Associations between the VDR Gene rs731236 (TaqI) Polymorphism and Bone Mineral Density in Postmenopausal Women from the RAC-OST-POL
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Examination
2.2. Bone Mineral Density Measurement
2.3. Biochemical and Genetic Analyses
2.4. Statistical Analysis
3. Results
3.1. General and Clinical Characteristics of the Study Group
3.2. VDR Gene Polymorphisms
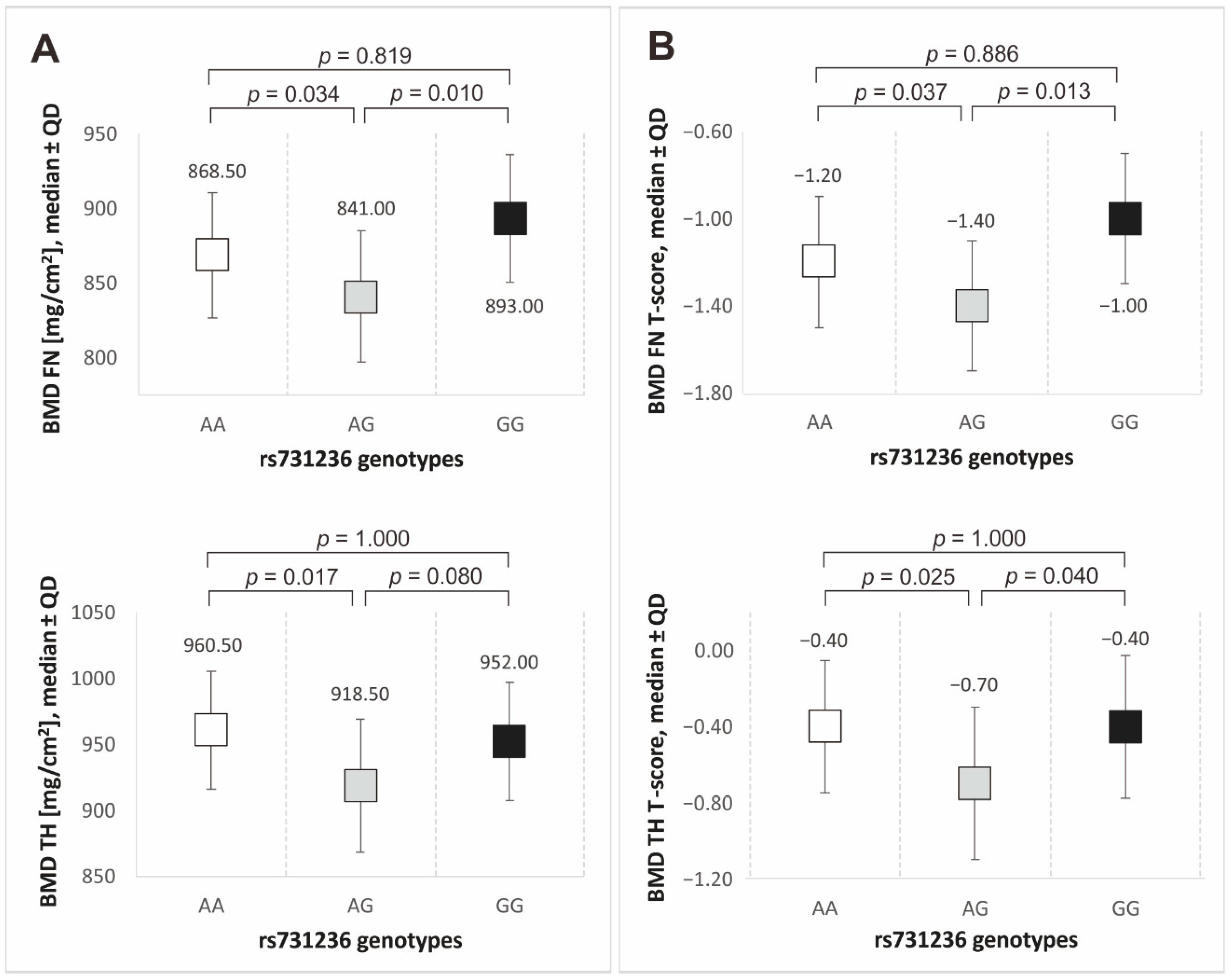
3.3. VDR Gene Polymorphisms and Bone Mineral Density
3.4. Correlates and Modifiers of Bone Mineral Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The North American Menopause Society. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Khosla, S.; Pacifici, R. Chapter 32—Estrogen Deficiency and the Pathogenesis of Osteoporosis; Academic Press: Cambridge, MA, USA, 2021; pp. 773–797. ISBN 9780128130735. [Google Scholar] [CrossRef]
- Bijelic, R.; Milicevic, S.; Balaban, J. Risk Factors for Osteoporosis in Postmenopausal Women. Med. Arch. 2017, 71, 25–28. [Google Scholar] [CrossRef]
- Alibasic, E.; Ramic, E.; Batic Mujanovic, O.; Avdibasic, E.; Husic, D.; Alic, A. Assessment of osteoporosis in family medicine obtained by ultrasound densitometry. Acta Inform. Med. 2013, 21, 274–276. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, G.; Wang, X.; Dong, Y.; Ding, F. Identification of New Genes and Loci Associated with Bone Mineral Density Based on Mendelian Randomization. Front. Genet. 2021, 12, 728563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Yu, X.H.; Bo, L.; Lei, S.F.; Deng, F.Y. Genetic Risk for Osteoporosis and the Benefit of Adherence to Healthy Lifestyles. Int. J. Public Health 2022, 67, 1605114. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S. Genetics of osteoporosis. Proc. Nutr. Soc. 2007, 66, 158–165. [Google Scholar] [CrossRef]
- Mondockova, V.; Kovacova, V.; Zemanova, N.; Babikova, M.; Martiniakova, M.; Galbavy, D.; Omelka, R. Vitamin D Receptor Gene Polymorphisms Affect Osteoporosis-Related Traits and Response to Antiresorptive Therapy. Genes 2023, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Uitterlinden, A.G. Genetics of osteoporosis. Endocr. Rev. 2010, 31, 629–662. [Google Scholar] [CrossRef]
- Duque, G.; El Abdaimi, K.; Macoritto, M.; Miller, M.M.; Kremer, R. Estrogens (E2) regulate expression and response of 1, 25-dihydroxyvitamin D3 receptors in bone cells: Changes with aging and hormone deprivation. Biochem. Biophys. Res. Commun. 2002, 299, 446–454. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Mickiewicz, A.; Fijałkowski, M. Pleotropic effect of vitamin D. Chor. Serca Naczyń 2019, 1, 45–52. [Google Scholar] [CrossRef]
- Stompór, T.; Ciechanowski, K.; Durlik, M.; Małyszko, J.; Nowicki, M.; Rutkowski, P.; Więcek, A.; Rutkowski, B. Agonists of vitamin D—Mechanisms of action selectivity. Forum Nefrol. 2012, 1, 89–97. [Google Scholar]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, B.; Visconti, V.V.; Ciccacci, C.; Falvino, A.; Gasbarra, E.; Iundusi, R.; Brandi, M.L.; Botta, A.; Tarantino, U. Role of the Vitamin D Receptor (VDR) in the Pathogenesis of Osteoporosis: A Genetic, Epigenetic and Molecular Pilot Study. Genes 2023, 14, 542. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, T.A.; Criss, Z.K.; Shroyer, N.F.; Abraham, B.P. Vitamin D Receptor Gene Single Nucleotide Polymorphisms and Association with Vitamin D Levels and Endoscopic Disease Activity in Inflammatory Bowel Disease Patients: A Pilot Study. Inflamm. Bowel. Dis. 2021, 27, 1263–1269. [Google Scholar] [CrossRef]
- Taha, I.M.; Allah, A.M.A.; El Tarhouny, S. Association of Vitamin D Gene Polymorphisms and Bone Mineral Density in Healthy young Saudi Females. Curr. Mol. Med. 2019, 19, 196–205. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef]
- Totonchi, H.; Rezaei, R.; Noori, S.; Azarpira, N.; Mokarram, P.; Imani, D. Vitamin D Receptor Gene Polymorphisms and the Risk of Metabolic Syndrome (MetS): A Meta-Analysis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 943–955. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Sakhr, H.M.; Hassan, M.H.; El-Amir, M.I.; Ameen, H.H. Vitamin D receptor rs7975232, rs731236 and rs1544410 single nucleotide polymorphisms, and 25-hydroxyvitamin D levels in Egyptian children with type 1 diabetes mellitus: Effect of vitamin D co-therapy. Diabetes Metab. Syndr. Obes. 2019, 12, 703–716. [Google Scholar] [CrossRef]
- Pluskiewicz, W.; Adamczyk, P.; Czekajło, A.; Grzeszczak, W.; Burak, W.; Drozdzowska, B. Epidemiological data on osteoporosis in women from the RAC-OST-POL study. J. Clin. Densitom. 2012, 15, 308–314. [Google Scholar] [CrossRef]
- Jakubowska-Pietkiewicz, E.; Młynarski, W.; Klich, I.; Fendler, W.; Chlebna-Sokół, D. Vitamin D receptor gene variability as a factor influencing bone mineral density in pediatric patients. Mol. Biol. Rep. 2012, 39, 6243–6250. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.; Yilmaz-Aydogan, H.; Uyar, M.; Isbir, T.; Seyhan, M.F.; Can, A. Evaluation of ERα and VDR gene polymorphisms in relation to bone mineral density in Turkish postmenopausal women. Mol. Biol. Rep. 2012, 39, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Kars, M.E.; Başak, A.N.; Onat, O.E.; Bilguvar, K.; Choi, J.; Itan, Y.; Çağla, R.C.; Palvadeau, R.; Casanova, J.L.; Cooper, D.N.; et al. The genetic structure of the Turkish population reveals high levels of variation and admixture. Proc. Natl. Acad. Sci. USA 2021, 118, e2026076118. [Google Scholar] [CrossRef] [PubMed]
- Hodoğlugil, U.; Mahley, R.W. Turkish population structure and genetic ancestry reveal relatedness among Eurasian populations. Ann. Hum. Genet. 2012, 76, 128–141. [Google Scholar] [CrossRef] [PubMed]
- González-Mercado, A.; Sánchez-López, J.Y.; Regla-Nava, J.A.; Gámez-Nava, J.I.; González-López, L.; Duran-Gonzalez, J.; Celis, A.; Perea-Díaz, F.J.; Salazar-Páramo, M.; Ibarra, B. Association analysis of vitamin D receptor gene polymorphisms and bone mineral density in postmenopausal Mexican-Mestizo women. Genet. Mol. Res. 2013, 12, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Marozik, P.; Rudenka, A.; Kobets, K.; Rudenka, E. Vitamin D Status, Bone Mineral Density, and VDR Gene Polymorphism in a Cohort of Belarusian Postmenopausal Women. Nutrients 2021, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; LeBoff, M.S.; Neale, R.E. Health Effects of Vitamin D Supplementation: Lessons Learned from Randomized Controlled Trials and Mendelian Randomization Studies. J. Bone Miner. Res. 2023, 38, 1391–1403. [Google Scholar] [CrossRef]
- Premaor, M.O.; Comim, F.V.; Compston, J.E. Obesity and fractures. Arq. Bras. Endocrinol. Metabol. 2014, 58, 470–477. [Google Scholar] [CrossRef]
- Cheung, Y.M.; Joham, A.; Marks, S.; Teede, H. The obesity paradox: An endocrine perspective. Intern. Med. J. 2017, 47, 727–733. [Google Scholar] [CrossRef]
- Tang, X.; Liu, G.; Kang, J.; Hou, Y.; Jiang, F.; Yuan, W.; Shi, J. Obesity and risk of hip fracture in adults: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e55077. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; Pérez Castrillón, J.L.; de Luis Román, D.A. Impact of obesity on bone metabolism. Endocrinol. Nutr. 2016, 63, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Díez-Pérez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Byberg, L.; Larsson, S.C.; Höijer, J.; Baron, J.A.; Michaëlsson, K. Prior loss of body mass index, low body mass index, and central obesity independently contribute to higher rates of fractures in elderly women and men. J. Bone Miner. Res. 2021, 36, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; He, L.; Sun, R.; Chen, S. Gender-specific associations between abdominal adipose mass and bone mineral density in the middle-aged US population. BMC Musculoskelet Disord. 2023, 24, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, L.; Yao, X.; Zhu, Z. The association between abdominal obesity and femoral neck bone mineral density in older adults. J. Orthop. Surg. Res. 2023, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.W.; Chen, H.; Zhang, S.B.; Yi, Y.Y.; Fang, X.Y.; Hu, T.; Ge, X.Y.; Wang, S.J. Association Between Abdominal Obesity and Subsequent Vertebral Fracture Risk. Pain Physician 2022, 25, E457–E468. [Google Scholar] [PubMed]
- Chiu, H.; Lee, M.Y.; Wu, P.Y.; Huang, J.C.; Chen, S.C. Development of Metabolic Syndrome Decreases Bone Mineral Density T-Score of Calcaneus in Foot in a Large Taiwanese Population Follow-Up Study. J. Pers. Med. 2021, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Broe, K.E.; Zhou, Y.; Boyd, S.K.; Cupples, L.A.; Hannan, M.T.; Lim, E.; McLean, R.R.; Samelson, E.J.; Bouxsein, M.L.; et al. Visceral Adipose Tissue Is Associated with Bone Microarchitecture in the Framingham Osteoporosis Study. J. Bone Miner. Res. 2017, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; El Said, S.M.; Adly, N.N.; Abdul-Rahman, S.A. The Relation Between Calcaneus Stiffness Index as a Measure of Bone Density and Body Mass Index in an Egyptian Cohort. J. Multidiscip. Healthcare 2019, 12, 1085–1090. [Google Scholar] [CrossRef]
- Sharifan, P.; Hassanzadeh, E.; Mohammadi-Bajgiran, M.; Dabbagh, V.R.; Aminifar, E.; Ghazizadeh, H.; Saffar-Soflaei, S.; Darroudi, S.; Tanbakouchi, D.; Fazl-Mashhadi, M.R.; et al. Effects of Vitamin D3 Fortified Low-fat Dairy Products on Bone Density Measures in Adults with Abdominal Obesity: A Randomized Clinical Trial. Arch. Bone Jt. Surg. 2022, 10, 601–610. [Google Scholar] [CrossRef]
- Gkastaris, K.; Goulis, D.G.; Potoupnis, M.; Anastasilakis, A.D.; Kapetanos, G. Obesity, osteoporosis and bone metabolism. J. Musculoskelet. Neuronal Interact. 2020, 20, 372–381. [Google Scholar] [PubMed]
- Fu, L.; Ma, J.; Yan, S.; Si, Q. A meta-analysis of VDR polymorphisms and postmenopausal osteoporosis. Endocr. Connect. 2020, 9, 882–889. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | |||
|---|---|---|---|
| General | Number of subjects, N (%) | 611 | (100.00) |
| Age [years], median ± QD | 65.82 | 6.29 | |
| BMI [kg/m2], median ± QD | 30.84 | 3.96 | |
| Overweight/obesity [BMI ≥ 25], n (%) | 533 | (87.23) | |
| Obesity [BMI ≥ 30], n (%) | 335 | (54.83) | |
| Waist circumference [cm], median ± QD | 94.00 | 8.50 | |
| Abdominal obesity [WC ≥ 88 cm], n (%) | 392 | (64.16) | |
| Cigarette smokers, n (%) | 70 | (11.46) | |
| Alcohol consumption [≥3 units/day], n (%) | 4 | (0.65) | |
| Vitamin D3 [ng/mL], median ± QD | 7.49 | 2.76 | |
| BMD parameters | BMD FN [mg/cm2], median ± QD | 855.00 | 87.00 |
| BMD FN T-score, median ± QD | −1.30 | 0.60 | |
| BMD TH [mg/cm2], median ± QD | 942.00 | 92.50 | |
| BMD TH T-score, median ± QD | −0.50 | 0.75 | |
| Comorbidities | Diabetes mellitus type 1, n (%) | 22 | (3.60) |
| Diabetes mellitus type 2, n (%) | 95 | (15.55) | |
| Glucocorticosteroid therapy, n (%) | 28 | (4.58) | |
| Rheumatoid arthritis, n (%) | 40 | (6.55) | |
| Thyroid gland diseases, n (%) | 6 | (0.98) | |
| Chronic kidney disease, n (%) | 7 | (1.15) |
| SNP | Position | Genotypes | n (%) | Alleles | n (%) | HWE p Value |
|---|---|---|---|---|---|---|
| rs731236 (TaqI) | chr12:47844974 | AA | 249 (40.75) | A | 773 (63.26) | 0.750 |
| AG | 275 (45.01) | G | 449 (36.74) | |||
| GG | 87 (14.24) | |||||
| AA + AG | 524 (85.76) | |||||
| AG + GG | 362 (59.25) | |||||
| rs7975232 (ApaI) | chr12:47845054 | AA | 154 (25.20) | A | 613 (50.16) | 0.987 |
| AC | 305 (49.92) | C | 609 (49.84) | |||
| CC | 152 (24.88) | |||||
| AA + AC | 459 (75.12) | |||||
| AC + CC | 457 (74.80) | |||||
| rs1544410 (BsmI) | chr12:47846052 | CC | 244 (39.93) | C | 763 (62.44) | 0.686 |
| CT | 275 (45.01) | T | 459 (37.56) | |||
| TT | 92 (15.06) | |||||
| CC + CT | 519 (84.94) | |||||
| CT + TT | 367 (60.07) |
| SNP | Parameter | Median | ±QD | Median | ±QD | p Mann–Whitney U Test |
|---|---|---|---|---|---|---|
| rs731236 | GG | AA/AG | ||||
| BMD FN [mg/cm2], median ± QD | 893.00 | 85.50 | 850.00 | 85.25 | 0.028 | |
| BMD FN T-score, median ± QD | −1.00 | 0.60 | −1.30 | 0.63 | 0.033 | |
| Correlates | BMD FN [mg/cm2] | BMD FN T-Score | BMD TH [mg/cm2] | BMD TH T-Score | Age [Years] | Vit. D3 [ng/mL] | BMI [kg/m2] | WC [cm] |
|---|---|---|---|---|---|---|---|---|
| BMD FN [mg/cm2] | - | 0.98 | 0.86 | 0.85 | −0.37 | 0.16 | 0.28 | 0.26 |
| BMD FN T-score | 0.98 | - | 0.86 | 0.87 | −0.36 | 0.15 | 0.28 | 0.26 |
| BMD TH [mg/cm2] | 0.86 | 0.86 | - | 0.98 | −0.33 | 0.13 | 0.45 | 0.41 |
| BMD TH T-score | 0.85 | 0.87 | 0.98 | - | −0.32 | 0.14 | 0.44 | 0.39 |
| Age [years] | −0.37 | −0.36 | −0.33 | −0.32 | - | −0.25 | 0.12 | 0.13 |
| Vit. D3 [ng/mL] | 0.16 | 0.15 | 0.13 | 0.14 | −0.25 | - | NS | NS |
| BMI [kg/m2] | 0.28 | 0.28 | 0.45 | 0.44 | 0.12 | NS | - | 0.87 |
| WC [cm] | 0.26 | 0.26 | 0.41 | 0.39 | 0.13 | NS | 0.87 | - |
| Modifier | BMD FN [mg/cm2] | p Mann–Whitney U Test | BMD TH [mg/cm2] | p Mann–Whitney U Test | |||
|---|---|---|---|---|---|---|---|
| Median | QD | Median | QD | ||||
| Obesity [BMI ≥ 30] | Yes | 884.50 | 41.63 | 0.000 | 988.00 | 49.63 | 0.000 |
| No | 825.00 | 78.00 | 896.00 | 88.50 | |||
| Abdominal obesity [WC ≥ 88 cm] | Yes | 873.00 | 82.50 | 0.000 | 969.50 | 94.00 | 0.000 |
| No | 812.00 | 79.00 | 883.00 | 96.00 | |||
| DM type 1 | Yes | 887.00 | 87.00 | 0.215 | 1016.50 | 143.50 | 0.008 |
| No | 855.00 | 85.50 | 941.00 | 91.50 | |||
| DM type 2 | Yes | 880.00 | 88.00 | 0.117 | 984.50 | 86.00 | 0.000 |
| No | 853.00 | 84.50 | 928.00 | 94.50 | |||
| RA | Yes | 812.00 | 63.00 | 0.098 | 875.50 | 77.50 | 0.011 |
| No | 858.00 | 87.00 | 946.00 | 93.50 | |||
| Calcium supplementation | Yes | 837.00 | 90.00 | 0.034 | 883.00 | 103.50 | 0.000 |
| No | 859.50 | 84.75 | 949.50 | 91.75 | |||
| Vitamin D3 supplementation | Yes | 815.00 | 83.00 | 0.012 | 876.00 | 94.50 | 0.000 |
| No | 861.00 | 85.00 | 952.00 | 91.50 | |||
| Anti-osteoporotic therapy | Yes | 832.50 | 85.50 | 0.017 | 876.50 | 105.00 | 0.000 |
| No | 859.00 | 84.50 | 951.00 | 91.00 | |||
| History of fractures > 40 year | Yes | 845.00 | 84.75 | 0.029 | 927.50 | 92.00 | 0.017 |
| No | 859.00 | 84.00 | 950.00 | 98.50 | |||
| Modifier | AA | AG | GG | p χ2 Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Additive Model | Recessive/Dominant | ||
| Obesity [BMI ≥ 30] | Yes | 129 | 38.51 | 158 | 47.16 | 48 | 14.33 | 0.430 | 0.944 |
| No | 120 | 43.48 | 117 | 42.39 | 39 | 14.13 | |||
| Abdominal obesity [WC ≥ 88 cm] | Yes | 157 | 40.05 | 177 | 45.15 | 58 | 14.80 | 0.932 | 0.754 |
| No | 72 | 41.38 | 78 | 44.83 | 24 | 13.79 | |||
| DM type 1 | Yes | 9 | 40.91 | 12 | 54.55 | 1 | 4.55 | 0.374 | 0.185 |
| No | 240 | 40.75 | 263 | 44.65 | 86 | 14.60 | |||
| DM type 2 | Yes | 37 | 38.95 | 45 | 47.37 | 13 | 13.68 | 0.881 | 0.866 |
| No | 212 | 41.09 | 230 | 44.57 | 74 | 14.34 | |||
| RA | Yes | 17 | 42.50 | 17 | 42.50 | 6 | 15.00 | 0.947 | 0.887 |
| No | 232 | 40.63 | 258 | 45.18 | 81 | 14.19 | |||
| Calcium supplementation | Yes | 39 | 43.82 | 34 | 38.20 | 16 | 17.98 | 0.311 | 0.275 |
| No | 210 | 40.23 | 241 | 46.17 | 71 | 13.60 | |||
| Vitamin D3 supplementation | Yes | 34 | 43.04 | 33 | 41.77 | 12 | 15.19 | 0.825 | 0.795 |
| No | 215 | 40.41 | 242 | 45.49 | 75 | 14.10 | |||
| Anti-osteoporotic therapy | Yes | 42 | 45.65 | 34 | 36.96 | 16 | 17.39 | 0.228 | 0.348 |
| No | 207 | 39.88 | 241 | 46.44 | 71 | 13.68 | |||
| History of fractures > 40 year | Yes | 62 | 36.47 | 78 | 45.88 | 30 | 17.65 | 0.221 | 0.134 |
| No | 187 | 42.40 | 197 | 44.67 | 57 | 12.93 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górczyńska-Kosiorz, S.; Tabor, E.; Niemiec, P.; Pluskiewicz, W.; Gumprecht, J. Associations between the VDR Gene rs731236 (TaqI) Polymorphism and Bone Mineral Density in Postmenopausal Women from the RAC-OST-POL. Biomedicines 2024, 12, 917. https://doi.org/10.3390/biomedicines12040917
Górczyńska-Kosiorz S, Tabor E, Niemiec P, Pluskiewicz W, Gumprecht J. Associations between the VDR Gene rs731236 (TaqI) Polymorphism and Bone Mineral Density in Postmenopausal Women from the RAC-OST-POL. Biomedicines. 2024; 12(4):917. https://doi.org/10.3390/biomedicines12040917
Chicago/Turabian StyleGórczyńska-Kosiorz, Sylwia, Elżbieta Tabor, Paweł Niemiec, Wojciech Pluskiewicz, and Janusz Gumprecht. 2024. "Associations between the VDR Gene rs731236 (TaqI) Polymorphism and Bone Mineral Density in Postmenopausal Women from the RAC-OST-POL" Biomedicines 12, no. 4: 917. https://doi.org/10.3390/biomedicines12040917
APA StyleGórczyńska-Kosiorz, S., Tabor, E., Niemiec, P., Pluskiewicz, W., & Gumprecht, J. (2024). Associations between the VDR Gene rs731236 (TaqI) Polymorphism and Bone Mineral Density in Postmenopausal Women from the RAC-OST-POL. Biomedicines, 12(4), 917. https://doi.org/10.3390/biomedicines12040917







