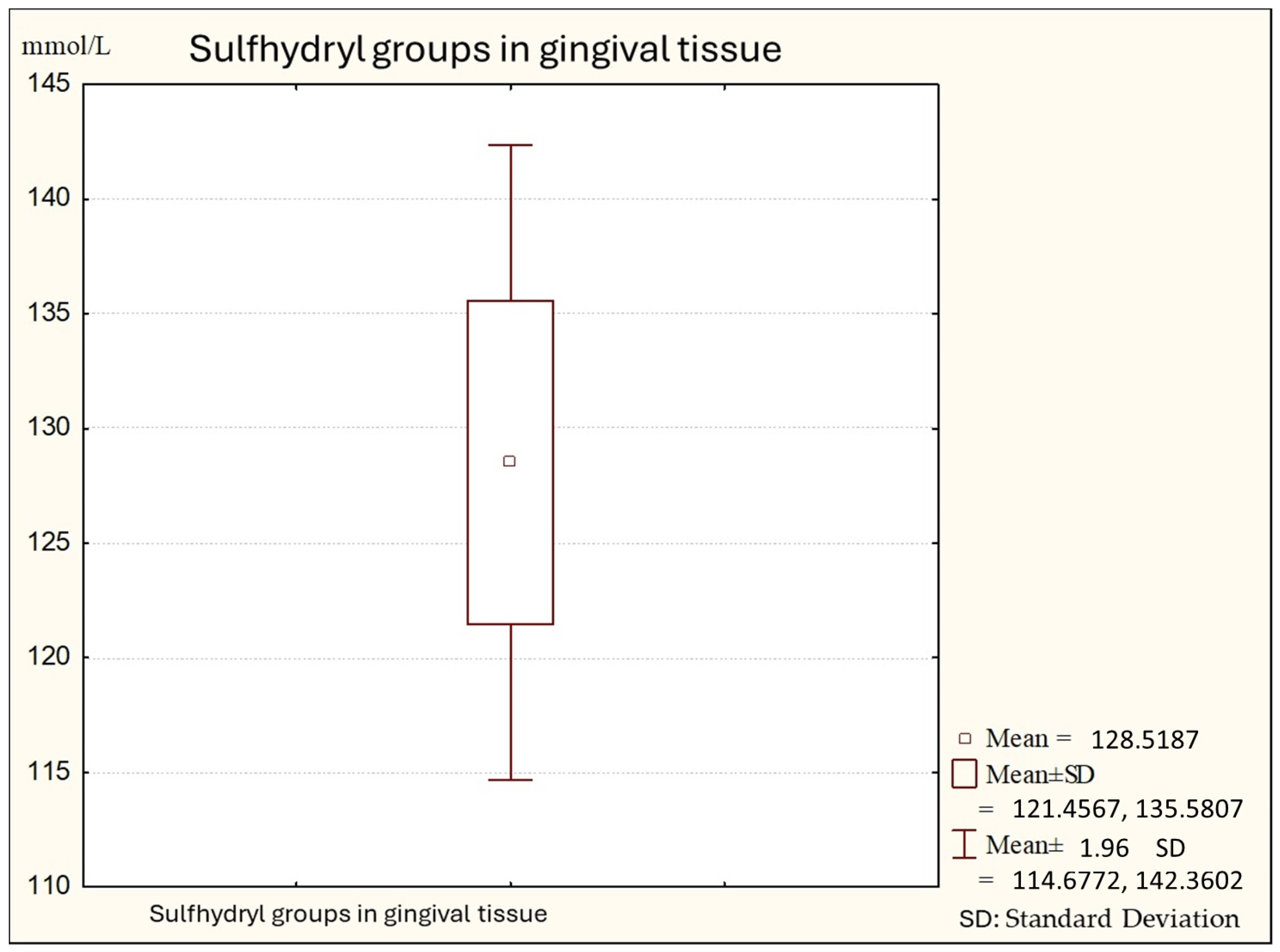
Figure 1.
Descriptive statistics of sulfhydryl group in the gingival tissue from healthy people (Group A).
This oxidative stress is associated with many diseases, from premature aging to more serious conditions such as heart disease, cancer, and neurodegenerative diseases [51,52,53]. ROS include oxygen-derived free radicals such as superoxide, hydroxyl, nitric oxide, hydrogen peroxide, and hypochlorous acid [54,55]. These molecules emerge in inflamed tissues and can cause damage to lipids, proteins, and deoxyribonucleic acid, eventually leading to tissue degradation. This phenomenon is believed to underlie inflammatory conditions affecting the periodontium, evident as gingivitis and periodontitis [56,57].
The primary (nicotinamine adenine dinucleotide) targets of highly reactive ROS are DNA, RNA, proteins, and lipids. Most damage stems from the hydroxyl radical generated through the Fenton reaction, reliant on iron or other divalent metal ions, along with a source of reducing equivalents, potentially NADH, to regenerate the metals, as detailed by Stadtman [58,59,60]. At a cellular level, exposure of proteins to ROS results in modifications of amino acid side chains, consequently altering protein structure and leading to functional disturbances within cellular metabolism [61]. Among the 20 amino acids constituting proteins, several are susceptible to direct modification via side chain reactions with ROS. Predominantly, those containing sulfhydryl groups (thiol groups) and those with aromatic side chain groups are affected.
Sulfhydryl groups show significant and diverse reactivity, easily oxidizing to form disulfides, or sulfenic or sulfonic acids, and easily undergoing processes such as alkylation, acylation, and thiol–disulfide exchange [62,63]. They react with heavy metal ions to form mercaptides and with aldehydes and ketones to form mercaptals and mercaptols, respectively. These groups play crucial roles in biochemical processes: the sulfhydryl group of coenzyme A, lipoic acid, and 4-phosphopantotheine participates in enzymatic reactions for the formation and transfer of acyl residues, which are essential for lipid and glucose metabolism. The sulfhydryl group of glutathione is critical in neutralizing foreign organic compounds, reducing peroxides, and acting as a coenzyme [64,65,66]. In addition, cysteine residues in proteins participate in catalytic effects, binding to substrates, and binding of coenzymes and metal ions. Enzymatic sulfhydryl groups catalyze the formation of intermediates with substrates or their residues, while in oxidative enzymes they facilitate electron and proton transfers from substrates to acceptors [67,68,69,70]. These reactions are critical for many metabolic pathways and biochemical processes crucial to cellular life. For example, the sulfhydryl group present in coenzyme A is essential for the synthesis and transport of fatty acids, while glutathione, with its sulfhydryl group, plays a key role in cell defense against oxidative stress and detoxification of foreign compounds. In addition, the sulfhydryl groups of proteins may be involved in the regulation of enzyme activity and cellular signal transduction, thus contributing to the overall functioning of the cell and the organism.
Blocking sulfhydryl groups results in partial or complete inhibition of the activity of many enzymes. Disulfide bonds (S–S), formed during protein biosynthesis through the oxidation of sulfhydryl groups, play a crucial role in stabilizing protein structures, including enzymes, antibodies, and various hormones [71,72]. Disruption of disulfide bonds leads to the denaturation of protein structures and the loss of biological activity [73,74,75,76]. These bonds significantly contribute to the tertiary structure of proteins.
Chemical and histochemical studies on epithelium suggest the potential significance of sulfhydryl and disulfide groups in gingival metabolism [77,78,79,80].
Antioxidants are a group of substances that prevent the oxidation of substrates by ROS, offering protection against oxidative stress. The thiol antioxidants include reduced glutathione, glutaredoxin, and N-acetyl cysteine [81,82,83]. Thiols are integral to the intraprotein structure and exist in equilibrium with disulfide groups. They prevent the irreversible unfolding of protein structures due to oxidative stress. Among the body’s antioxidants, thiols constitute a major portion and play a crucial role in defending against reactive oxygen species. Total thiols comprise intracellular and extracellular forms, existing as free or bound to proteins, including oxidized or reduced glutathione. Among protein-bound thiols, albumin predominantly binds to the sulfhydryl group at its cysteine-34 portion. Thiols contribute significantly to defense against free radicals, involving detoxification, signal transduction, apoptosis, and various molecular-level functions [84,85], especially in the detection of low-molecular-weight thiols in gingival cervical fluid (GCF) as a defense against ROS-mediated damage.
Antioxidants, thus, are molecules that counteract the harmful action of free radicals and ROS, helping to protect cells from oxidative damage [86,87,88]. In periodontitis, where oxidative stress can play a significant role in inflammation and disease progression, antioxidants can play an important role in reducing these harmful effects because they help neutralize ROS, thus reducing oxidative stress in inflamed gum tissue and limiting inflammation and associated tissue damage [89,90,91]. They also influence the inflammatory mediators involved in periodontitis, helping to regulate the inflammatory response and limiting the worsening of the condition. This happens through various mechanisms [92,93]:
- Neutralizing free radicals that activate the inflammatory cascade, stimulating the production of inflammatory mediators such as proinflammatory cytokines (such as TNF-α and IL-1β) [2,94] and tumor necrosis factors. By neutralizing ROS, antioxidants can reduce this inflammatory response [95,96];
- By inhibiting intracellular signaling pathways involved in the inflammatory response. They can block the activation of certain transcription factors that regulate the gene expression of inflammatory mediators, thus reducing their production [97,98,99];
- By modulating the activity of cells involved in the inflammatory response such as macrophages and lymphocytes, reducing the production of proinflammatory cytokines, and, instead, increasing the production of anti-inflammatory molecules [100,101];
- Reducing oxidative stress, and therefore ROS levels, which support the inflammatory response [102].
Antioxidants exert a regulatory effect on the inflammatory response in periodontitis, helping to limit the worsening of the condition and promoting a more favorable environment for the healing of inflamed tissues [103]. However, it is important to emphasize that understanding the specific mechanisms involved requires further research to more precisely define the role of antioxidants in the management of periodontitis [104,105,106,107]. They support the healing of damaged gingival tissues, facilitating repair and recovery [108,109,110]. In addition to antioxidants, some natural substances have been studied for their effectiveness in managing periodontitis and reducing gingival inflammation; coenzyme Q10, vitamin C, tea tree essential oil, aloe vera, phenols, turmeric, and ginger [62]. The mechanisms by which antioxidants exert their beneficial effects on periodontitis are multiple and complex. Antioxidants work by neutralizing free radicals and reducing oxidative stress in inflamed tissues. This process helps limit cellular damage and preserve the structural integrity of tissues, thereby promoting healing. In addition, antioxidants can modulate gene expression and inflammatory cytokine production, thereby influencing the immune and inflammatory response in the context of periodontitis.
Coenzyme Q10, for example, is involved in cellular energy production processes and has demonstrated antioxidant and anti-inflammatory properties that may contribute to improved periodontal health. Vitamin C is known for its role in collagen synthesis and wound healing, as well as acting as a powerful antioxidant. Tea tree essential oil has demonstrated antimicrobial and anti-inflammatory activity, which may help control the infection and inflammation associated with periodontitis. Aloe vera is known for its soothing and healing properties, which may promote the healing of inflamed gum tissue. Phenols, found, for example, in green tea, have demonstrated antioxidant and anti-inflammatory properties that may help control gingival inflammation. Turmeric and ginger, on the other hand, have been studied for their potential anti-inflammatory and antioxidant properties, which could be useful in the treatment of periodontitis.
In addition, antioxidants may interact with other treatments used for the management of periodontitis, enhancing their effectiveness and reducing their side effects. For example, the combined use of antioxidants and antibiotic treatments may help reduce bacterial load in inflamed tissues and improve clinical outcomes of treatment. However, further research is needed to fully understand the role of antioxidants in the management of periodontitis and to identify the best therapeutic strategies based on these compounds.
These natural substances can be considered as adjuncts to conventional therapy for periodontitis. However, studies on the relationship between antioxidant status and periodontitis have produced discordant results [111,112,113]: not all studies have observed significant differences in total antioxidant levels between saliva samples from healthy subjects and those with periodontitis. This study aims to further investigate the role of sulfhydryl groups (thiols) in the periodontal environment by exploring their variations in the initial and clinical manifestations of periodontal disease [7,114]. Ultimately, the study of sulfhydryl groups in the periodontal environment represents a promising area of research that could lead to new insights into the pathogenesis and management of periodontal disease. Understanding the molecular mechanisms involved in the physiological and pathological processes of periodontal tissue could pave the way for new diagnostic, therapeutic, and preventive strategies aimed at improving periodontal health and patients’ quality of life.
2. Materials and Methods
This research encompassed a cohort of 90 individuals aged between 18 and 64 years. Assessment of the subjects’ periodontal status involved evaluating the Plaque Index (PI) developed by Silness and Loe in 1964 [115,116] and the Gingival Index (GI) devised by Loe and Silness in 1963 [115,117,118], as well as conducting measurements of pocket probing depths and clinical attachment levels. Table 1 shows how 90 participants were divided into three groups according to certain criteria: those with gingivitis (Group B), those with periodontal health (Group A), and those with a periodontitis diagnosis (Group C). [119,120].

Table 1.
Participants categorized into distinct groups based on specific criteria: health (Group A), gingivitis (Group B), periodontitis (Group C).
According to the stratification and degree of periodontitis in the new classification, the three groups of patients were divided into:
Group A—Individuals with periodontal health (periodontal health): absence of periodontitis;
Group B—Patients with gingivitis (gingivitis): incipient and moderate periodontitis;
Group C—Patients diagnosed with periodontitis (periodontitis): severe periodontitis and advanced periodontitis.
To analyze the biochemical composition and molecular characteristics of gingival tissue, including levels of sulfhydryls, which are important for understanding the redox state and antioxidant activity of gingival tissue in healthy conditions and periodontal disease, marginal and interproximal gingival tissue from all oral cavity districts, taken during gingival biopsy procedures, was used.
The quantification of sulfhydryl (thiol) groups within gingival tissue was conducted using a spectrophotometric method employing dithionitrobenzoic (DTNB) [93,121]. This compound reacts with accessible sulfhydryl (thiol) groups present in proteins, reducing them to stable intermediate compounds known as mixed disulfides, specifically protein S–S compounds, denoted as 5-mercapto-2-nitrobenzoate (MNB) [21,122]. The measurement of MNB, taken after 5 min at 412 nm using a Spectronic 10 UV spectrophotometer (Rochester, NY, USA), was compared against a glutathione standard and quantified in terms of micromoles per liter, following the method outlined by Mochink [123,124,125].
Statistical analysis was performed using the SPSS 7.1 statistical software (SPSS statistical software version 7.1, Chicago, IL, USA) for data processing and analysis [126,127]. The normality of the distribution was determined using the Shapiro–Wilk test, the significance of the differences was estimated with the student’s t-test, and differences were taken to be significant at p < 0.05.
3. Results
Figure 1 shows the average values of sulfhydryl groups (thiol) in gingival tissue from healthy subjects. The average value in this subject is 147.74 (minimal values: 121.00; maximal: 173.00), with a standard deviation of 11.28.
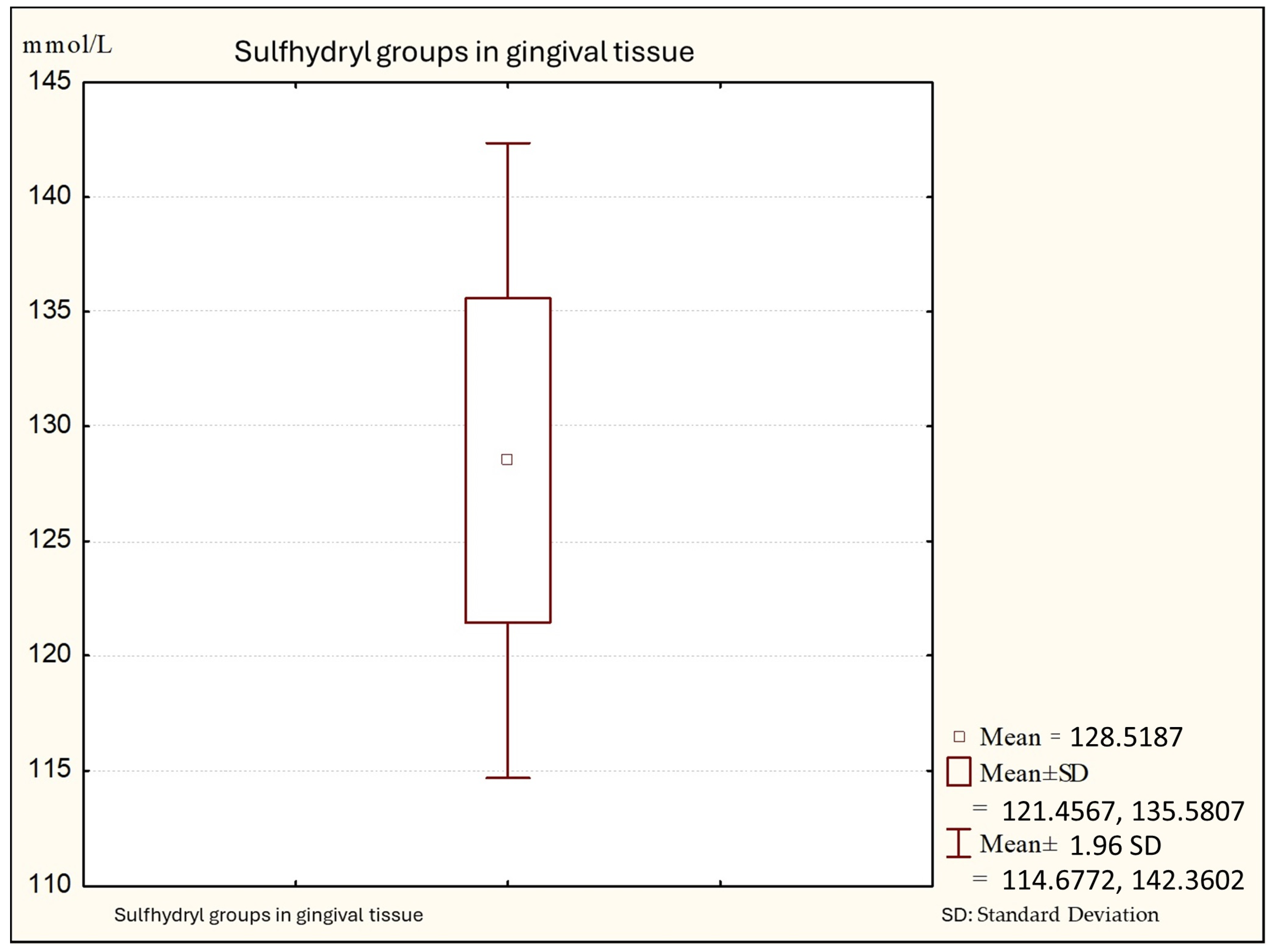
Figure 2 shows that the average values of sulfhydryl groups (thiol) in gingival tissue from patients with gingivitis are 128.88 (minimal values: 110.14; maximal: 140.00 +95%), with a standard deviation of 7.06.
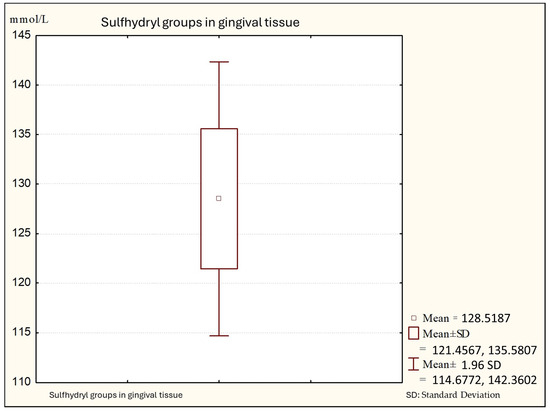
Figure 2.
Descriptive statistics of sulfhydryl group in the gingival tissue from people with gingivitis (Group B).
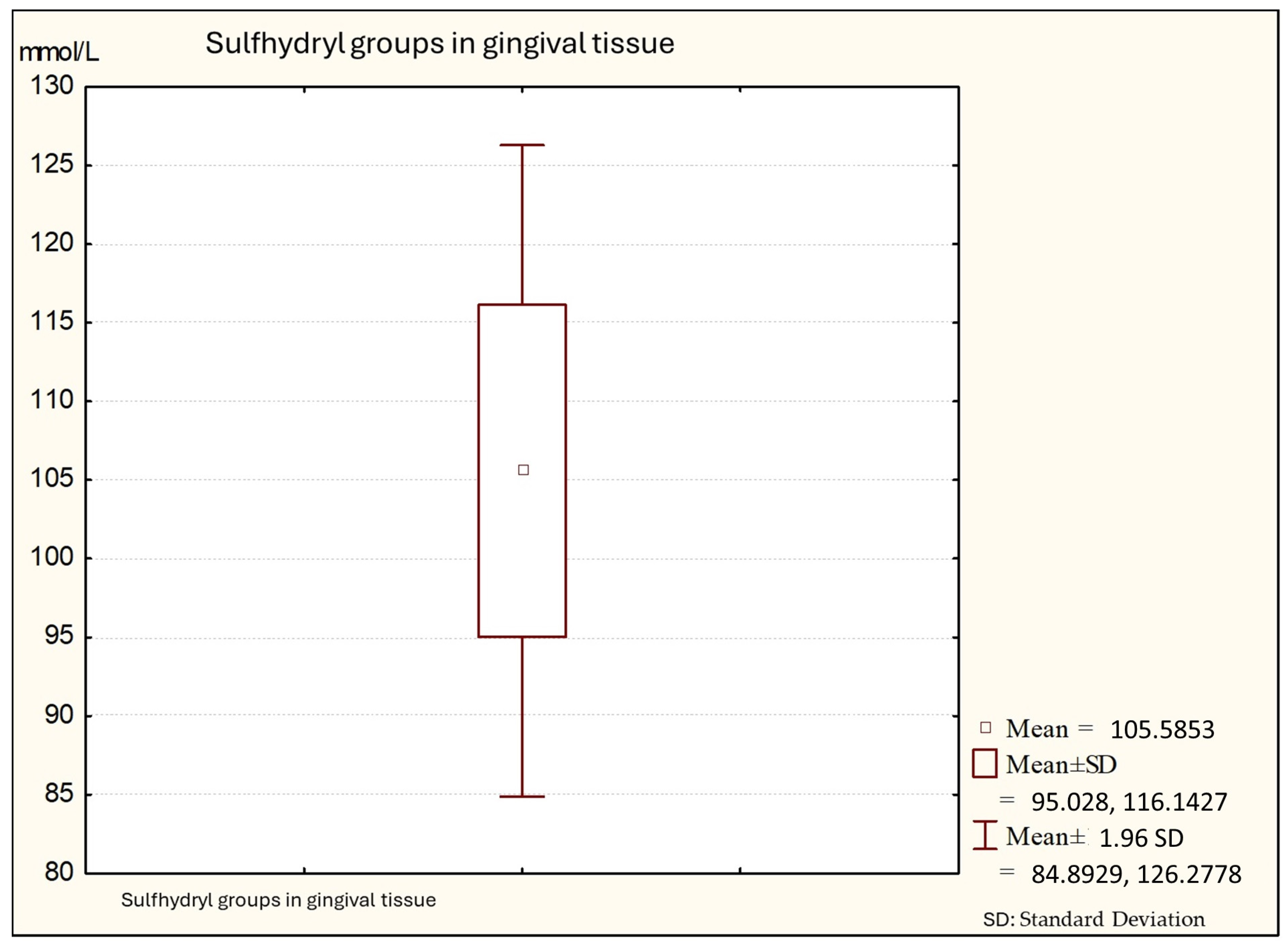
Figure 3 shows that the average values of sulfhydryl groups (thiol) in gingival tissue from patients with periodontitis is 101.64 (minimal values: 91.34; maximal: 130.14), with a standard deviation of 10.56.
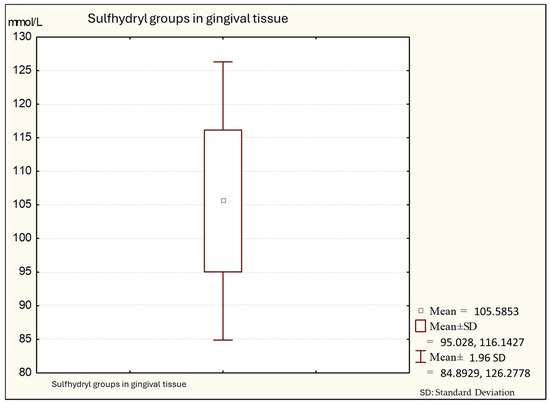
Figure 3.
Descriptive statistics of sulfhydryl group in the gingival tissue from people with periodontitis (Group C).
The average values of sulfhydryl are summarized in Table 2.

Table 2.
Average values of sulfhydryl group (thiol) in the gingival tissue of healthy persons (Group A), from people with gingivitis (Group B), and from people with periodontitis (Group C).
Table 3 and Table 4 show the concentration, in mmol/L, of the sulfhydryl (thiol) group in gingival tissue, between examined groups (healthy, gingivitis, and periodontitis).

Table 3.
Concentration of the sulfhydryl (thiol) group in gingival tissue.

Table 4.
Concentration of the sulfhydryl (thiol) group in Group A (healthy), Group B (gingivitis), and Group C (periodontitis).
Table 4 is a representation of the results of the statistical analysis in which the three groups were compared. “M = 105.59, M = 128.52, M = 147.74” means were calculated for each group. The numbers in the table, under the letters A, B, and C, are the results of the significance values (p-value) of the analysis, which, in this context, are all zero (“0.000”).
The sulfhydryl (thiol) group concentrations differed significantly across groups, with healthy individuals (control Group A) exhibiting the highest mean value (x = 147.74 mmol/L), followed by individuals with gingival inflammation (Group B) (x = 128.52 mmol/L), and the lowest levels observed in patients with periodontal disease (Group C) (x = 105.59 mmol/L) (p < 0.001). Additionally, post hoc analysis revealed statistically significant differences in sulfhydryl group concentrations among all groups (F = 138.91; p < 0.001).
The average concentration of sulfhydryl groups (thiols) was significantly different among patient groups. The highest concentration was observed in subjects with periodontal health, followed by those with gingivitis, and then those with periodontitis. This difference was evaluated as statistically significant (F = 138.91, p < 0.001).
4. Discussion
Healthy individuals (Group A) exhibited the highest average sulfhydryl group concentration (147.74 mmol/L), significantly higher than both gingivitis (Group B) and periodontitis (Group C) patients.
Gingivitis patients (Group B) showed an intermediate average sulfhydryl group concentration (128.52 mmol/L), significantly lower than healthy individuals (Group A) but higher than periodontitis patients (Group C).
Periodontitis patients (Group C) displayed the lowest average sulfhydryl group concentration (105.59 mmol/L), significantly lower than both healthy individuals (Group A) and gingivitis patients (Group B). A summary of results is provided in Table 5.

Table 5.
Summary of results.
The inflammatory and immune response induced by sub-gingival plaque is the most important factor in the development of this disease [128,129,130]. Development mechanisms of inflammatory-destructive processes in periodontal tissues are not well understood. The disorder is probably multifactorial and it is characterized by the generation of ROS (Figure 4) (Waddington) [129], by activated phagocytes at gingival tissue (Baelum) [131], (Katsuragi H) [132], which can initiate the destruction of connective tissue. Oxidative stress refers to the imbalance due to excess ROS or oxidants over the capability of the cell to mount an effective antioxidant response (Kantarci) [133,134,135,136].
Figure 4.
Different physical, chemical, and biological agents can influence the spontaneous generation or physiological production of ROS. These include ionizing and UV radiations, air pollution, various types of pollutants, and foods rich in animal fats, but also aromatic hydrocarbons, pesticides, drugs, bacteria, and stress.
The pathological events which lead to the destruction of the periodontal tissues during gingival inflammation and periodontal destruction have been related to the effect of an imbalance between oxidants and antioxidants in patients with gingivitis and periodontitis (Sakalilioglu) [61,129,137]. In the health of the periodontium, a balance between oxidizing substances (such as free radicals and other reactive molecules) and antioxidants is essential for maintaining a healthy environment [138,139,140]. Gum inflammation and periodontal diseases, such as gingivitis and periodontitis, involve inflammatory processes that can generate excessive free radicals and other oxidizing molecules [141,142]. These molecules, if present in excessive or unbalanced quantities, can damage the periodontal tissues, leading to the destruction of the supporting tissues of the teeth [143,144,145]. An imbalance between these oxidizing and antioxidant substances, which normally protect tissues by neutralizing free radicals and maintaining balance, can promote the progression of gum and periodontal diseases, damaging the tissues and contributing to their destruction [146,147,148].
There are many enzymatic antioxidant defense mechanisms to protect against ROS effects in vivo: SOD, GPx, Cathalasa, uric acid, ascorbate, alpha-tocopherol, urate, thiols, and albumin (Sculley, Nagler, Amerongen) [144,149,150,151]. Chaplle et al. [81,152,153], found lower total antioxidant concentration in the saliva of periodontitis patients when compared to health controls [24,154]. Salivary antioxidant levels (SOD, GPx, reduced GHS, ascorbic acid, alfa tocopherol, were observed to the lower in periodontal patients (Canacki) [146,155,156,157]. In the present study, sulfhydryl (thiol), in gingival tissue, was observed in reduced quantities in the patients with gingivitis and periodontitis (groups B and C), compared with the people from healthy group (Group A) [158,159]. Testing the significance of differences in the concentration of sulfhydryl (thiol) group between the examined groups by post hoc test showed statistically significant differences in the values of this parameter among all groups of patients (F: 138.91; p < 0.001; p = 0.000) [160]. The lower nonconcentrate of sulfhydryl (thiol group in the gingivitis and periodontitis groups can be attributed to the presence of periodontopathic pathogens that readily degrade them from hydrogen sulfide, which can be toxic [129,158,161]. Similar observations were made by Thomas [162,163], Patel [164], Duarte [165], and Novakovic [166]. They found that gingival tissue analysis revealed that the antioxidative capacity in patients with periodontitis was decreased when compared to the control group [128,167]. This study reported a significant increase in glutathione peroxidase (GSH-Px) and glutathione S-transferase (GST), while a decrease in glutathione (GSH) and total glutathione levels in gingival tissue and serum from an experimental group when compared to the control group [168,169,170]. The ubiquitous tripeptide GSH and other amino thiols are also involved in the production of volatile sulfur responsible for the bad breath in periodontopathic patients (Bald and Glowacki) [171,172,173].
The intricate interplay between sulfhydryl (thiol) groups and periodontal health is paramount in understanding the underlying oxidative stress contributing to gingivitis and periodontitis [174,175]. Sulfhydryl groups, specifically within glutathione, serve pivotal roles in neutralizing foreign organic compounds and reducing peroxides [176,177]. They act as significant coenzymes and are present in cysteine residues of amino acids within proteins [142,178]. The blocking or alteration of sulfhydryl groups can lead to considerable enzyme activity inhibition, underscoring their critical importance in protein structure stabilization via disulfide bonds [179,180].
This study yielded compelling evidence demonstrating significantly diminished levels of sulfhydryl (thiol) groups within gingival tissue among patients afflicted with gingivitis and periodontitis when compared to healthy individuals (control group), corroborating the vital role of these groups in combating free radicals [181,182,183]. These findings highlight the pivotal involvement of sulfhydryl groups not only in detoxification processes but also in signal transduction, apoptosis, and various molecular-level functions [184,185,186].
The statistical analyses performed in this study revealed a statistically significant decrease in sulfhydryl (thiol) group concentrations among individuals with gingivitis and periodontitis in comparison to the control group [164,187]. These findings align with previous studies that have emphasized the vital role of antioxidative mechanisms, such as sulfhydryl groups, in mitigating periodontal disease progression [7,188,189].
5. Conclusions
Oxidative stress in the pathogenesis of gingivitis and periodontitis is the result of an alteration in redox balance and tissues of the innate immunity system. Gingival tissue analysis of the sulfhydryl (thiols) group revealed that the antioxidative activity in patients with gingivitis and periodontitis was decreased when compared to the control group. The observed decline in the antioxidative capacity of sulfhydryl groups in patients with periodontitis compared to the control group suggests a potential avenue for therapeutic intervention. Strategies aimed at restoring or enhancing sulfhydryl group levels within gingival tissues might hold promise as adjunctive therapies in managing and ameliorating the progression of gingivitis and periodontitis. Our study strengthens the growing body of evidence implicating sulfhydryl (thiol) groups in the pathophysiology of periodontal diseases. Further investigations into modulating these antioxidative mechanisms could unveil novel therapeutic avenues to alleviate the burden of these prevalent oral inflammatory conditions. This expanded conclusion aims to encapsulate the significance of sulfhydryl groups, their alterations in periodontal disease, and the potential implications for therapeutic interventions.
Author Contributions
Conceptualization, F.I., A.M.I., S.C., G.D., M.N., I.G., A.P., L.F. and A.D.I.; methodology, K.F., A.B.-F., L.F., A.P., S.C., M.N., I.G. and A.M.I.; software, A.D.I., A.P. and G.D.; validation, A.M.I., L.F., G.D., A.D.I. and F.I.; formal analysis, A.D.I., F.I., L.F., A.M.I., A.P. and G.D.; resources, A.D.I., A.M.I., A.P., A.B.-F., K.F., S.C., M.N., I.G. and G.D.; data curation, A.M.I., F.I., A.M.I., A.D.I., K.F., A.B.-F. and G.D.; writing—original draft preparation, F.I., A.D.I., A.M.I., L.F., K.F., A.B.-F. and G.D.; writing—review and editing, A.P., L.F., F.I., G.D., A.M.I. and A.D.I.; visualization, A.M.I., F.I., A.D.I., L.F., G.D. and A.P.; supervision, G.D., S.C., K.F., A.D.I., A.M.I., A.P. and F.I.; project administration, G.D., L.F., A.M.I., A.D.I. and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethical Committee for Biomedical Research of upon request submitted n.02-2379/11 dated 20 September 2019 by Prof. Dr. Kenan Ferati, who has approved medical research on “The probiotic effects of the incidences of absorption health capabilities nutrition” No. 02-231912. Committee for ethical issues President Dr. Fatmit Rushani, Signed number Prot. 02-237912, dated 20 September 2019, Committee for ethical issues at the clinical institute of health public, Clinic Hospital of Tetovo, Medical Science Faculty, State University of Tetova, Tetova Republic of North Macedonia, dated 20 September 2019 approved. This study was executed in accordance with the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Deoxyribonucleic acid (DNA), degrees of freedom (Df), dithionitrobenzoic (DTNB), F-statistic (F), gingival cervical fluid (GCF), Gingival Index (GI), glutathione (GSH), glutathione peroxidase (GSH-Px), glutathione s-transferase (GST), interleukin-1β (IL), mean (M), 5-Mercapto-2-Nitrobenzoate (MNB), mean square (MS), nicotinamine adenine dinucleotide (NADH), oxidative stress (OS), P-value (P), Plaque Index (PI), reactive oxygen species (ROS), ribonucleic acid (RNA), sum of squares (SS),tumor necrosis factor-A (TNF-α).
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primer 2017, 3, 17038. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Cazzolla, A.P.; Di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; Topi, S.; Malcangi, G.; Hazballa, D.; Scarano, A.; et al. The Integumentary System and Its Microbiota between Health and Disease. J. Biol. Regul. Homeost. Agents 2021, 35, 303–321. [Google Scholar] [CrossRef]
- Janani, R.G.; Asokan, S.; Geetha Priya, P.R. Effect of Custom-Made Probiotic Chocolates on Streptococcus Mutans, Plaque pH, Salivary pH and Buffering Capacity in Children—A Randomised Controlled Trial. Oral Health Prev. Dent. 2019, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pol’ovyĭ, P.V. The status of acid-base homeostasis in oral fluid during gestation. Lik. Sprava 2013, 7, 77–80. [Google Scholar]
- Rathee, M.; Jain, P. Gingivitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rapone, B.; Ferrara, E.; Montemurro, N.; Converti, I.; Loverro, M.; Loverro, M.T.; Gnoni, A.; Scacco, S.; Siculella, L.; Corsalini, M.; et al. Oral Microbiome and Preterm Birth: Correlation or Coincidence? A Narrative Review. Open Access Maced. J. Med. Sci. 2020, 8, 123–132. [Google Scholar] [CrossRef]
- Khocht, A.; Seyedain, M.; Hardan, S.; Gaughan, J.; Suzuki, J.B. Salivary Thiol Levels and Periodontal Parameters Assessed with a Chromogenic Strip. Gen. Dent. 2013, 61, 50–54. [Google Scholar]
- Grant, W.B.; van Amerongen, B.M.; Boucher, B.J. Periodontal Disease and Other Adverse Health Outcomes Share Risk Factors, Including Dietary Factors and Vitamin D Status. Nutrients 2023, 15, 2787. [Google Scholar] [CrossRef]
- Visentin, D.; Gobin, I.; Maglica, Ž. Periodontal Pathogens and Their Links to Neuroinflammation and Neurodegeneration. Microorganisms 2023, 11, 1832. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current Concepts in the Pathogenesis of Periodontitis: From Symbiosis to Dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Asikainen, S.; Saarela, M.; Karjalainen, J.; Jousimies-Somer, H. The Oral Gram-Negative Anaerobic Microflora in Young Children: Longitudinal Changes from Edentulous to Dentate Mouth. Oral Microbiol. Immunol. 1994, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Asikainen, S.; Jousimies-Somer, H. The Early Colonization of Gram-Negative Anaerobic Bacteria in Edentulous Infants. Oral Microbiol. Immunol. 1992, 7, 28–31. [Google Scholar] [CrossRef]
- Kolenbrander, P.E. Surface Recognition among Oral Bacteria: Multigeneric Coaggregations and Their Mediators. Crit. Rev. Microbiol. 1989, 17, 137–159. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Intrageneric Coaggregation among Strains of Human Oral Bacteria: Potential Role in Primary Colonization of the Tooth Surface. Appl. Environ. Microbiol. 1990, 56, 3890–3894. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; London, J. Adhere Today, Here Tomorrow: Oral Bacterial Adherence. J. Bacteriol. 1993, 175, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Basic, A.; Blomqvist, M.; Dahlén, G.; Svensäter, G. The Proteins of Fusobacterium Spp. Involved in Hydrogen Sulfide Production from L-Cysteine. BMC Microbiol. 2017, 17, 61. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial Shift and Periodontitis. Periodontology 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Di Cosola, M.; Inchingolo, A.M.; Greco Lucchina, A.; Malcangi, G.; Pettini, F.; Scarano, A.; Bordea, I.R.; Hazballa, D.; Lorusso, F.; et al. Correlation between Occlusal Trauma and Oral Microbiota: A Microbiological Investigation. J. Biol. Regul. Homeost. Agents 2021, 35, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L.; Santacroce, L.; Dipalma, G.; Haxhirexha, K.; Topi, S.; Cantore, S.; Altini, V.; Pacifici, A.; De Vito, D.; Pettini, F.; et al. Gender Medicine: The Impact of Probiotics on Male Patients. Clin. Ter. 2021, 171, e8–e15. [Google Scholar] [CrossRef]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis From a Microbial and Host Perspective Relative to Oral Health and Disease. Front. Microbiol. 2021, 12, 617485. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, F.; Charitos, I.A.; Di Cosola, M.; Cazzolla, A.P. Focus on the Cariogenic Process: Microbial and Biochemical Interactions with Teeth and Oral Environment. J. Biol. Regul. Homeost. Agents 2021, 35. [Google Scholar] [CrossRef]
- Lim, J.J.; Grinstein, S.; Roth, Z. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front. Cell. Infect. Microbiol. 2017, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral Piercing and Oral Diseases: A Short Time Retrospective Study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil Kinetics in Health and Disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Serra, P.; Francesco, I.; Di Carmine, M.; Tari, S.R.; Leo, L.; Lorusso, F. Current Status of Peri-Implant Diseases: A Clinical Review for Evidence-Based Decision Making. J. Funct. Biomater. 2023, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.M.; Dohan Ehrenfest, D. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 2: Bone Graft, Implant and Reconstructive Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Anelli, M.G.; Quercia, E.; Piancino, M.G.; Di Comite, M. Temporomandibular Disorders and Oral Features in Early Rheumatoid Arthritis Patients: An Observational Study. Int. J. Med. Sci. 2019, 16, 253–263. [Google Scholar] [CrossRef]
- Crincoli, V.; Di Comite, M.; Guerrieri, M.; Rotolo, R.P.; Limongelli, L.; Tempesta, A.; Iannone, F.; Rinaldi, A.; Lapadula, G.; Favia, G. Orofacial Manifestations and Temporomandibular Disorders of Sjögren Syndrome: An Observational Study. Int. J. Med. Sci. 2018, 15, 475–483. [Google Scholar] [CrossRef]
- Crincoli, V.; Scivetti, M.; Di Bisceglie, M.B.; Pilolli, G.P.; Favia, G. Unusual Case of Adverse Reaction in the Use of Sodium Hypochlorite during Endodontic Treatment: A Case Report. Quintessence Int. Berl. Ger. 2008, 39, e70–e73. [Google Scholar]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J. Clin. Med. 2022, 11, 2491. [Google Scholar] [CrossRef]
- Bavetta, G.; Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, G.; Gargiulo Isacco, C.; Scarano, A.; De Vito, D.; et al. A Retrospective Study on Insertion Torque and Implant Stability Quotient (ISQ) as Stability Parameters for Immediate Loading of Implants in Fresh Extraction Sockets. BioMed Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.T.; Huynh, T.D.; Inchingolo, F.; Dipalma, G.; Inchingolo, A.D.; Cantore, S.; Paduanelli, G.; Nguyen, K.C.D.; Ballini, A.; Isacco, C.G.; et al. Human Chondrocytes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Seeded on a Dermal-Derived Collagen Matrix Sheet: Our Preliminary Results for a Ready to Go Biotechnological Cartilage Graft in Clinical Practice. Stem Cells Int. 2021, 2021, 6664697. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Mummolo, S.; Tecco, S.; Continenza, M.A.; Marzo, G. Histological Characterization of Sacco’s Concentrated Growth Factors Membrane. Int. J. Morphol. 2017, 35, 114–119. [Google Scholar] [CrossRef]
- Campanella, V.; Libonati, A.; Nardi, R.; Angotti, V.; Gallusi, G.; Montemurro, E.; D’Amario, M.; Marzo, G. Single Tooth Anesthesia versus Conventional Anesthesia: A Cross-over Study. Clin. Oral Investig. 2018, 22, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Tecco, S.; Caterini, E.; Casalena, F.; Quinzi, V.; Mattei, A.; Marzo, G. Alcohol-Free Essential Oils Containing Mouthrinse Efficacy on Three-Day Supragingival Plaque Regrowth: A Randomized Crossover Clinical Trial. Trials 2017, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Marrapodi, M.M.; Mascolo, A.; di Mauro, G.; Mondillo, G.; Pota, E.; Rossi, F. The Safety of Blinatumomab in Pediatric Patients with Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 929122. [Google Scholar] [CrossRef] [PubMed]
- Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Lucchese, A.; Romano, A.; Della Vella, F.; Di Stasio, D.; Serpico, R.; Petruzzi, M. Oral Microbiota Features in Subjects with Down Syndrome and Periodontal Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9251. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Isola, G.; Cicciù, M. Conservative Treatment of Temporomandibular Joint Condylar Fractures: A Systematic Review Conducted According to PRISMA Guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J. Oral Rehabil. 2023, 50, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. The Association between Parent Education Level, Oral Health, and Oral-Related Sleep Disturbance. An Observational Crosssectional Study. Eur. J. Paediatr. Dent. 2023, 24, 218–223. [Google Scholar] [CrossRef]
- Uzunçıbuk, H.; Marrapodi, M.M.; Meto, A.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Prevalence of temporomandibular disorders in clear aligner patients using orthodontic intermaxillary elastics assessed with diagnostic criteria for temporomandibular disorders (DC/TMD) axis II evaluation: A cross-sectional study. J. Oral Rehabil. 2024, 51, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Marrapodi, M.M.; Cicciù, M. Online Bruxism-related information: Can people understand what they read? A Cross-Sectional Study. J. Oral Rehabil. 2023, 50, 1211–1216. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Post-traumatic Stress, Prevalence of Temporomandibular Disorders in War Veterans: Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Almeida, L.E.; Ronsivalle, V.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Obesity Patients: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2023, 50, 1544–1553. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Ronsivalle, V.; Cicciù, M. Children Oral Health and Parents Education Status: A Cross Sectional Study. BMC Oral Health 2023, 23, 787. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Malcangi, G.; Converti, I.; Palermo, A.; Mancini, A.; Maggiore, M.; Tartaglia, G.; Ferrara, E.; Vecchiet, F.; Lorusso, F.; Scarano, A.; et al. Chewing and Cognitive Performance: What We Know. J. Biol. Regul. Homeost. Agents 2022, 36, 193–204. [Google Scholar]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhang, Y.; Ren, X.; Zhang, R.; Cheng, R.; Hu, T. A Method for Porphyromonas Gingivalis Based on Recombinase Polymerase Amplification and Lateral Flow Strip Technology. Anal. Biochem. 2024, 687, 115425. [Google Scholar] [CrossRef] [PubMed]
- McCambridge, J.; Golder, S. Alcohol, Cardiovascular Disease and Industry Funding: A Co-Authorship Network Analysis of Epidemiological Studies. Addict. Behav. 2024, 151, 107932. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Bullon, P.; Wilson, M.; Newman, H. Oxidative Injury and Inflammatory Periodontal Diseases: The Challenge of Anti-Oxidants to Free Radicals and Reactive Oxygen Species. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1999, 10, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, Z.; Jamoussi, K.; Zeghal, K.M. Free radicals and antioxidants: Physiology, human pathology and therapeutic aspects (part II). Therapie 1998, 53, 315–339. [Google Scholar] [PubMed]
- Stadtman, E.R. Protein Oxidation and Aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Vilar-Rojas, C.; Guzman-Grenfell, A.M.; Hicks, J.J. Participation of Oxygen-Free Radicals in the Oxido-Reduction of Proteins. Arch. Med. Res. 1996, 27, 1–6. [Google Scholar]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef]
- Phillips, L.; Chu, L.; Kolodrubetz, D. Multiple Enzymes Can Make Hydrogen Sulfide From Cysteine in Treponema Denticola. Anaerobe 2020, 64, 102231. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Mancini, A.; Inchingolo, F.; Inchingolo, A.D.; Di Venere, D.; Dipalma, G.; et al. Damage from Carbonated Soft Drinks on Enamel: A Systematic Review. Nutrients 2023, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, D.E.; Marino, S.M.; Gladyshev, V.N. Functional Diversity of Cysteine Residues in Proteins and Unique Features of Catalytic Redox-Active Cysteines in Thiol Oxidoreductases. Mol. Cells 2008, 26, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A. On the Evolution of Coenzyme Biosynthesis. Nat. Prod. Rep. 2022, 39, 2175–2199. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; D’Ettorre, E.; Nico, A.; Inchingolo, F.; Dipalma, G.; Tomassone, D.; Serlenga, E.M.; Dalagni, G.; et al. New Translational Trends in Personalized Medicine: Autologous Peripheral Blood Stem Cells and Plasma for COVID-19 Patient. J. Pers. Med. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.C.; van der Donk, W.A. Go It Alone: Four Electron Oxidations by Mononuclear Non-Heme Iron Enzymes. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2017, 22, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Margon, A.; Parente, G.; Piantanida, M.; Cantone, P.; Leita, L. Novel Investigation on Ammonium Thiosulphate (ATS) as an Inhibitor of Soil Urease and Nitrification. Agric. Sci. 2015, 6, 1502–1512. [Google Scholar] [CrossRef]
- Kovaleva, E.G.; Lipscomb, J.D. Versatility of Biological Non-Heme Fe(II) Centers in Oxygen Activation Reactions. Nat. Chem. Biol. 2008, 4, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.R.; Sherma, N.D. Techniques for the Analysis of Cysteine Sulfhydryls and Oxidative Protein Folding. Antioxid. Redox Signal. 2014, 21, 511–531. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemical Basis for Pharmacological and Therapeutic Actions of Penicillamine. In Protein Crosslinking; Friedman, M., Ed.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 1977; Volume 86, pp. 649–673. ISBN 978-1-4757-9115-0. [Google Scholar]
- Gupta, V.; Carroll, K.S. Sulfenic Acid Chemistry, Detection and Cellular Lifetime. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.L.; Carroll, K.S. The Redox Biochemistry of Protein Sulfenylation and Sulfinylation. J. Biol. Chem. 2013, 288, 26480–26488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shi, R.; Wu, J.; Luo, X.; Liu, X. Point-of-Care Detection of Salivary Nitrite Based on the Surface Plasmon-Assisted Catalytic Coupling Reaction of Aromatic Amines. Biosensors 2021, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Van Scott, E.J. Sulfhydryl Groups and Disulfide Linkages in Normal and Pathological Keratinization. Arch. Dermatol. 1954, 70, 141. [Google Scholar] [CrossRef] [PubMed]
- Van Scott, E.J.; Flesch, P. Sulfhydryl Disulfide in Keratinization. Science 1954, 119, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, D.; Cooreman, K.; Coucke, P.; Nsabumukunzi, S.; Reyniers, P.; Kluyskens, P.; Gillis, E. A Quantitative Histochemical Study of Sulphydryl and Disulphide Content during Normal Epidermal Keratinization. Histochem. J. 1982, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Giroud, A.; Leblond, C.P. The Keratinization of Epidermis and Its Derivatives, Especially the Hair, as Shown by x-Ray Diffraction and Histochemical Studies. Ann. N. Y. Acad. Sci. 1951, 53, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The Role of Thiols in Antioxidant Systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Halperson, E.; Weintraub, M. Oral Langerhans Cell Histiocytosis in an Infant. J. Dent. Child. Chic. Ill 2018, 85, 75–78. [Google Scholar]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Boccellino, M. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Role of Free Radicals and Antioxidants in the Pathogenesis of the Inflammatory Periodontal Diseases. Clin. Mol. Pathol. 1996, 49, M247. [Google Scholar] [CrossRef]
- Kanwal, A.; Iqbal, A.; Arshad, R.; Akhtar, S.; Razzaq, S.; Ahmad, N.M.; Shahnaz, G. Formulation and Evaluation of Novel Thiolated Intra Pocket Periodontal Composite Membrane of Doxycycline. AAPS PharmSciTech 2019, 20, 325. [Google Scholar] [CrossRef]
- Amou, T.; Hinode, D.; Yoshioka, M.; Grenier, D. Relationship between Halitosis and Periodontal Disease—Associated Oral Bacteria in Tongue Coatings. Int. J. Dent. Hyg. 2014, 12, 145–151. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef]
- Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive Oxygen Species in Periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately Loaded Dental Implants Bioactivated with Platelet-Rich Plasma (PRP) Placed in Maxillary and Mandibular Region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar] [CrossRef]
- Van Dyke, T.E. Pro-Resolving Mediators in the Regulation of Periodontal Disease. Mol. Aspects Med. 2017, 58, 21–36. [Google Scholar] [CrossRef]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Suh, M.K.; Carson, J.S.; Dale, M.A.; Meisinger, T.M.; Fitzgerald, M.; Opperman, P.J.; Luo, J.; Pipinos, I.I.; Xiong, W.; et al. IL-1β (Interleukin-1β) and TNF-α (Tumor Necrosis Factor-α) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Signorini, L.; Inchingolo, A.D.; Saini, R.; Gnoni, A.; Scacco, S.; Santacroce, L. Probiotics May Improve Serum Folate Availability in Pregnant Women: A Pilot Study. Open Access Maced. J. Med. Sci. 2020, 8, 1124–1130. [Google Scholar] [CrossRef]
- Marretta, S.M.; Leesman, M.; Burgess-Cassler, A.; McClure, G.D.; Buelow, M.; Finn, M. Pilot Evaluation of a Novel Test Strip for the Assessment of Dissolved Thiol Levels, as an Indicator of Canine Gingival Health and Periodontal Status. Can. Vet. J. 2012, 53, 1260–1265. [Google Scholar]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Sharifi-Rad, J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Malcangi, G.; Patano, A.; Di Pede, C.; Netti, A.; Ciocia, A.M.; Corriero, A.; Semjonova, A.; et al. A Systematic Review of Positional Plagiocephaly Prevention Methods for Patients in Development. Appl. Sci. 2022, 12, 11172. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S. Microbial Etiological Agents of Destructive Periodontal Diseases. Periodontology 1994, 5, 78–111. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The Bacterial Etiology of Destructive Periodontal Disease: Current Concepts. J. Periodontol. 1992, 63, 322–331. [Google Scholar] [CrossRef]
- Mathur, A.; Mathur, L.; Manohar, B.; Mathur, H.; Shankarapillai, R.; Shetty, N.; Bhatia, A. Antioxidant Therapy as Monotherapy or as an Adjunct to Treatment of Periodontal Diseases. J. Indian Soc. Periodontol. 2013, 17, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.L.; Duarte, N.N.; Nascimento, P.C.; Magno, M.B.; Fagundes, N.C.F.; Flores-Mir, C.; Monteiro, M.C.; Rösing, C.K.; Maia, L.C.; Lima, R.R. Antioxidants as Adjuvants in Periodontitis Treatment: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 9187978. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2024, 16, 113. [Google Scholar] [CrossRef]
- Merle, C.L.; Lenzen, C.; Schmalz, G.; Ziebolz, D. Systematic Review on Protocols of Coenzyme Q10 Supplementation in Non-Surgical Periodontitis Therapy. Nutrients 2023, 15, 1585. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Hussain, A.; Tebyaniyan, H. Green Alternatives as Antimicrobial Agents in Mitigating Periodontal Diseases: A Narrative Review. Microorganisms 2023, 11, 1269. [Google Scholar] [CrossRef]
- Moore, S.; Calder, K.A.; Miller, N.J.; Rice-Evans, C.A. Antioxidant Activity of Saliva and Periodontal Disease. Free Radic. Res. 1994, 21, 417–425. [Google Scholar] [CrossRef]
- Al-Abdaly, M.M.A.A.; Alharbi, F.S.T.; Almoalem, A.M.; Awaji, N.A.T. The Influence of Kidney Stones and Salivary Uric Acid on Dental Calculus Formation and Periodontal Status among Some Saudi Patients Aged 25–70 Years. Int. J. Clin. Med. 2020, 11, 565–578. [Google Scholar] [CrossRef]
- Queck, K.E.; Chapman, A.; Herzog, L.J.; Shell-Martin, T.; Burgess-Cassler, A.; McClure, G.D. Oral-Fluid Thiol-Detection Test Identifies Underlying Active Periodontal Disease Not Detected by the Visual Awake Examination. J. Am. Anim. Hosp. Assoc. 2018, 54, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Loe, H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, C.; Battaglia, T.; Niccoli, A. Periodontal pathology during pregnancy. Clin. Ter. 2003, 154, 105–109. [Google Scholar] [PubMed]
- Ringsdorf, W.M.; Powell, B.J.; Knight, L.A.; Cheraskin, E. Periodontal Status and Pregnancy. Am. J. Obstet. Gynecol. 1962, 83, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Loee, H. Periodontal changes in pregnancy. J. Periodontol. 1965, 36, 209–217. [Google Scholar] [CrossRef]
- Raj, S.C.; Panda, S.M.; Dash, M.; Patnaik, K.; Mohanty, D.; Katti, N.; Mahapatra, A.; Mishra, D.; Praharaj, K. Association of Human Interleukin-35 Level in Gingival Crevicular Fluid and Serum in Periodontal Health, Disease, and after Nonsurgical Therapy: A Comparative Study. Contemp. Clin. Dent. 2018, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Medina-Navarro, R.; Durán-Reyes, G.; Díaz-Flores, M.; Vilar-Rojas, C. Protein Antioxidant Response to the Stress and the Relationship between Molecular Structure and Antioxidant Function. PLoS ONE 2010, 5, e8971. [Google Scholar] [CrossRef]
- Motchnik, P.A.; Frei, B.; Ames, B.N. Measurement of Antioxidants in Human Blood Plasma. Methods Enzymol. 1994, 234, 269–279. [Google Scholar] [CrossRef]
- Martins, C.S.; Leitao, R.F.; Costa, D.V.; Melo, I.M.; Santos, G.S.; Lima, V.; Brito, G.A. Topical HPMC/S-Nitrosoglutathione Solution Decreases Inflammation and Bone Resorption in Experimental Periodontal Disease in Rats. PLoS ONE 2016, 11, e0153716. [Google Scholar]
- De Luca, C.; Filosa, A.; Grandinetti, M.; Maggio, F.; Lamba, M.; Passi, S. Blood Antioxidant Status and Urinary Levels of Catecholamine Metabolites in Beta-Thalassemia. Free Radic. Res. 1999, 30, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, G. Statistical Methods for Medical and Biological Students. Stat. Methods Med. Biol. Stud. 1940, 2, 358–359. [Google Scholar]
- Nagaiah, M.; Ayyanar, K. Software for Data Analysis in SPSS On over View. Available online: https://www.researchgate.net/publication/348740813_Software_for_Data_Analysis_in_SPSS_On_over_view (accessed on 2 January 2024).
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L. Dental plaque–induced gingival conditions. J. Periodontol. 2018, 89, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive Oxygen Species: A Potential Role in the Pathogenesis of Periodontal Diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, R.; Dolci, C.; Boccalari, E.; Inchingolo, F.; Paghi, A.; Strambini, L.; Galimberti, D.; Tartaglia, G.M. Salivary biomarkers of neurodegenerative and demyelinating diseases and biosensors for their detection. Ageing Res. Rev. 2022, 76, 101587. [Google Scholar] [CrossRef] [PubMed]
- Baelum, V.; Lopez, R. Periodontal Epidemiology: Towards Social Science or Molecular Biology? Commun. Dent. Oral Epidemiol. 2004, 32, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, H.; Ohtake, M.; Kurasawa, I.; Saito, K. Intracellular Production and Extracellular Release of Oxygen Radicals by PMNs and Oxidative Stress on PMNs during Phagocytosis of Periodontopathic Bacteria. Odontology 2003, 91, 13–18. [Google Scholar] [CrossRef]
- Kantarci, A.; Oyaizu, K.; Van Dyke, T.E. Neutrophil-Mediated Tissue Injury in Periodontal Disease Pathogenesis: Findings from Localized Aggressive Periodontitis. J. Periodontol. 2003, 74, 66–75. [Google Scholar] [CrossRef]
- Kubar, A.; Saygun, I.; Ozdemir, A.; Yapar, M.; Slots, J. Real-Time Polymerase Chain Reaction Quantification of Human Cytomegalovirus and Epstein-Barr Virus in Periodontal Pockets and the Adjacent Gingiva of Periodontitis Lesions. J. Periodontal Res. 2005, 40, 97–104. [Google Scholar] [CrossRef]
- Seidman, M.D. Effects of Dietary Restriction and Antioxidants on Presbyacusis. Laryngoscope 2000, 110, 727–738. [Google Scholar] [CrossRef]
- Canakçi, C.F.; Tatar, A.; Canakçi, V.; Cicek, Y.; Oztas, S.; Orbak, R. New Evidence of Premature Oxidative DNA Damage: Mitochondrial DNA Deletion in Gingival Tissue of Patients with Periodontitis. J. Periodontol. 2006, 77, 1894–1900. [Google Scholar] [CrossRef]
- Buranasin, P.; Kominato, H.; Mizutani, K.; Mikami, R.; Saito, N.; Takeda, K.; Iwata, T. Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients. Antioxidants 2023, 12, 1787. [Google Scholar] [CrossRef]
- Nugala, B.; Namasi, A.; Emmadi, P.; Krishna, P.M. Role of Green Tea as an Antioxidant in Periodontal Disease: The Asian Paradox. J. Indian Soc. Periodontol. 2012, 16, 313. [Google Scholar] [CrossRef]
- Pendyala, G.; Thomas, B.; Kumari, S. The Challenge of Antioxidants to Free Radicals in Periodontitis. J. Indian Soc. Periodontol. 2008, 12, 79–83. [Google Scholar] [CrossRef]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; Del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in Human Health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Nagler, R.M.; Klein, I.; Zarzhevsky, N.; Drigues, N.; Reznick, A.Z. Characterization of the Differentiated Antioxidant Profile of Human Saliva. Free Radic. Biol. Med. 2002, 32, 268–277. [Google Scholar] [CrossRef]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the Human Immune Cell Network at the Gingival Barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1323–1328. [Google Scholar]
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; Dipalma, G.; Inchingolo, F.; Saini, R. Clinical Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar]
- Bokor-Bratić, M. Clinical significance of analysis of immunoglobulin A levels in saliva. Med. Pregl. 2000, 53, 164–168. [Google Scholar]
- Amerongen, A.V.N.; Veerman, E.C.I. Saliva--the Defender of the Oral Cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Sculley, D.V.; Langley-Evans, S.C. Salivary Antioxidants and Periodontal Disease Status. Proc. Nutr. Soc. 2002, 61, 137–143. [Google Scholar] [CrossRef]
- Azizi, A.; Sarlati, F.; Parchakani, A.; Alirezaei, S. Evaluation of Whole Saliva Antioxidant Capacity in Patients with Periodontal Diseases. Open J. Stomatol. 2014, 4, 228–231. [Google Scholar] [CrossRef][Green Version]
- Palermo, A.; Giannotti, L.; Di Chiara Stanca, B.; Ferrante, F.; Gnoni, A.; Nitti, P.; Calabriso, N.; Demitri, C.; Damiano, F.; Batani, T.; et al. Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation. Int. J. Mol. Sci. 2022, 23, 15164. [Google Scholar] [CrossRef]
- Loesche, W.J.; Grossman, N.S. Periodontal Disease as a Specific, Albeit Chronic, Infection: Diagnosis and Treatment. Clin. Microbiol. Rev. 2001, 14, 727–752, table of contents. [Google Scholar] [CrossRef]
- Corridore, D.; Saccucci, M.; Zumbo, G.; Fontana, E.; Lamazza, L.; Stamegna, C.; Di Carlo, G.; Vozza, I.; Guerra, F. Impact of Stress on Periodontal Health: Literature Revision. Healthcare 2023, 11, 1516. [Google Scholar] [CrossRef]
- Talmaç, A.C.; Çalişir, M.; Talmaç, A.C.; Çalişir, M. Antioxidants and Periodontal Diseases. In Gingival Disease—A Professional Approach for Treatment and Prevention; IntechOpen: London, UK, 2019; ISBN 978-1-83962-348-6. [Google Scholar]
- Canakci, C.F.; Cicek, Y.; Yildirim, A.; Sezer, U.; Canakci, V. Increased Levels of 8-Hydroxydeoxyguanosine and Malondialdehyde and Its Relationship with Antioxidant Enzymes in Saliva of Periodontitis Patients. Eur. J. Dent. 2009, 3, 100–106. [Google Scholar] [CrossRef]
- Karim, S.; Pratibha, P.K.; Kamath, S.; Bhat, G.S.; Kamath, U.; Dutta, B.; Sharma, N.; Archana, B.; Bhat, K.M.; Guddattu, V. Superoxide Dismutase Enzyme and Thiol Antioxidants in Gingival Crevicular Fluid and Saliva. Dent. Res. J. 2012, 9, 266–272. [Google Scholar]
- Afacan, B.; Öztürk, V.Ö.; Emingil, G.; Köse, T.; Mitsakakis, K.; Bostanci, N. Salivary Secretory Leukocyte Protease Inhibitor Levels in Patients with Stage 3 Grade C Periodontitis: A Comparative Cross-Sectional Study. Sci. Rep. 2022, 12, 21267. [Google Scholar] [CrossRef]
- How to Interpret Contradictory Results between ANOVA and Multiple Pairwise Comparisons?|XLSTAT Help Center. Available online: https://help.xlstat.com/6741-how-interpret-contradictory-results-between-anova-and (accessed on 3 January 2024).
- Shackelford, R.E.; Kaufmann, W.K.; Paules, R.S. Oxidative Stress and Cell Cycle Checkpoint Function. Free Radic. Biol. Med. 2000, 28, 1387–1404. [Google Scholar] [CrossRef]
- Ashutosh, W.; Verghese, Y.; Mohammed, A.; Devanna, R.; Bhardwaj, R.; Sahu, A.; Babaji, P. A Comparative Evaluation of Nickel-Titanium Wires and Clear Aligners in the Management of Mandibular Incisor Crowding. J. Orthod. Sci. 2023, 12, 21. [Google Scholar] [CrossRef]
- Thomas, B.; Ramesh, A.; Suresh, S.; Prasad, B.R. A Comparative Evaluation of Antioxidant Enzymes and Selenium in the Serum of Periodontitis Patients with Diabetes Mellitus Type 2. Contemp. Clin. Dent. 2013, 4, 176–180. [Google Scholar] [CrossRef]
- Patel, S.P.; Rao, N.S.; Pradeep, A.R. Effect of Nonsurgical Periodontal Therapy on Crevicular Fluid and Serum Glutathione Peroxidase Levels. Dis. Markers 2012, 32, 632842. [Google Scholar] [CrossRef]
- Duarte, P.M.; Napimoga, M.H.; Fagnani, E.C.; Santos, V.R.; Bastos, M.F.; Ribeiro, F.V.; Araújo, V.C.; Demasi, A.P.D. The Expression of Antioxidant Enzymes in the Gingivae of Type 2 Diabetics with Chronic Periodontitis. Arch. Oral Biol. 2012, 57, 161–168. [Google Scholar] [CrossRef]
- Novakovic, N.; Todorovic, T.; Rakic, M.; Milinkovic, I.; Dozic, I.; Jankovic, S.; Aleksic, Z.; Cakic, S. Salivary Antioxidants as Periodontal Biomarkers in Evaluation of Tissue Status and Treatment Outcome. J. Periodontal Res. 2014, 49, 129–136. [Google Scholar] [CrossRef]
- Bains, V.K.; Bains, R. The Antioxidant Master Glutathione and Periodontal Health. Dent. Res. J. 2015, 12, 389. [Google Scholar] [CrossRef]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal Jelly Modulates Oxidative Stress and Apoptosis in Liver and Kidneys of Rats Treated with Cisplatin. Oxid. Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef]
- Belge Kurutas, E.; Ciragil, P.; Gul, M.; Kilinc, M. The Effects of Oxidative Stress in Urinary Tract Infection. Mediat. Inflamm. 2005, 2005, 242–244. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef]
- Głowacki, R.; Bald, E. Fully Automated Method for Simultaneous Determination of Total Cysteine, Cysteinylglycine, Glutathione and Homocysteine in Plasma by HPLC with UV Absorbance Detection. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2009, 877, 3400–3404. [Google Scholar] [CrossRef]
- Bald, E.; Głowacki, R. Analysis of Saliva for Glutathione and Metabolically Related Thiols by Liquid Chromatography with Ultraviolet Detection. Amino Acids 2005, 28, 431–433. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Chwatko, G.; Głowacki, R.; Bald, E. Determination of Endogenous Thiols and Thiol Drugs in Urine by HPLC with Ultraviolet Detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3300–3308. [Google Scholar] [CrossRef]
- Greabu, M.; Totan, A.; Miricescu, D.; Radulescu, R.; Virlan, J.; Calenic, B. Hydrogen Sulfide, Oxidative Stress and Periodontal Diseases: A Concise Review. Antioxidants 2016, 5, 3. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, W.; Pei, Y.; Yang, G. H(2)S Signaling in Redox Regulation of Cellular Functions. Can. J. Physiol. Pharmacol. 2013, 91, 8–14. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef] [PubMed]
- Cysteine—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/food-science/cysteine (accessed on 3 January 2024).
- Inchingolo, A.M.; Patano, A.; Piras, F.; Mancini, A.; Inchingolo, A.D.; Paduanelli, G.; Inchingolo, F.; Palermo, A.; Dipalma, G.; Malcangi, G. Interconnection between Microbiota–Gut–Brain Axis and Autism Spectrum Disorder Comparing Therapeutic Options: A Scoping Review. Microorganisms 2023, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The Basics of Thiols and Cysteines in Redox Biology and Chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Shin, S.-I.; Hong, J.-Y. Investigation of Volatile Sulfur Compound Level and Halitosis in Patients with Gingivitis and Periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, M.; Beushausen, M.; Feng, C.; Beech, A.; Baur, D. Halitosis as a Product of Hepatic Disease. South Afr. Dent. J. 2014, 69, 364–367. [Google Scholar]
- Casu, C.; Mosaico, G.; Natoli, V.; Scarano, A.; Lorusso, F.; Inchingolo, F. Microbiota of the Tongue and Systemic Connections: The Examination of the Tongue as an Integrated Approach in Oral Medicine. Hygiene 2021, 1, 56–68. [Google Scholar] [CrossRef]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- López-Pelegrín, M.; Ksiazek, M.; Karim, A.Y.; Guevara, T.; Arolas, J.L.; Potempa, J.; Gomis-Rüth, F.X. A Novel Mechanism of Latency in Matrix Metalloproteinases. J. Biol. Chem. 2015, 290, 4728–4740. [Google Scholar] [CrossRef]
- Pacifici, A.; Pacifici, L.; Nuzzolese, M.; Cascella, G.; Ballini, A.; Santacroce, L.; Dipalma, G.; Aiello, E.; Amantea, M.; Saini, R.; et al. The Alteration of Stress-Related Physiological Parameters after Probiotics Administration in Oral Surgeons with Different Degrees of Surgical Experience. Clin. Ter. 2020, 171, e197–e208. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.T.; Chu, P.M.; Tuan, V.P.; Te, J.S.L.; Lee, I.T. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).