Disruption of Electroencephalogram Coherence between Cortex/Striatum and Midbrain Dopaminergic Regions in the Knock-Out Mice with Combined Loss of Alpha, Beta, and Gamma Synucleins
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Electrode Implantation and Recording of EEG
2.3. EEG Spectral Coherence Computation
2.4. Statistics
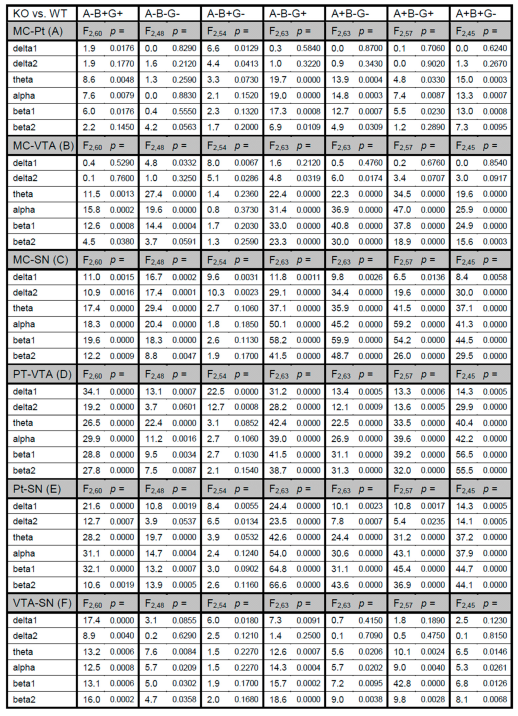
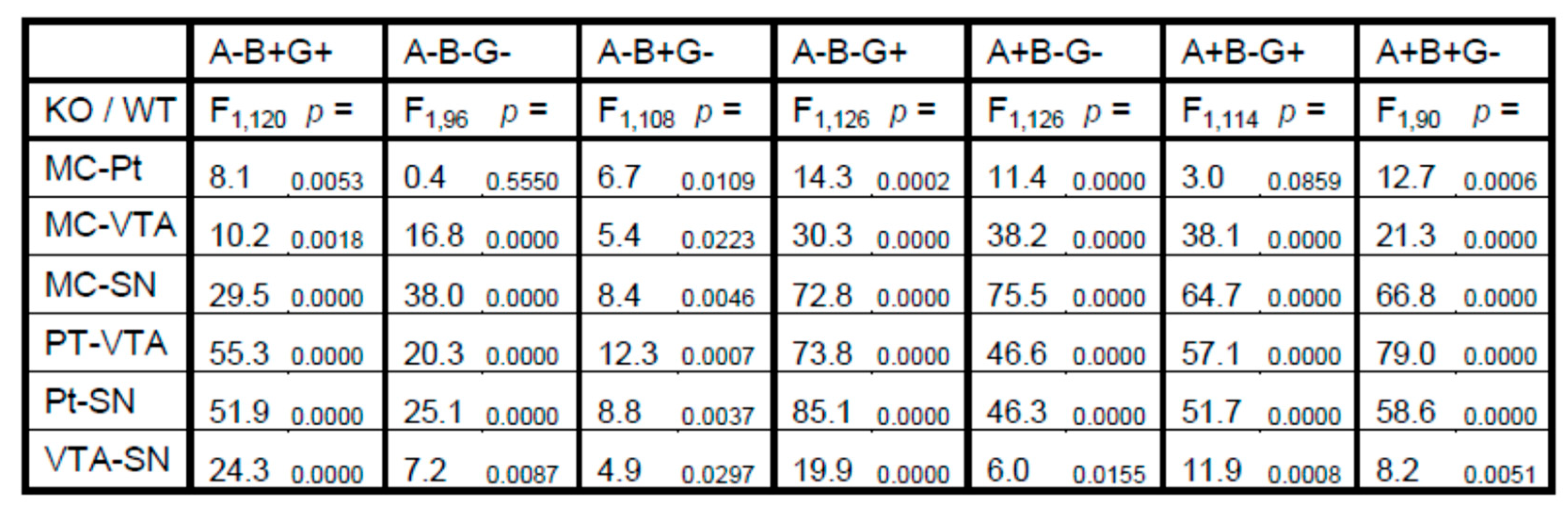
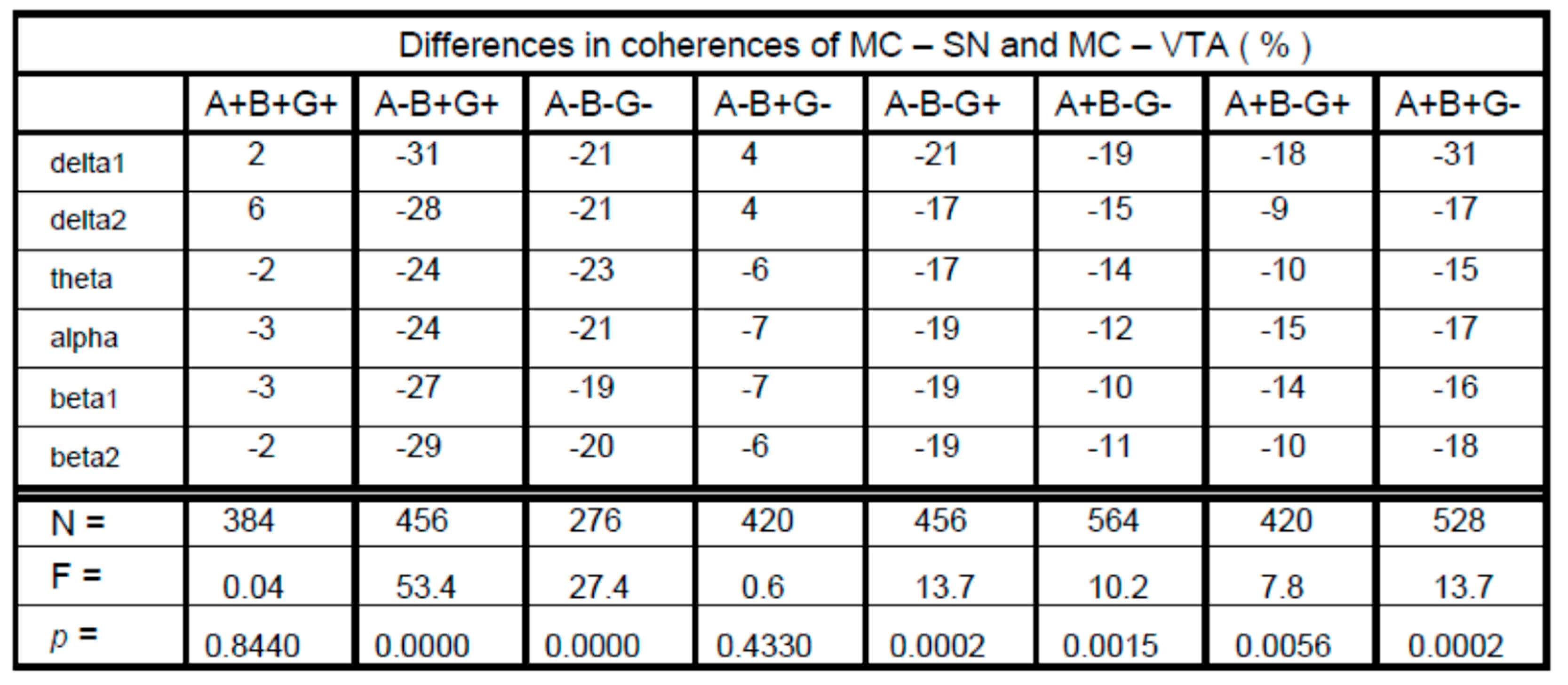
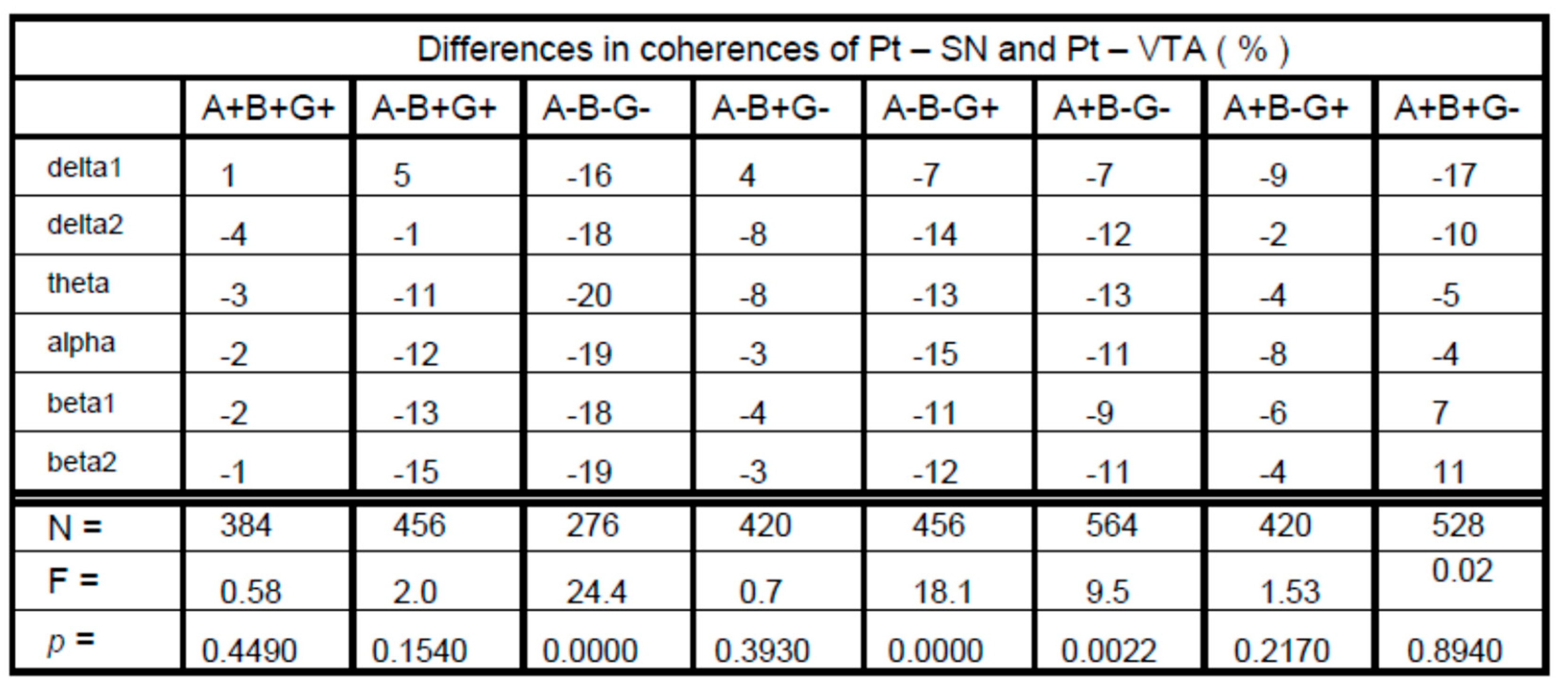
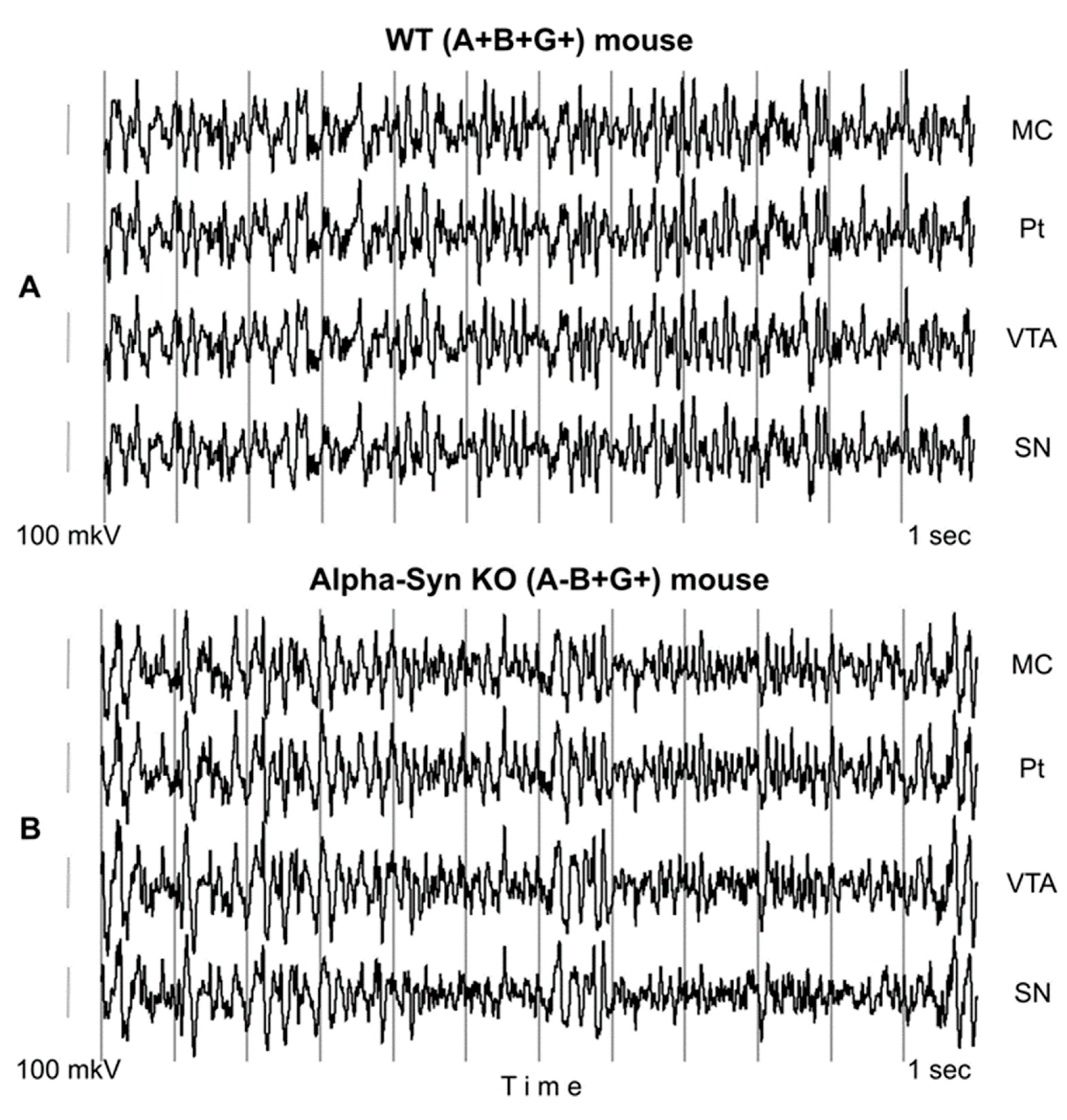
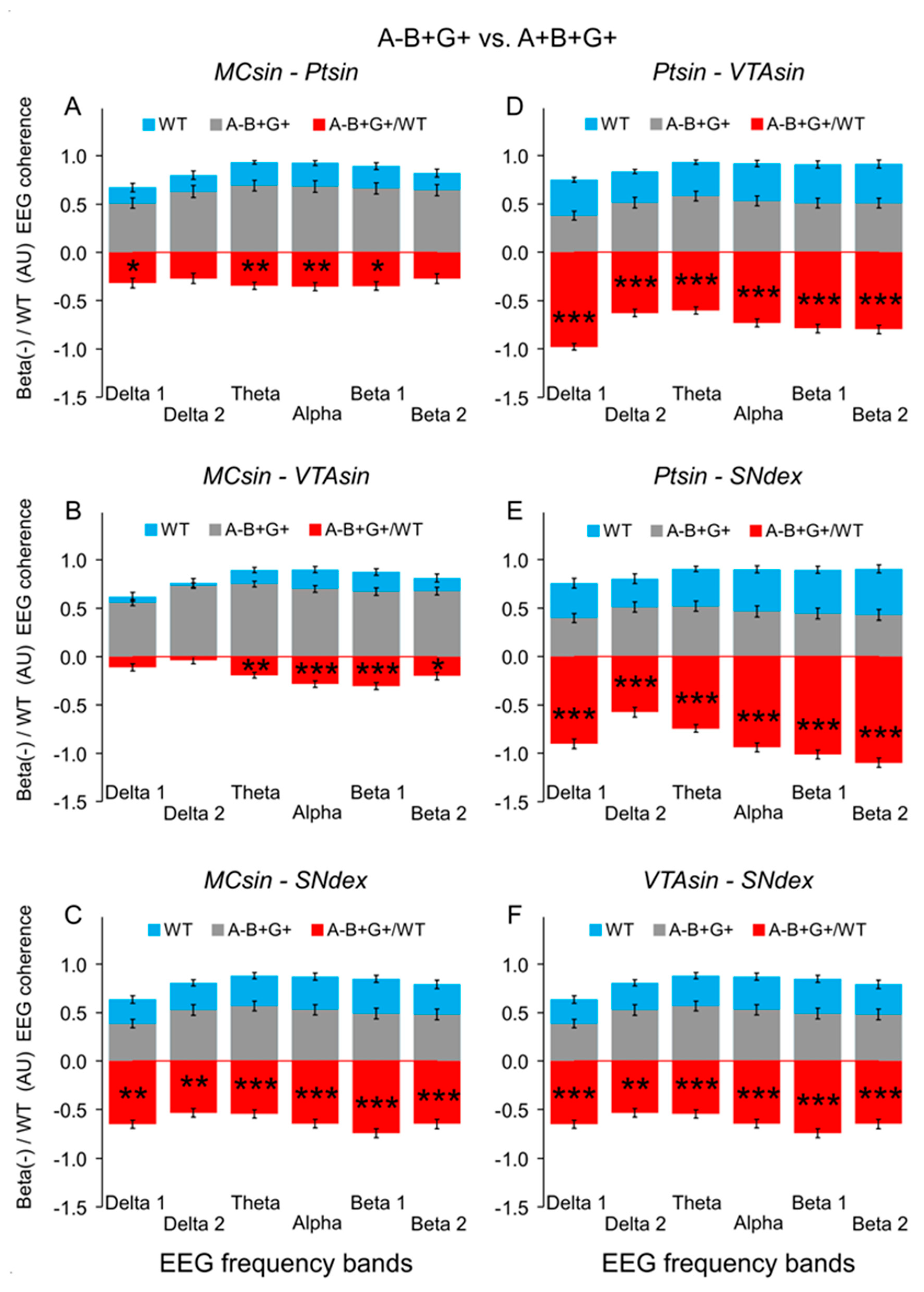
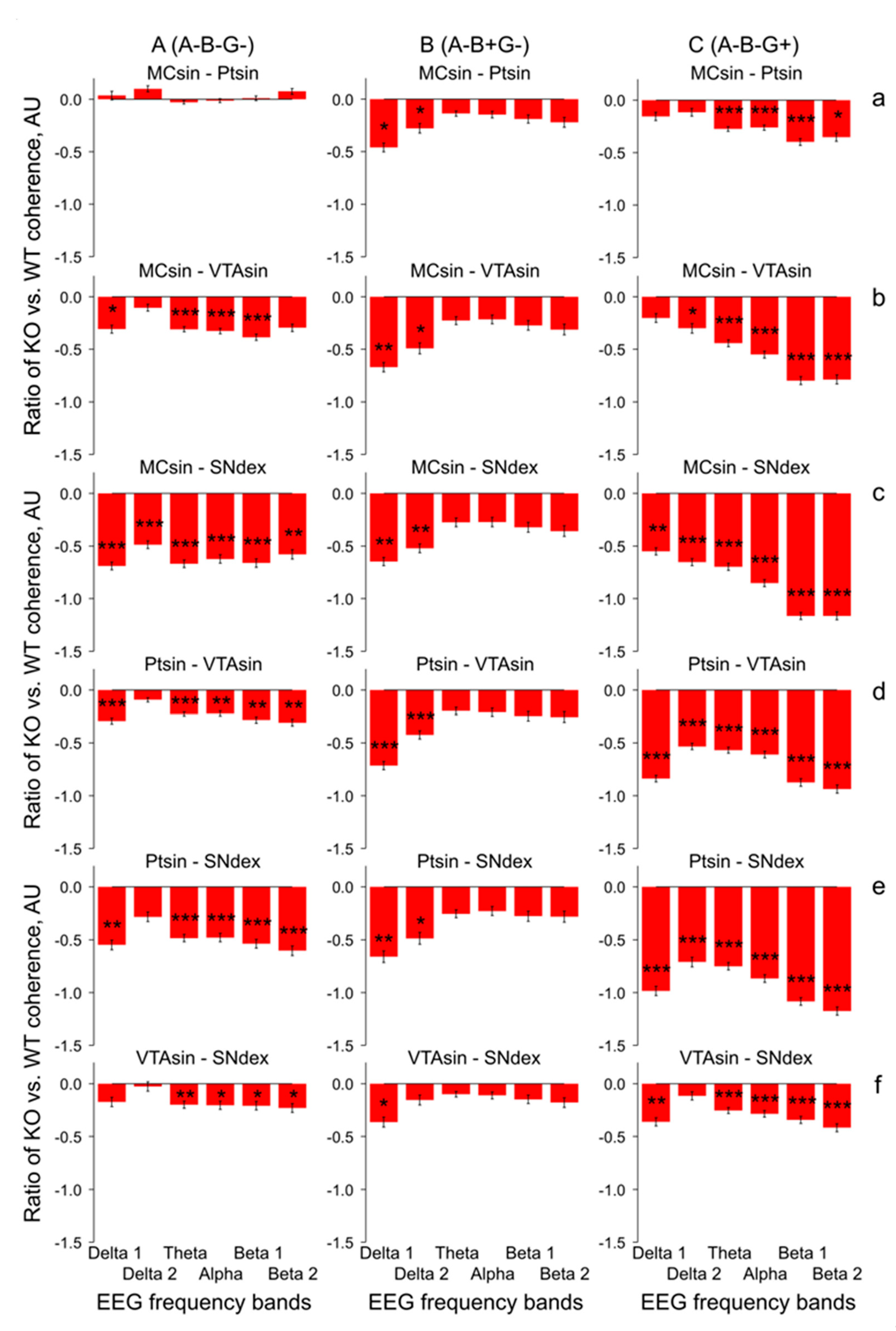
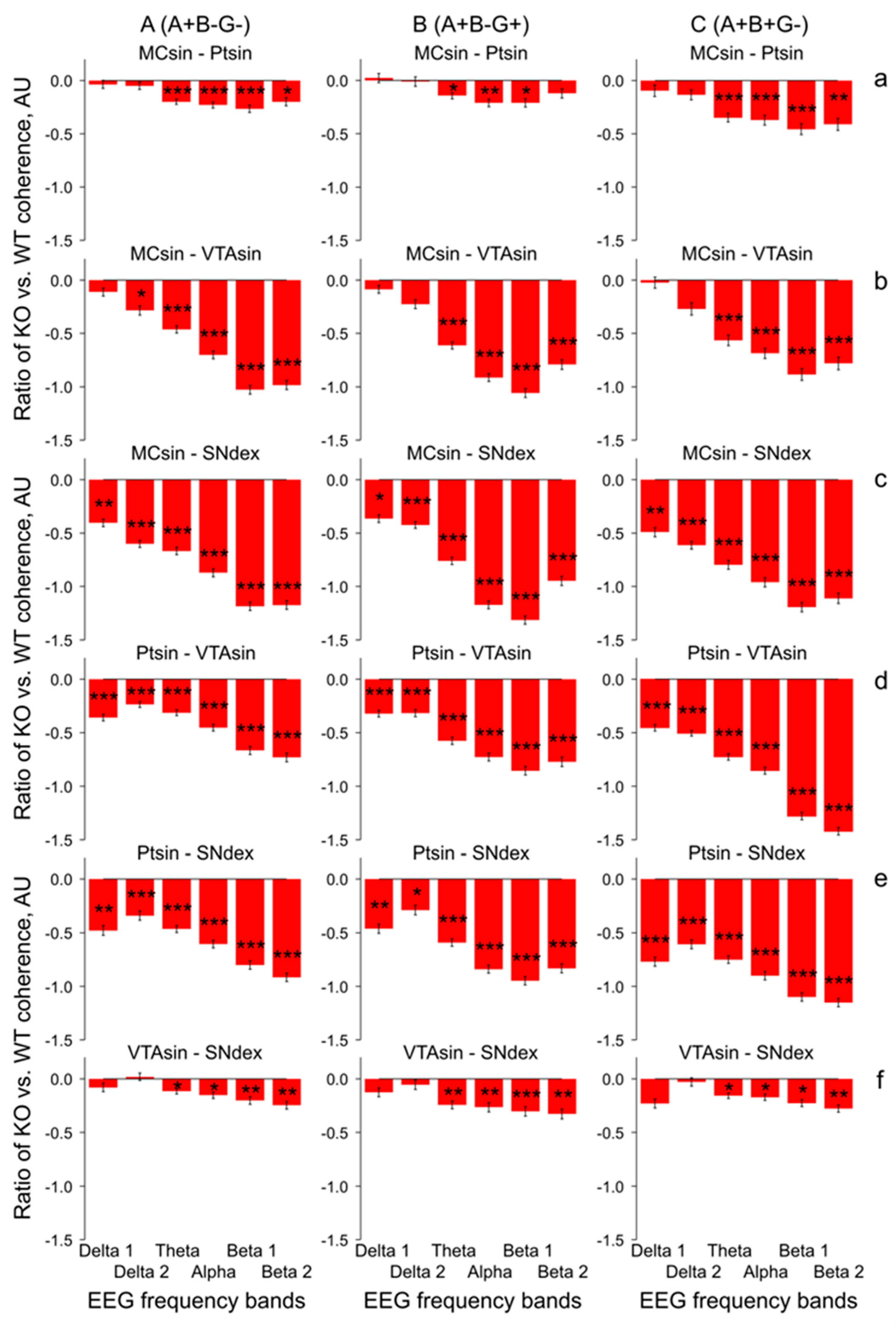
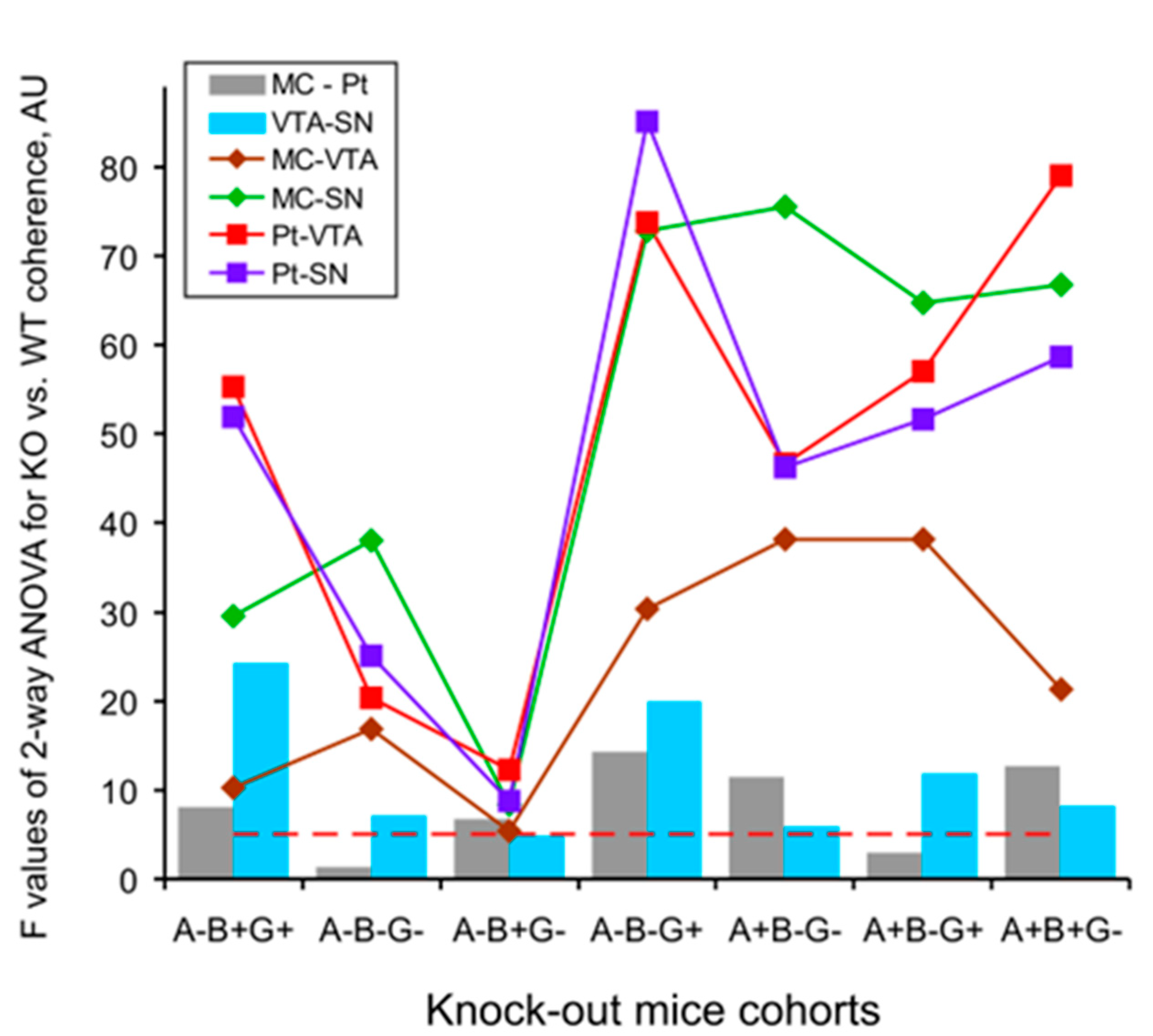
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Spillantini, M.G.; Goedert, M. The α-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann. N. Y. Acad. Sci. 2000, 920, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Brás, I.C.; Outeiro, T.F. Alpha-synuclein: Mechanisms of release and pathology progression in synucleinopathies. Cells 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S. Synucleins. The New Encyclopedia of Neuroscience; Squire, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 833–837. [Google Scholar]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fornai, F.; Kwon, H.-B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.; Millership, S.J.; Peters, O.M.; Connor-Robson, N.; Chaprov, K.; Kopylov, A.T.; Montoya, A.; Kramer, H.; Withers, D.J.; Buchman, V.L. β-synuclein potentiates synaptic vesicle dopamine uptake and rescues dopaminergic neurons from MPTP-induced death in the absence of other synucleins. J. Biol. Chem. 2021, 297, 101375. [Google Scholar] [CrossRef] [PubMed]
- Courte, J.; Bousset, L.; Boxberg, Y.V.; Villard, C.; Melki, R.; Peyrin, J.M. The expression level of alpha-synuclein in different neuronal populations is the primary determinant of its prion-like seeding. Sci. Rep. 2020, 10, 4895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Zhang, Y.; Yau, Y.; Zeighami, Y.; Larcher, K.; Misic, B.; Dagher, A. Local vulnerability and global connectivity jointly shape neurodegenerative disease propagation. PLoS Biol. 2019, 17, e3000495. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, C.; Rahayel, S.; Vo, A.; Morys, F.; Shafiei, G.; Abbasi, N.; Markello, R.D.; Gan-Or, Z.; Misic, B.; Dagher, A. Brain atrophy progression in Parkinson’s disease is shaped by connectivity and local vulnerability. Brain Commun. 2021, 3, fcab269. [Google Scholar] [CrossRef] [PubMed]
- Zeighami, Y.; Ulla, M.; Iturria-Medina, Y.; Dadar, M.; Zhang, Y.; Larcher, K.M.-H.L.; Fonov, V.; Evans, A.C.; Collins, D.L.; Dagher, A. Network structure of brain atrophy in de novo Parkinson’s disease. eLife 2015, 4, e08440. [Google Scholar] [CrossRef]
- Womelsdorf, T.; Schoffelen, J.M.; Oostenveld, R.; Singer, W.; Desimone, R.; Engel, A.K.; Fries, P. Modulation of neuronal interactions through neuronal synchronization. Science 2007, 316, 1609–1612. [Google Scholar] [CrossRef]
- Sharma, M.; Burré, J. α-Synuclein in synaptic function and dysfunction. Trends Neurosci. 2023, 46, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Waninger, S.; Berka, C.; Stevanovic Karic, M.; Korszen, S.; Mozley, P.D.; Henchcliffe, C.; Kang, Y.; Hesterman, J.; Mangoubi, T.; Verma, A. Neurophysiological biomarkers of Parkinson’s disease. J. Parkinsons Dis. 2020, 10, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Nimmrich, V.; Draguhn, A.; Axmacher, N. Neuronal network oscillations in neurodegenerative diseases. Neuromolecular Med. 2015, 17, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Vorobyov, V.; Deev, A.; Sengpiel, F.; Nebogatikov, V.; Ustyugov, A.A. Cortical and striatal Eeectroencephalograms and apomorphine effects in the FUS mouse model of amyotrophic lateral sclerosis. J. Alzheimers Dis. 2021, 81, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Vorobyov, V.; Deev, A.; Chaprov, K.; Ustyugov, A.A.; Lysikova, E. Age-related modifications of electroencephalogram coherence in mice models of Alzheimer’s disease and amyotrophic lateral sclerosis. Biomedicines 2023, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Vorobyov, V.; Deev, A.; Sukhanova, I.; Morozova, O.; Oganesyan, Z.; Chaprov, K.; Buchman, V.L. Loss of the synuclein family members differentially affects baseline- and apomorphine-associated EEG determinants in single-, double- and triple-knockout mice. Biomedicines 2022, 10, 3128. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.P.; Buell, A.K.; Michaels, T.C.T.; Meisl, G.; Carozza, J.; Flagmeier, P.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M.; Galvagnion, C. β-synuclein suppresses both the initiation and amplification steps of α-synuclein aggregation via competitive binding to surfaces. Sci. Rep. 2016, 6, 36010. [Google Scholar] [CrossRef] [PubMed]
- Ducas, V.C.; Rhoades, E. Quantifying interactions of β-synuclein and γ-synuclein with model membranes. J. Mol. Biol. 2012, 423, 528–539. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of α-synuclein is modulated by β- and γ-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef] [PubMed]
- Pavia-Collado, R.; Rodríguez-Aller, R.; Alarcón-Arís, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofré, M.; Sgambato, V.; Bortolozzi, A. Up and down γ-synuclein transcription in dopamine neurons translates into changes in dopamine neurotransmission and behavioral performance in mice. Int. J. Mol. Sci. 2022, 23, 1807. [Google Scholar] [CrossRef] [PubMed]
- Ludtmann, M.H.R.; Angelova, P.R.; Ninkina, N.N.; Gandhi, S.; Buchman, V.L.; Abramov, A.Y. Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J. Neurosci. 2016, 36, 10510–10521. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Paladini, C.A.; Roeper, J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 2014, 282, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hage, T.A.; Khaliq, Z.M. Tonic firing rate controls dendritic Ca2+ signaling and synaptic gain in substantia nigra dopamine neurons. J. Neurosci. 2015, 35, 5823–5836. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Del-Fava, F.; Hasue, R.H.; Shammah-Lagnado, S.J. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience 2008, 153, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Aransay, A.; Rodríguez-López, C.; García-Amado, M.; Clascá, F.; Prensa, L. Long-range projection neurons of the mouse ventral tegmental area: A single-cell axon tracing analysis. Front. Neuroanat. 2015, 9, 59. [Google Scholar] [CrossRef]
- Liss, B.; Haeckel, O.; Wildmann, J.; Miki, T.; Seino, S.; Roeper, J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat. Neurosci. 2005, 8, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Senior, S.L.; Ninkina, N.; Deacon, R.; Bannerman, D.; Buchman, V.L.; Cragg, S.J.; Wade-Martins, R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur. J. Neurosci. 2008, 27, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Oster, A.; Faure, P.; Gutkin, B.S. Mechanisms for multiple activity modes of VTA dopamine neurons. Front. Comput. Neurosci. 2015, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Iskhakova, L.; Rappel, P.; Deffains, M.; Fonar, G.; Marmor, O.; Paz, R.; Israel, Z.; Eitan, R.; Bergman, H. Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations. Nat. Commun. 2021, 12, 7026. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.P.; Demaro, J.A., III; Knoten, A.; Hoshi, M.; Pehek, E.; Johnson, E.M., Jr.; Gereau, R.W., IV; Jain, S. Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. J. Neurosci. 2013, 33, 17095–17107. [Google Scholar] [CrossRef]
- Kostrzewa, R.M.; Kostrzewa, J.P.; Brown, R.W.; Nowak, P.; Brus, R. Dopamine receptor supersensitivity: Development, mechanisms, presentation, and clinical applicability. Neurotox. Res. 2008, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; Wade-Martins, R. α-synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nordengen, K.; Morland, C. From synaptic hysiology to synaptic pathology: The enigma of α-synuclein. Int. J. Mol. Sci. 2024, 25, 986. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Peters, O.; Millership, S.; Ninkina, N.; Doig, N.; Connor-Robson, N.; Threlfell, S.; Kooner, G.; Deacon, R.M.; Bannerman, D.M.; et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011, 31, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.; Peters, O.M.; Connor-Robson, N.; Lytkina, O.; Sharfeddin, E.; Buchman, V.L. Contrasting effects of α-synuclein and γ-synuclein on the phenotype of cysteine string protein α (CSPα) null mutant mice suggest distinct function of these proteins in neuronal synapses. J. Biol. Chem. 2012, 287, 44471–44477. [Google Scholar] [CrossRef] [PubMed]
- McDowell, K.A.; Shin, D.; Roos, K.P.; Chesselet, M.F. Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J. Parkinsons Dis. 2014, 4, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Virdee, K.; McArthur, S.; Dalley, J.W. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 2014, 282, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne-Proulx, J.; Coulombe, K.; Morissette, M.; Rieux, M.; Calon, F.; Di Paolo, T.; Soulet, D. Sex and age differences in a progressive synucleinopathy mouse model. Biomolecules 2023, 13, 977. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyov, V.; Deev, A.; Chaprov, K.; Ninkina, N. Disruption of Electroencephalogram Coherence between Cortex/Striatum and Midbrain Dopaminergic Regions in the Knock-Out Mice with Combined Loss of Alpha, Beta, and Gamma Synucleins. Biomedicines 2024, 12, 881. https://doi.org/10.3390/biomedicines12040881
Vorobyov V, Deev A, Chaprov K, Ninkina N. Disruption of Electroencephalogram Coherence between Cortex/Striatum and Midbrain Dopaminergic Regions in the Knock-Out Mice with Combined Loss of Alpha, Beta, and Gamma Synucleins. Biomedicines. 2024; 12(4):881. https://doi.org/10.3390/biomedicines12040881
Chicago/Turabian StyleVorobyov, Vasily, Alexander Deev, Kirill Chaprov, and Natalia Ninkina. 2024. "Disruption of Electroencephalogram Coherence between Cortex/Striatum and Midbrain Dopaminergic Regions in the Knock-Out Mice with Combined Loss of Alpha, Beta, and Gamma Synucleins" Biomedicines 12, no. 4: 881. https://doi.org/10.3390/biomedicines12040881
APA StyleVorobyov, V., Deev, A., Chaprov, K., & Ninkina, N. (2024). Disruption of Electroencephalogram Coherence between Cortex/Striatum and Midbrain Dopaminergic Regions in the Knock-Out Mice with Combined Loss of Alpha, Beta, and Gamma Synucleins. Biomedicines, 12(4), 881. https://doi.org/10.3390/biomedicines12040881




