Saliva as a Diagnostic Tool for Early Detection of Exercise-Induced Oxidative Damage in Female Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Blood and Saliva Sampling
2.3. Quantification of Lipid Peroxidation Products
2.4. Statistical Analysis
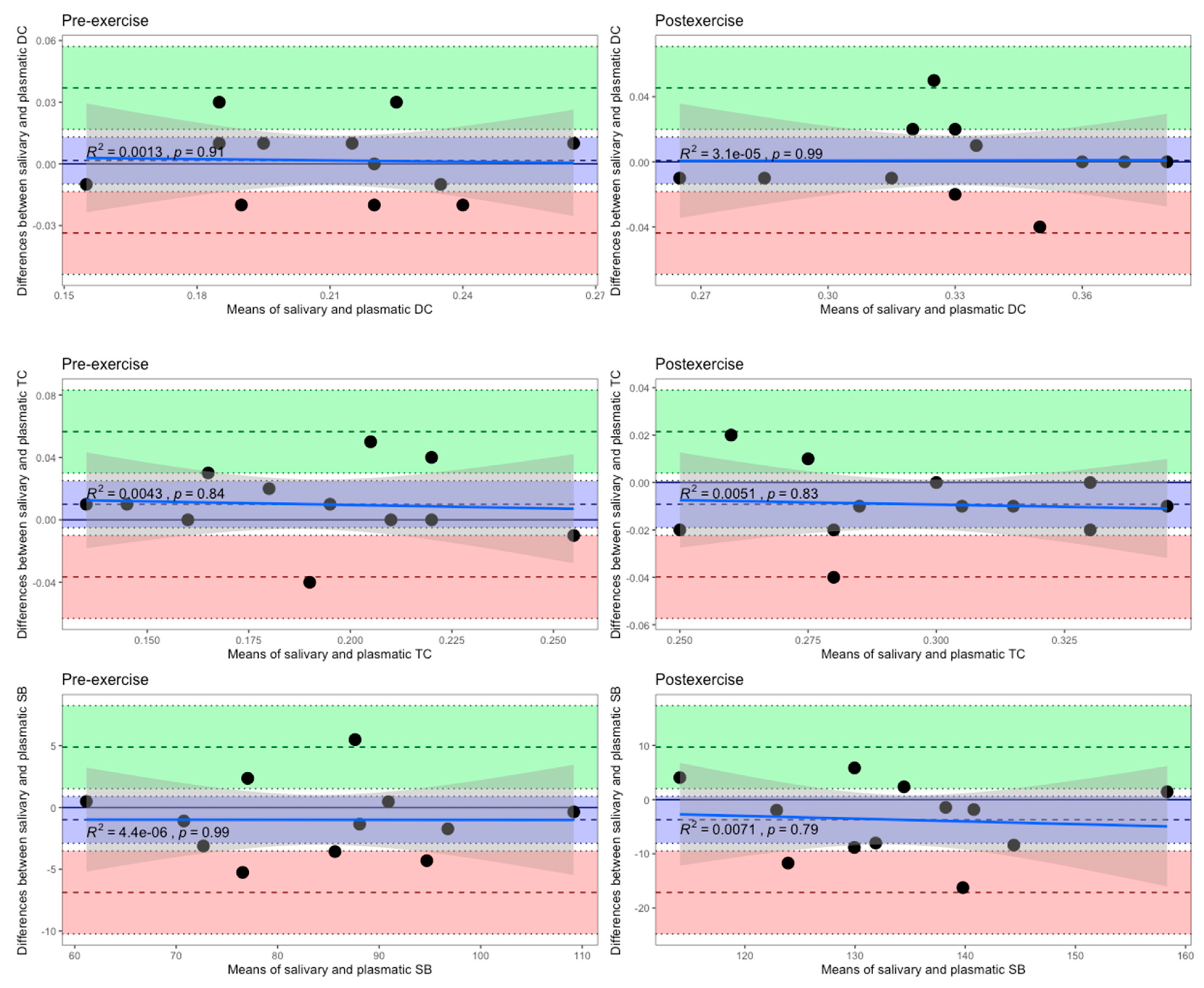
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Chen, Y.W.; Lip, G.Y.; Fisher, J.P.; Aldred, S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. 2016, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. 2011, 110, 730–737. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef]
- Ovchinnikov, A.N.; Paoli, A.; Seleznev, V.V.; Deryugina, A.V. Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise. J. Clin. Med. 2022, 11, 3098. [Google Scholar] [CrossRef]
- Sant’Anna, M.; Casimiro-Lopes, G.; Boaventura, G.; Marques, S.T.; Sorenson, M.M.; Simão, R.; Pinto, V.S. Anaerobic exercise affects the saliva antioxidant/oxidant balance in high performance pentathlon athletes. Hum. Mov. 2016, 17, 50–55. [Google Scholar] [CrossRef]
- Rodrigues de Araujo, V.; Lisboa, P.; Boaventura, G.; Caramez, F.; Pires, L.; Oliveira, E.; Moura, E.; Casimiro-Lopes, G. Acute high-intensity exercise test in soccer athletes affects salivary biochemical markers. Free Radic. Res. 2018, 52, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.V.; Giolo, J.S.; Teixeira, R.R.; Vilela, D.D.; Peixoto, L.G.; Justino, A.B.; Caixeta, D.C.; Puga, G.M.; Espindola, F.S. Salivary and Plasmatic Antioxidant Profile following Continuous, Resistance, and High-Intensity Interval Exercise: Preliminary Study. Oxid. Med. Cell. Longev. 2019, 2019, 5425021. [Google Scholar] [CrossRef]
- Ovchinnikov, A.N.; Paoli, A.; Seleznev, V.V.; Deryugina, A.V. Royal jelly plus coenzyme Q10 supplementation improves high-intensity interval exercise performance via changes in plasmatic and salivary biomarkers of oxidative stress and muscle damage in swimmers: A randomized, double-blind, placebo-controlled pilot trial. J. Int. Soc. Sports Nutr. 2022, 19, 239–257. [Google Scholar]
- Alves, R.C.C.; Ferreira, R.O.; Frazão, D.R.; de Souza Né, Y.G.; Mendes, P.F.S.; Marañón-Vásquez, G.; Royes, L.F.F.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. The Relationship between Exercise and Salivary Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Costello, J.T. Realising the Potential of Urine and Saliva as Diagnostic Tools in Sport and Exercise Medicine. Sports Med. 2017, 47, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Seljeskog, E.; Hervig, T.; Mansoor, M.A. A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin. Biochem. 2006, 39, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free. Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, H.F.; Reid, R.G.; Yousef, S.; Boyle, S.P. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J. Lipid Res. 2013, 54, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. S1), 10–23. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Il’yasova, D.; Morrow, J.D.; Ivanova, A.; Wagenknecht, L.E. Epidemiological marker for oxidant status: Comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann. Epidemiol. 2004, 14, 793–797. [Google Scholar] [CrossRef]
- Soffler, C.; Campbell, V.L.; Hassel, D.M. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation: A comparison of enzyme immunoassays with gas chromatography-mass spectrometry in domestic animal species. J. Vet. Diagn. Investig. 2010, 22, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Suchy, M.T. Assessment of urinary F2-isoprostanes in experimental and clinical studies: Mass spectrometry versus ELISA. Hypertension 2012, 60, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Quantitative analysis of biomarkers, drugs and toxins in biological samples by immunoaffinity chromatography coupled to mass spectrometry or tandem mass spectrometry: A focused review of recent applications. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 133–148. [Google Scholar] [CrossRef]
- Dimova, M.; Tugai, A.; Tugai, T.; Iutynska, G.; Dordevic, D.; Kushkevych, I. Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. Int. J. Mol. Sci. 2022, 23, 11415. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Endogenous Intoxication and Saliva Lipid Peroxidation in Patients with Lung Cancer. Diagnostics 2016, 6, 39. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Corongiu, F.P.; Banni, S. Detection of conjugated dienes by second derivative ultraviolet spectrophotometry. Methods Enzymol. 1994, 233, 303–310. [Google Scholar]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-Hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; González-Sánchez, M.; Díaz-Del Cerro, E.; Valera, G.; Carracedo, J.; Guerra-Pérez, N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech. Ageing Dev. 2023, 211, 111797. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.N.; Deryugina, A.V.; Paoli, A. Salivary Concentrations of Lipid Peroxidation Products Represent Plasma Concentrations in Athletes before and after Exercise. Med. Sci. Sports Exerc. 2022, 54 (Suppl. S9), 436. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Volchegorskiĭ, I.A.; Nalimov, A.G.; Iarovinskiĭ, B.G.; Lifshits, R.I. Comparison of various approaches to the determination of the products of lipid peroxidation in heptane-isopropanol extracts of blood. Vopr. Meditsinskoi Khimii 1989, 35, 127–131. [Google Scholar]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases–Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary Biomarkers of Oxidative Stress and Inflammation in Stroke Patients: From Basic Research to Clinical Practice. Oxid. Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef] [PubMed]
- Veljovic, T.; Djuric, M.; Mirnic, J.; Gusic, I.; Maletin, A.; Ramic, B.; Neskovic, I.; Vukoje, K.; Brkic, S. Lipid Peroxidation Levels in Saliva and Plasma of Patients Suffering from Periodontitis. J. Clin. Med. 2022, 11, 3617. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Jamurtas, A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef]
- Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef]
- Čižmárová, B.; Tomečková, V.; Hubková, B.; Hurajtová, A.; Ohlasová, J.; Birková, A. Salivary Redox Homeostasis in Human Health and Disease. Int. J. Mol. Sci. 2022, 23, 10076. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Karowicz-Bilinska, A.; Plodzidym, M.; Krol, J.; Lewinska, A.; Bartosz, G. Changes of markers of oxidative stress during menstrual cycle. Redox Rep. 2008, 13, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Beckendorf, L.; Linke, W.A. Emerging importance of oxidative stress in regulating striated muscle elasticity. J. Muscle Res. Cell Motil. 2015, 36, 25–36. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic. Biol. Med. 2013, 65, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Ciamporcero, E.S.; Daga, M.; Ullio, C.; Arcaro, A.; Cetrangolo, G.P.; Ferretti, C.; Dianzani, C.; Lepore, A.; et al. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid. Redox Signal. 2015, 22, 1681–1702. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57 (Suppl. S5), 715S–725S. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Schaur, R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]

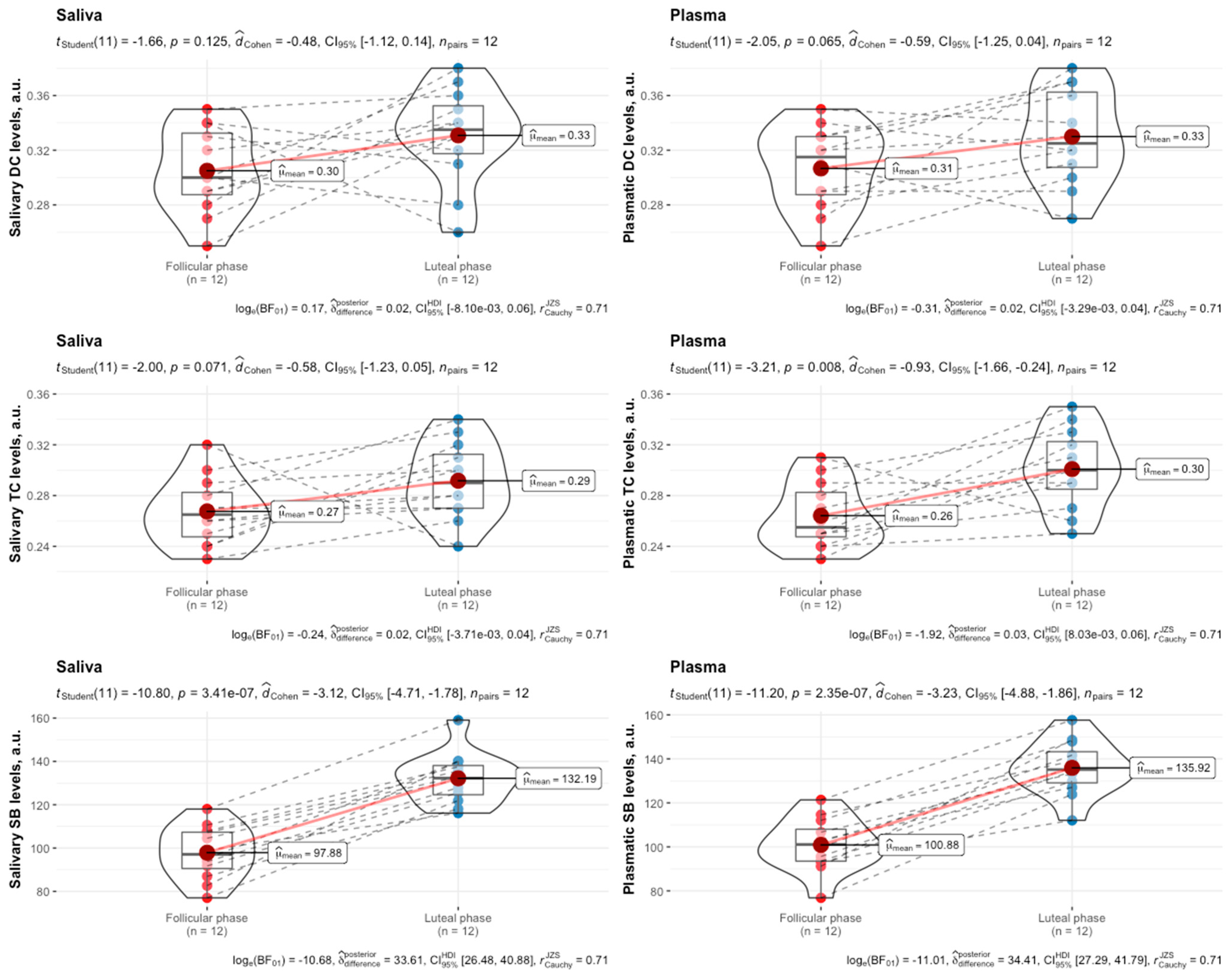


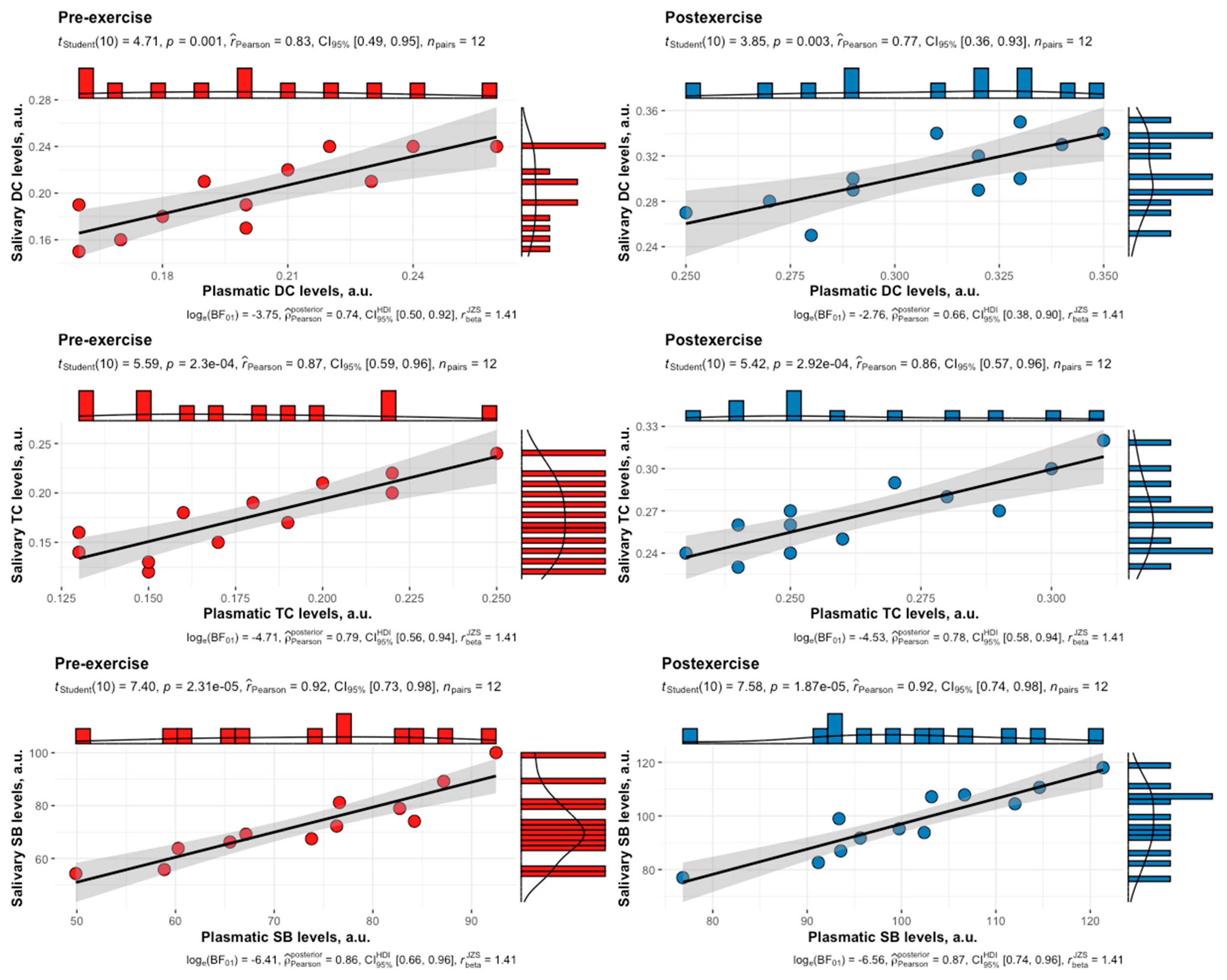
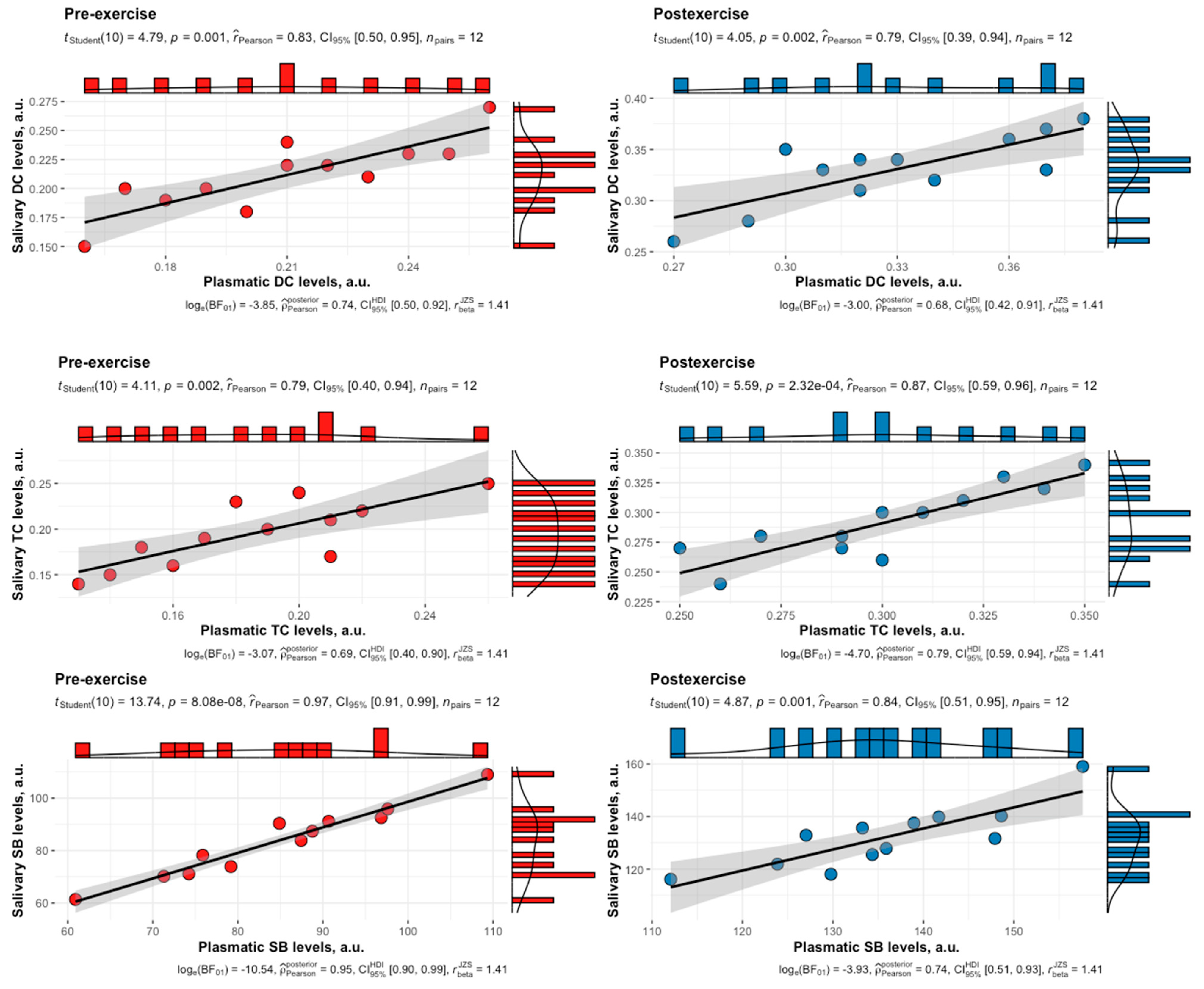
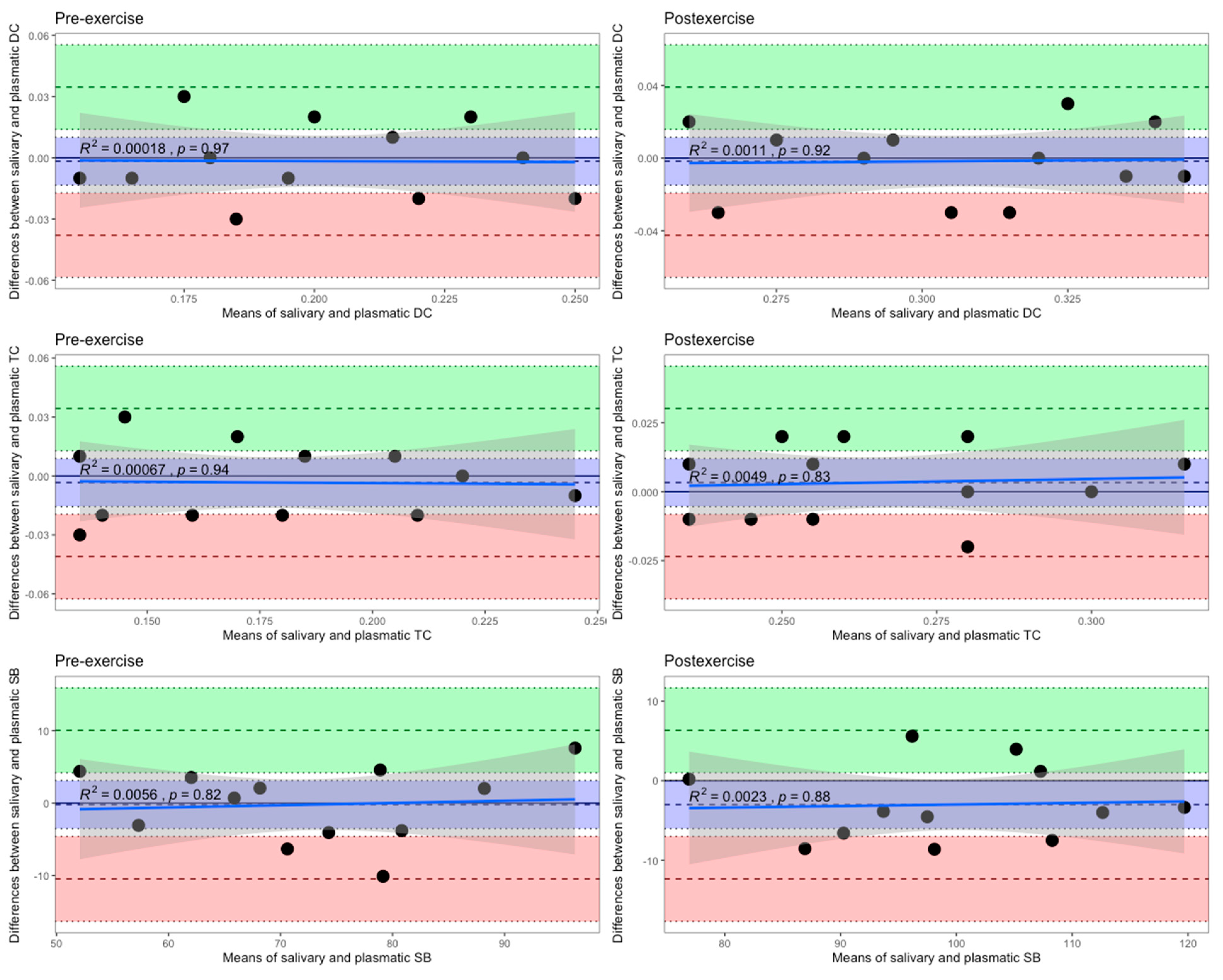
| Follicular Phase | Luteal Phase | |
|---|---|---|
| FINA points, a.u. | 452.67 ± 31.85 | 454.25 ± 28.83 |
| LPO Product | Phase | Time Point | r | a [95% CI] | b [95% CI] |
|---|---|---|---|---|---|
| DC | F | Pre | 0.83 | −0.0002 [−0.08, 0.08] | 0.99 [0.68, 1.46] |
| DC | L | Pre | 0.83 | 0.006 [−0.07, 0.09] | 0.98 [0.67, 1.43] |
| DC | F | Post | 0.77 | −0.008 [−0.15, 0.13] | 1.02 [0.66, 1.57] |
| DC | L | Post | 0.79 | −0.0003 [−0.14, 0.14] | 1.00 [0.66, 1.53] |
| TC | F | Pre | 0.87 | −0.001 [−0.06, 0.06] | 0.99 [0.70, 1.39] |
| TC | L | Pre | 0.79 | 0.02 [−0.06, 0.10] | 0.96 [0.63, 1.46] |
| TC | F | Post | 0.86 | −0.006 [−0.10, 0.09] | 1.04 [0.73, 1.47] |
| TC | L | Post | 0.87 | 0.001 [−0.10, 0.10] | 0.97 [0.69, 1.36] |
| SB | F | Pre | 0.92 | −2.38 [−23.48, 18.71] | 1.03 [0.78, 1.35] |
| SB | L | Pre | 0.97 | −0.96 [−14.49, 12.58] | 1.00 [0.85, 1.17] |
| SB | F | Post | 0.92 | −4.91 [−32.97, 23.15] | 1.02 [0.78, 1.33] |
| SB | L | Post | 0.84 | 2.41 [−47.61, 52.43] | 0.95 [0.66, 1.39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovchinnikov, A.N.; Paoli, A. Saliva as a Diagnostic Tool for Early Detection of Exercise-Induced Oxidative Damage in Female Athletes. Biomedicines 2024, 12, 1006. https://doi.org/10.3390/biomedicines12051006
Ovchinnikov AN, Paoli A. Saliva as a Diagnostic Tool for Early Detection of Exercise-Induced Oxidative Damage in Female Athletes. Biomedicines. 2024; 12(5):1006. https://doi.org/10.3390/biomedicines12051006
Chicago/Turabian StyleOvchinnikov, Aleksandr N., and Antonio Paoli. 2024. "Saliva as a Diagnostic Tool for Early Detection of Exercise-Induced Oxidative Damage in Female Athletes" Biomedicines 12, no. 5: 1006. https://doi.org/10.3390/biomedicines12051006
APA StyleOvchinnikov, A. N., & Paoli, A. (2024). Saliva as a Diagnostic Tool for Early Detection of Exercise-Induced Oxidative Damage in Female Athletes. Biomedicines, 12(5), 1006. https://doi.org/10.3390/biomedicines12051006










