Physiological Consequences of Nonsense-Mediated Decay and Its Role in Adaptive Responses
Abstract
1. Introduction
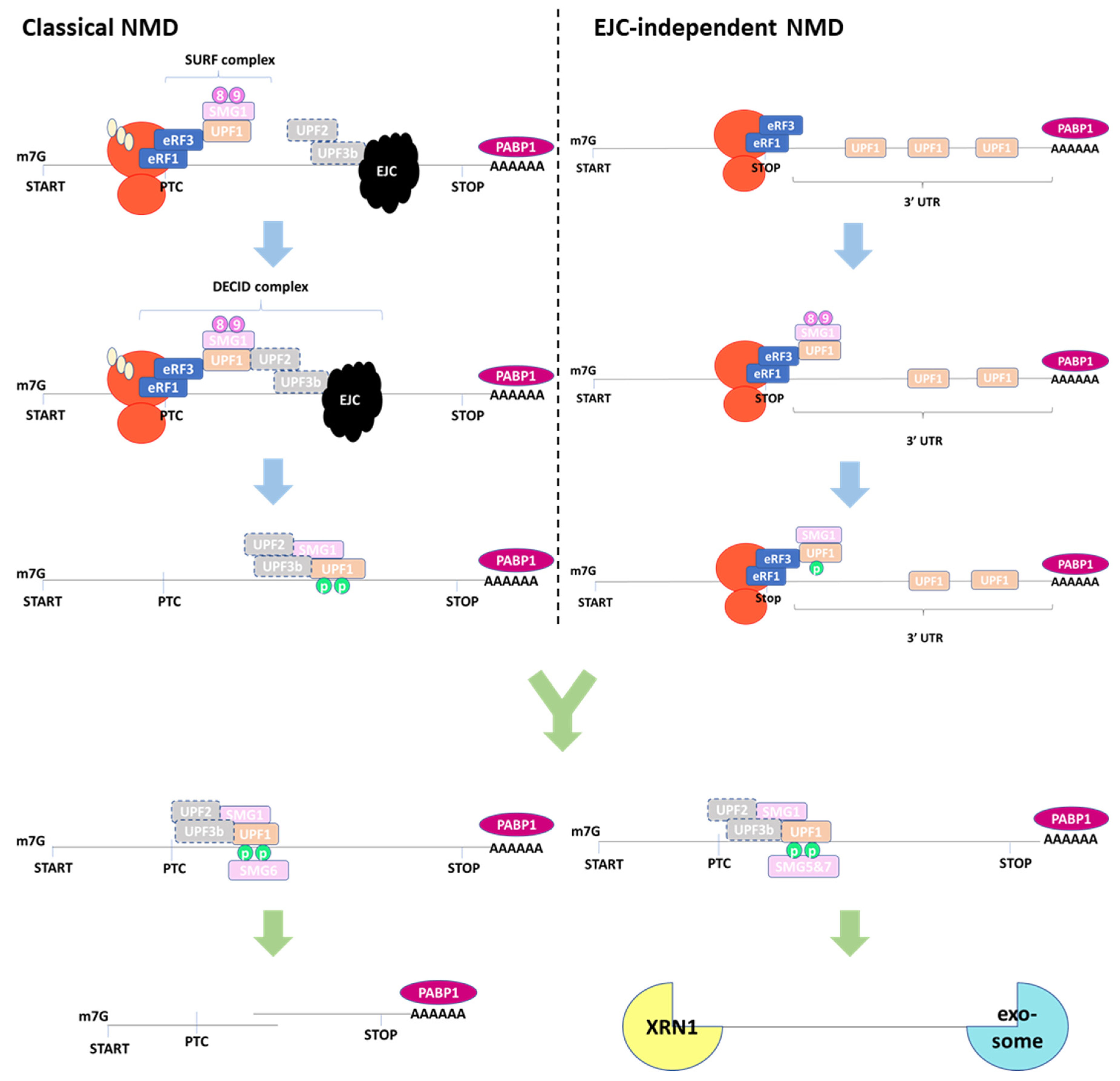
2. NMD Pathways
2.1. EJC-Dependent NMD
2.2. EJC-Independent NMD
2.3. AS-NMD
3. Tissue-Specific NMD Efficiency Variation
4. Physiological Consequences of NMD
4.1. Growth and Development
4.2. Disease
4.3. Stress Responses
4.4. Longevity
5. Genetic Tools for Studying NMD and the Physiological Outcomes
5.1. Genetic Techniques
5.2. Genetic Models
6. Perspectives and Concluding Remarks
Funding
Conflicts of Interest
References
- Hug, N.; Longman, D.; Cáceres, J.F. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 2016, 44, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.M.; Singh, G.; Lykke-Andersen, J. Upf1 ATPase-Dependent mRNP Disassembly Is Required for Completion of Nonsense- Mediated mRNA Decay. Cell 2010, 143, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Li, W.; Hoque, M.; Popp, M.W.-L.; Ermolenko, D.N.; Tian, B.; Maquat, L.E. A post-translational regulatory switch on UPF1 controls targeted mRNA degradation. Genes Dev. 2014, 28, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wilkinson, M.F. Regulation of nonsense-mediated mRNA decay. Wiley Interdiscip. Rev. RNA 2012, 3, 807–828. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.P.B.; Davies, B. SMG1 is an ancient nonsense-mediated mRNA decay effector. Plant J. 2013, 76, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Popp, M.W.; Maquat, L.E. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat. Rev. Mol. Cell Biol. 2019, 20, 406–420, Correction in Nat. Rev. Mol. Cell Biol. 2019, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Izumi, N.; Kashima, I.; Ohnishi, T.; Saari, B.; Katsuhata, Y.; Muramatsu, R.; Morita, T.; Iwamatsu, A.; Hachiya, T.; et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 2009, 23, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.B.; Lykke-Andersen, S.; Mühlemann, O.; Jensen, T.H. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat. Struct. Mol. Biol. 2008, 16, 49–55. [Google Scholar] [CrossRef]
- Anders, K.R.; Grimson, A.; Anderson, P. SMG-5, required for C.elegans nonsense-mediated mRNA decay, associates with SMG-2 and protein phosphatase 2A. EMBO J. 2003, 22, 641–650. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Serin, G.; Ohara, O.; Maquat, L.E. Characterization of human Smg5/7a: A protein with similarities to Caenorhabditis elegans SMG5 and SMG7 that functions in the dephosphorylation of Upf1. RNA 2003, 9, 77–87. [Google Scholar] [CrossRef]
- Ohnishi, T.; Yamashita, A.; Kashima, I.; Schell, T.; Anders, K.R.; Grimson, A.; Hachiya, T.; Hentze, M.W.; Anderson, P.; Ohno, S. Phosphorylation of hUPF1 Induces Formation of mRNA Surveillance Complexes Containing hSMG-5 and hSMG-7. Mol. Cell 2003, 12, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Okada-Katsuhata, Y.; Yamashita, A.; Kutsuzawa, K.; Izumi, N.; Hirahara, F.; Ohno, S. N- and C-terminal Upf1 phosphorylations create binding platforms for SMG-6 and SMG-5:SMG-7 during NMD. Nucleic Acids Res. 2011, 40, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Bonneau, F.; Schüssler, S.; Eppinger, E.; Conti, E. Phospho-dependent and phospho-independent interactions of the helicase UPF1 with the NMD factors SMG5–SMG7 and SMG6. Nucleic Acids Res. 2014, 42, 9447–9460. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Karousis, E.D.; Bourquin, J.; Bruggmann, R.; Mühlemann, O. Transcriptome-wide identification of NMD-targeted human mRNAs reveals extensive redundancy between SMG6- and SMG7-mediated degradation pathways. RNA 2016, 23, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Malabat, C.; Feuerbach, F.; Ma, L.; Saveanu, C.; Jacquier, A. Quality control of transcription start site selection by nonsense-mediated-mRNA decay. eLife 2015, 4, e06722. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Shaffer, D.; Snow, S.S.; Kapahi, P.; Rogers, A.N. Dietary restriction induces posttranscriptional regulation of longevity genes. Life Sci. Alliance 2019, 2, e201800281. [Google Scholar] [CrossRef] [PubMed]
- Riehs, N.; Akimcheva, S.; Puizina, J.; Bulankova, P.; Idol, R.A.; Siroky, J.; Schleiffer, A.; Schweizer, D.; Shippen, D.E.; Riha, K. ArabidopsisSMG7 protein is required for exit from meiosis. J. Cell Sci. 2008, 121, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Maquat, L.E. UPFront and center in RNA decay: UPF1 in nonsense-mediated mRNA decay and beyond. RNA 2019, 25, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Redon, S.; Raftopoulou, C.; Wischnewski, H.; Gagos, S.; Azzalin, C.M. Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. EMBO J. 2011, 30, 4047–4058. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Lingner, J. The Double Life of UPF1 in RNA and DNA Stability Pathways. Cell Cycle 2006, 5, 1496–1498. [Google Scholar] [CrossRef]
- Gewandter, J.S.; Bambara, R.A.; O’reilly, M.A. The RNA surveillance protein SMG1 activates p53 in response to DNA double-strand breaks but not exogenously oxidized mRNA. Cell Cycle 2011, 10, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.E.; Erdmann, N.; Cruickshank, J.; Goldman, H.; Gill, R.; Robinson, M.O.; Harrington, L. Functional Conservation of the Telomerase Protein Est1p in Humans. Curr. Biol. 2003, 13, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, P.; Höss, M.; Azzalin, C.M.; Nabholz, M.; Bucher, P.; Lingner, J. A Human Homolog of Yeast Est1 Associates with Telomerase and Uncaps Chromosome Ends When Overexpressed. Curr. Biol. 2003, 13, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.J.; Séraphin, B. Genomic-scale quantitative analysis of yeast pre-mRNA splicing: Implications for splice-site recognition. RNA 1999, 5, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Bühler, M.; Steiner, S.; Mohn, F.; Paillusson, A.; Mühlemann, O. EJC-independent degradation of nonsense immunoglobulin-μ mRNA depends on 3′ UTR length. Nat. Struct. Mol. Biol. 2006, 13, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Imamachi, N.; Salam, K.A.; Suzuki, Y.; Akimitsu, N. A GC-rich sequence feature in the 3′ UTR directs UPF1-dependent mRNA decay in mammalian cells. Genome Res. 2016, 27, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Zünd, D.; Gruber, A.R.; Zavolan, M.; Mühlemann, O. Translation-dependent displacement of UPF1 from coding sequences causes its enrichment in 3′ UTRs. Nat. Struct. Mol. Biol. 2013, 20, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Soler, S.; Lambert, N.J.; Zahler, A.M. Global analysis of alternative splicing uncovers developmental regulation of nonsense-mediated decay in C. elegans. RNA 2009, 15, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Filichkin, S.A.; Priest, H.D.; Givan, S.A.; Shen, R.; Bryant, D.W.; Fox, S.E.; Wong, W.-K.; Mockler, T.C. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res. 2009, 20, 45–58. [Google Scholar] [CrossRef]
- Hansen, K.D.; Lareau, L.F.; Blanchette, M.; Green, R.E.; Meng, Q.; Rehwinkel, J.; Gallusser, F.L.; Izaurralde, E.; Rio, D.C.; Dudoit, S.; et al. Genome-Wide Identification of Alternative Splice Forms Down-Regulated by Nonsense-Mediated mRNA Decay in Drosophila. PLoS Genet. 2009, 5, e1000525. [Google Scholar] [CrossRef]
- Ni, J.Z.; Grate, L.; Donohue, J.P.; Preston, C.; Nobida, N.; O’brien, G.; Shiue, L.; Clark, T.A.; Blume, J.E.; Ares, M. Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 2007, 21, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Harris, K.S.; Roth, M.B. smg mutants affect the expression of alternatively spliced SR protein mRNAs in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1997, 94, 9782–9785. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Green, R.E.; Brenner, S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA 2002, 100, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ottens, F.; Gehring, N.H. Physiological and pathophysiological role of nonsense-mediated mRNA decay. Pflügers Arch. Eur. J. Physiol. 2016, 468, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- McGlincy, N.J.; Smith, C.W. Alternative splicing resulting in nonsense-mediated mRNA decay: What is the meaning of nonsense? Trends Biochem. Sci. 2008, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wollerton, M.C.; Gooding, C.; Wagner, E.J.; Garcia-Blanco, M.A.; Smith, C.W. Autoregulation of Polypyrimidine Tract Binding Protein by Alternative Splicing Leading to Nonsense-Mediated Decay. Mol. Cell 2004, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sureau, A.; Gattoni, R.; Dooghe, Y.; Stévenin, J.; Soret, J. SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. EMBO J. 2001, 20, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Jumaa, H.; Nielsen, P.J. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J. 1997, 16, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Mitrovich, Q.M.; Anderson, P. Unproductively spliced ribosomal protein mRNAs are natural targets of mRNA surveillance in C. elegans. Genes Dev. 2000, 14, 2173–2184. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, Y.; Han, W.; Song, F.; Shen, X.; Zhao, J.; Zuo, J.; Saffen, D.; Chen, W.; Wang, Z.; et al. Autoregulation of RBM10 and cross-regulation of RBM10/RBM5 via alternative splicing-coupled nonsense-mediated decay. Nucleic Acids Res. 2017, 45, 8524–8540. [Google Scholar] [CrossRef]
- Jangi, M.; Boutz, P.L.; Paul, P.; Sharp, P.A. Rbfox2 controls autoregulation in RNA-binding protein networks. Genes Dev. 2014, 28, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.F.; Freddi, S.; Nattrass, G.; Savarirayan, R. Tissue-specific RNA surveillance? Nonsense-mediated mRNA decay causes collagen X haploinsufficiency in Schmid metaphyseal chondrodysplasia cartilage. Hum. Mol. Genet. 2003, 12, 217–225. [Google Scholar] [CrossRef]
- Linde, L.; Boelz, S.; Neu-Yilik, G.; Kulozik, A.E.; Kerem, B. The efficiency of nonsense-mediated mRNA decay is an inherent character and varies among different cells. Eur. J. Hum. Genet. 2007, 15, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Singer, R.H.; Sato, H.; Singer, R.H. Cellular variability of nonsense-mediated mRNA decay. Nat. Commun. 2021, 12, 7203. [Google Scholar] [CrossRef]
- Zetoune, A.B.; Fontanière, S.; Magnin, D.; Anczuków, O.; Buisson, M.; Zhang, C.X.; Mazoyer, S. Comparison of nonsense-mediated mRNA decay efficiency in various murine tissues. BMC Genet. 2008, 9, 83. [Google Scholar] [CrossRef]
- Watts, J.S.; Harrison, H.F.; Omi, S.; Guenthers, Q.; Dalelio, J.; Pujol, N.; Watts, J.L. New Strains for Tissue-Specific RNAi Studies in Caenorhabditis elegans. G3 Genes|Genomes|Genetics 2020, 10, 4167–4176. [Google Scholar] [CrossRef]
- Taylor, R.; Kebaara, B.W.; Nazarenus, T.; Jones, A.; Yamanaka, R.; Uhrenholdt, R.; Wendler, J.P.; Atkin, A.L. Gene Set Coregulated by the Saccharomyces cerevisiae Nonsense-Mediated mRNA Decay Pathway. Eukaryot. Cell 2005, 4, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Altamura, N.; Groudinsky, O.; Dujardin, G.; Slonimski, P.P. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J. Mol. Biol. 1992, 224, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Kebaara, B.W.; Atkin, A.L. Long 3′-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Res. 2009, 37, 2771–2778. [Google Scholar] [CrossRef]
- Wang, X.; Okonkwo, O.; Kebaara, B.W. Physiological basis of copper tolerance of Saccharomyces cerevisiae nonsense-mediated mRNA decay mutants. Yeast 2013, 30, 179–190. [Google Scholar] [CrossRef]
- Johansson, M.J.; Jacobson, A. Nonsense-mediated mRNA decay maintains translational fidelity by limiting magnesium uptake. Genes Dev. 2010, 24, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Leeds, P.; Wood, J.M.; Lee, B.S.; Culbertson, M.R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, J.; Papp, A.; Pulak, R.; Ambros, V.; Anderson, P. A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics 1989, 123, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Pulak, R.; Anderson, P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993, 7, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.E.; Son, H.G.; Park, H.-E.H.; Jung, Y.; Kwon, S.; Lee, S.-J.V. Caenorhabditis elegans algn-2 Is Critical for Longevity Conferred by Enhanced Nonsense-Mediated mRNA Decay. iScience 2020, 23, 101713. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, K.; Yoshina, S.; Shen, X.; Han, J.; DeSantis, M.R.; Xiong, M.; Mitani, S.; Kaufman, R.J. RNA surveillance is required for endoplasmic reticulum homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 8079–8084. [Google Scholar] [CrossRef] [PubMed]
- Son, H.G.; Seo, M.; Ham, S.; Hwang, W.; Lee, D.; An, S.W.A.; Artan, M.; Seo, K.; Kaletsky, R.; Arey, R.N.; et al. RNA surveillance via nonsense-mediated mRNA decay is crucial for longevity in daf-2/insulin/IGF-1 mutant C. elegans. Nat. Commun. 2017, 8, 14749. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.S.; Sharma, R.D.; Jain, V.; Siddiqui, A.A.; Mukhopadhyay, A. Differential alternative splicing coupled to nonsense-mediated decay of mRNA ensures dietary restriction-induced longevity. Nat. Commun. 2017, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Metzstein, M.M.; Krasnow, M.A. Functions of the Nonsense-Mediated mRNA Decay Pathway in Drosophila Development. PLoS Genet. 2006, 2, e180. [Google Scholar] [CrossRef]
- Wittkopp, N.; Huntzinger, E.; Weiler, C.; Saulière, J.; Schmidt, S.; Sonawane, M.; Izaurralde, E. Nonsense-Mediated mRNA Decay Effectors Are Essential for Zebrafish Embryonic Development and Survival. Mol. Cell. Biol. 2009, 29, 3517–3528. [Google Scholar] [CrossRef]
- Medghalchi, S.M. Rent1, a trans-effector of nonsense-mediated mRNA decay, is essential for mammalian embryonic viability. Hum. Mol. Genet. 2001, 10, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, Y.; Wang, P.; Guachalla, L.M.; Sun, B.; Joerss, T.; Chen, Y.; Groth, M.; Krueger, A.; Platzer, M.; et al. Smg6/Est1 licenses embryonic stem cell differentiation via nonsense-mediated mRNA decay. EMBO J. 2015, 34, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Pan, Q.; Reilly, P.T.; Elia, A.J.; McCracken, S.; Wakeham, A.C.; Itie-Youten, A.; Blencowe, B.J.; Mak, T.W. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc. Natl. Acad. Sci. USA 2010, 107, 12186–12191. [Google Scholar] [CrossRef] [PubMed]
- Weischenfeldt, J.; Damgaard, I.; Bryder, D.; Theilgaard-Mönch, K.; Thoren, L.A.; Nielsen, F.C.; Jacobsen, S.E.W.; Nerlov, C.; Porse, B.T. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. 2008, 22, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Tang, C.; Yuan, S.; Porse, B.T.; Yan, W. UPF2, a nonsense-mediated mRNA decay factor, is required for prepubertal Sertoli cell development and male fertility by ensuring fidelity of the transcriptome. Development 2015, 142, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.B. Hypoxic Inhibition of Nonsense-Mediated RNA Decay Regulates Gene Expression and the Integrated Stress Response. Mol. Cell. Biol. 2008, 28, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Anazi, S.; Ben-Omran, T.; Seidahmed, M.Z.; Caddle, L.B.; Palmer, K.; Ali, R.; Alshidi, T.; Hagos, S.; Goodwin, L.; et al. Mutations in SMG9, Encoding an Essential Component of Nonsense-Mediated Decay Machinery, Cause a Multiple Congenital Anomaly Syndrome in Humans and Mice. Am. J. Hum. Genet. 2016, 98, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Rahikkala, E.; Urpa, L.; Ghimire, B.; Topa, H.; Kurki, M.I.; Koskela, M.; Airavaara, M.; Hämäläinen, E.; Pylkäs, K.; Körkkö, J.; et al. A novel variant in SMG9 causes intellectual disability, confirming a role for nonsense-mediated decay components in neurocognitive development. Eur. J. Hum. Genet. 2022, 30, 619–627. [Google Scholar] [CrossRef]
- Lou, C.-H.; Chousal, J.; Goetz, A.; Shum, E.Y.; Brafman, D.; Liao, X.; Mora-Castilla, S.; Ramaiah, M.; Cook-Andersen, H.; Laurent, L.; et al. Nonsense-Mediated RNA Decay Influences Human Embryonic Stem Cell Fate. Stem Cell Rep. 2016, 6, 844–857. [Google Scholar] [CrossRef]
- Jolly, L.A.; Homan, C.C.; Jacob, R.; Barry, S.; Gecz, J. The UPF3B gene, implicated in intellectual disability, autism, ADHD and childhood onset schizophrenia regulates neural progenitor cell behaviour and neuronal outgrowth. Hum. Mol. Genet. 2013, 22, 4673–4687. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Raymond, F.L.; Nguyen, L.S.; Rodriguez, J.; Hackett, A.; Vandeleur, L.; Smith, R.; Shoubridge, C.; Edkins, S.; Stevens, C.; et al. Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation. Nat. Genet. 2007, 39, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Kim, H.-G.; Rosenfeld, J.A.; Shen, Y.; Gusella, J.F.; Lacassie, Y.; Layman, L.C.; Shaffer, L.G.; Gécz, J. Contribution of copy number variants involving nonsense-mediated mRNA decay pathway genes to neuro-developmental disorders. Hum. Mol. Genet. 2013, 22, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Jolly, L.; Shoubridge, C.; Chan, W.K.; Huang, L.; Laumonnier, F.; Raynaud, M.; Hackett, A.; Field, M.; Rodriguez, J.; et al. Transcriptome profiling of UPF3B/NMD-deficient lymphoblastoid cells from patients with various forms of intellectual disability. Mol. Psychiatry 2011, 17, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.T.; Sharifi, N.A.; Meyers, J.L.; Martinez-Murillo, F.; Dietz, H.C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 2004, 36, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, G.; Horvath, P.; Schweingruber, C.; Zünd, D.; McInerney, G.; Merits, A.; Mühlemann, O.; Azzalin, C.; Helenius, A. The Host Nonsense-Mediated mRNA Decay Pathway Restricts Mammalian RNA Virus Replication. Cell Host Microbe 2014, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.R.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.-H.; Albert, P.S.; et al. Genetic regulation of OAS1 nonsense-mediated decay underlies association with COVID-19 hospitalization in patients of European and African ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.E.; Wilkinson, M. Stress and the nonsense-mediated RNA decay pathway. Cell. Mol. Life Sci. 2017, 74, 3509–3531. [Google Scholar] [CrossRef]
- Popp, M.W.; Maquat, L.E. Nonsense-mediated mRNA Decay and Cancer. Curr. Opin. Genet. Dev. 2017, 48, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Han, Z.; Teng, Z.; Jin, C.; Yuan, H.; Zhang, S.; Sun, K.; Wang, Y. Downregulated RBM5 Enhances CARM1 Expression and Activates the PRKACA/GSK3β Signaling Pathway through Alternative Splicing-Coupled Nonsense-Mediated Decay. Cancers 2023, 16, 139. [Google Scholar] [CrossRef]
- Kuzmiak, H.A.; Maquat, L.E. Applying nonsense-mediated mRNA decay research to the clinic: Progress and challenges. Trends Mol. Med. 2006, 12, 306–316. [Google Scholar] [CrossRef]
- Nasif, S.; Contu, L.; Muhlemann, O. Beyond quality control: The role of nonsense-mediated mRNA decay (NMD) in regulating gene expression. Semin. Cell Dev. Biol. 2018, 75, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kõks, S.; Keermann, M.; Reimann, E.; Prans, E.; Abram, K.; Silm, H.; Kõks, G.; Kingo, K. Psoriasis-Specific RNA Isoforms Identified by RNA-Seq Analysis of 173,446 Transcripts. Front. Med. 2016, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Karam, R.; Lou, C.; Kroeger, H.; Huang, L.; Lin, J.H.; Wilkinson, M.F. The unfolded protein response is shaped by the NMD pathway. Embo Rep. 2015, 16, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Fujimura, M.; Yamashita, A. Endoplasmic reticulum stress preconditioning attenuates methylmercury-induced cellular damage by inducing favorable stress responses. Sci. Rep. 2013, 3, 2346. [Google Scholar] [CrossRef] [PubMed]
- Wengrod, J.; Martin, L.; Wang, D.; Frischmeyer-Guerrerio, P.; Dietz, H.C.; Gardner, L.B. Inhibition of Nonsense-Mediated RNA Decay Activates Autophagy. Mol. Cell. Biol. 2013, 33, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Horrocks, J.; Mir, D.A.; Cox, M.; Ruzga, M.; Rollins, J.; Rogers, A.N. The integrated stress response protects against ER stress but is not required for altered translation and lifespan from dietary restriction in Caenorhabditis elegans. Front. Cell Dev. Biol. 2023, 11, 1263344. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.S.; Li, S.; Wilkinson, M.F. A splicing-dependent regulatory mechanism that detects translation signals. EMBO J. 1996, 15, 5965–5975. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Longman, D.; Plasterk, R.H.; Johnstone, I.L.; Cáceres, J.F. Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway. Genes Dev. 2007, 21, 1075–1085. [Google Scholar] [CrossRef]
- Domeier, M.E.; Morse, D.P.; Knight, S.W.; Portereiko, M.; Bass, B.L.; Mango, S.E. A Link Between RNA Interference and Nonsense-Mediated Decay in Caenorhabditis elegans. Science 2000, 289, 1928–1930. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Harel, I.; Brunet, A. The African Turquoise Killifish: A Model for Exploring Vertebrate Aging and Diseases in the Fast Lane. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gil Nam, H.; Valenzano, D.R. The short-lived African turquoise killifish: An emerging experimental model for ageing. Dis. Model. Mech. 2016, 9, 115–129. [Google Scholar] [CrossRef] [PubMed]

| Phenotypes | Species | In Vitro or In Vivo | References |
|---|---|---|---|
| Growth rates of NMD mutants is reduced on nonfermentable carbon sources | S. cerevisiae | [47,48] | |
| NMD mutants are sensitive to Calcofluor white (cell wall disruptor) | S. cerevisiae | [49] | |
| NMD mutants are more tolerant to toxic concentrations of copper | S. cerevisiae | [50] | |
| NMD regulates magnesium homeostasis | S. cerevisiae | [51] | |
| UPF1 is not essential for growth | S. cerevisiae | [52] | |
| NMD mutants have abnormal morphogenesis on the genitalia and reduced offspring numbers | C. elegans | In vivo | [53] |
| NMD mutants rescued the worms from unc-54 (r293) movement defects | C. elegans | In vivo | [54] |
| algn-2, a positive regulator of NMD, is essential for longevity | C. elegans | In vivo | [55] |
| smg-1, -4, and -6 defects in C. elegans and depletion of SMG6 in HeLa cells cause endoplasmic reticulum stress | C. elegans; H. sapiens | In vitro; In vivo | [56] |
| NMD is required for longevity through the insulin-like signaling pathway | C. elegans | In vivo | [57] |
| NMD coupled with alternative splicing is required for longevity from dietary restriction (DR) | C. elegans | In vivo | [58] |
| smg-6 and smg-7 mutants showed reduced lifespan under DR | C. elegans | In vivo | [16] |
| NMD mutants cause lethality during larval development | Drosophila | In vivo | [59] |
| NMD is essential for zebrafish embryonic development and survival | D. rerio | In vivo | [60] |
| RENT1/UPF1, UPF2, SMG1, and SMG6 are essential for mammalian embryonic viability | M. musculus | In vitro; In vivo | [61,62,63,64] |
| Deletion of UPF2 led to extinction of hematopoietic stem and progenitor cells | M. musculus | In vivo | [64] |
| Upf2 ablation leads to testicular atrophy and male sterility in embryonic Sertoli cells | M. musculus | In vitro | [65] |
| NMD is required in hypoxic stress response | M. musculus | In vitro | [66] |
| SMG9 mutation causes a multiple congenital anomaly syndrome and intellectual disability | H. sapiens; M. musculus | In vivo | [67,68] |
| NMD involves in specifying the developmental fate of embryonic stem cells | H. sapiens | In vitro | [69] |
| Mutation in UPF3B cause intellectual disability (ID); Mutation in UPF2 is associated with ID and neuro-developmental disorders | H. sapiens | In vivo | [70,71,72,73] |
| NMD is inhibited by amino acid starvation and transcripts that promote amino acid homeostasis is upregulated | H. sapiens | In vitro | [74] |
| NMD has antiviral activity | H. sapiens | In vitro | [75] |
| NMD affects COVID-19 susceptibility via regulating OAS1 expression | H. sapiens | In vitro; In vivo | [76] |
| NMD is downregulated to restore homeostasis under endoplasmic reticulum stress | Mammals | Review | [77] |
| In cancer, some types of tumors use NMD to downregulate tumor-suppressor mRNAs by selecting for destruction-inducing mutations; other types of tumors disable NMD by NMD factor mutations, which favors the tumor cells to adapt to microenvironment | Mammals | Review | [78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Sharma, R.; Rogers, A.N. Physiological Consequences of Nonsense-Mediated Decay and Its Role in Adaptive Responses. Biomedicines 2024, 12, 1110. https://doi.org/10.3390/biomedicines12051110
Ma Z, Sharma R, Rogers AN. Physiological Consequences of Nonsense-Mediated Decay and Its Role in Adaptive Responses. Biomedicines. 2024; 12(5):1110. https://doi.org/10.3390/biomedicines12051110
Chicago/Turabian StyleMa, Zhengxin, Ratna Sharma, and Aric N. Rogers. 2024. "Physiological Consequences of Nonsense-Mediated Decay and Its Role in Adaptive Responses" Biomedicines 12, no. 5: 1110. https://doi.org/10.3390/biomedicines12051110
APA StyleMa, Z., Sharma, R., & Rogers, A. N. (2024). Physiological Consequences of Nonsense-Mediated Decay and Its Role in Adaptive Responses. Biomedicines, 12(5), 1110. https://doi.org/10.3390/biomedicines12051110






