The Role of Natural Products in Diabetic Retinopathy
Abstract
1. Introduction
2. Method
- Natural extracts from plants, animals, or medical herbs were considered.
- The effectiveness of natural products in treating diabetic retinopathy was validated through in vitro, in vivo, or human clinical trials.
- The results included effects, therapeutic potentials, signaling mechanisms, etc.
- Studies not relevant to natural products were excluded.
- Natural products lacking clear chemical structures were omitted.
- Natural products with multiple components that did not specify the active components were excluded.
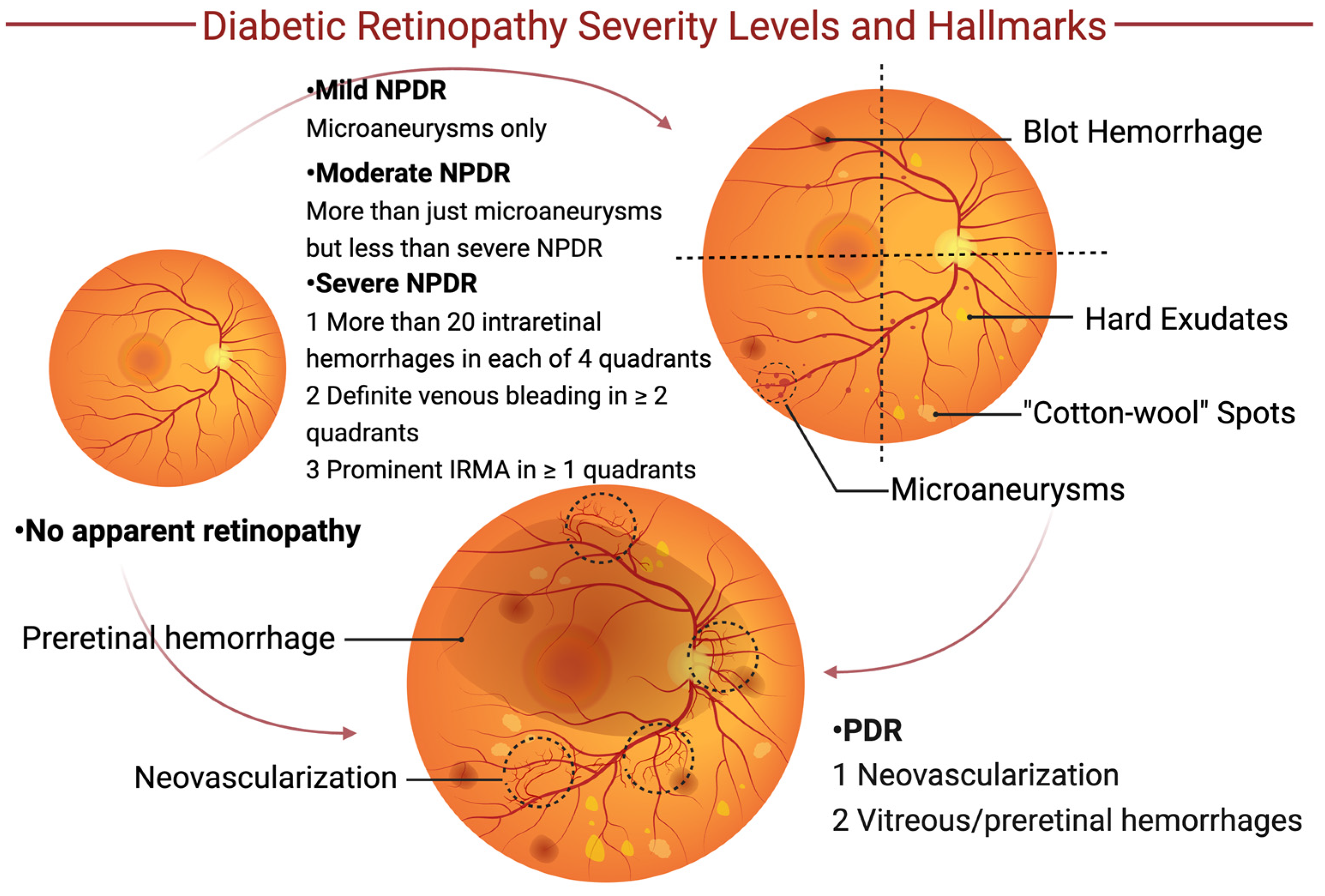
3. Diabetic Retinopathy
3.1. Pathophysiology of Diabetic Retinopathy
3.1.1. Inflammation
3.1.2. Retinal Micro-Vasculopathy
3.1.3. Oxidative Stress
3.1.4. Retinal Neurodegeneration
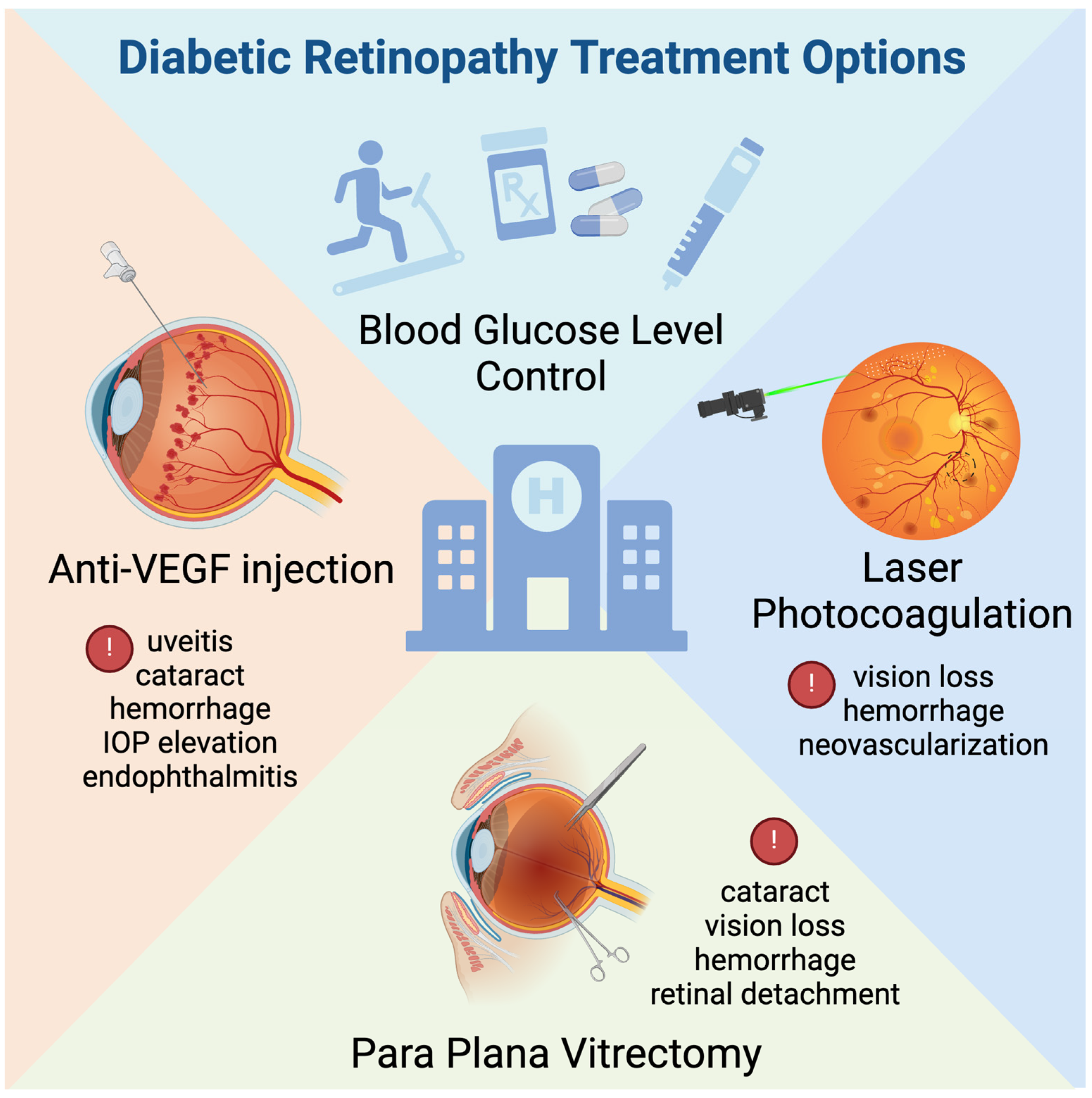
3.2. Current Therapy for Diabetic Retinopathy
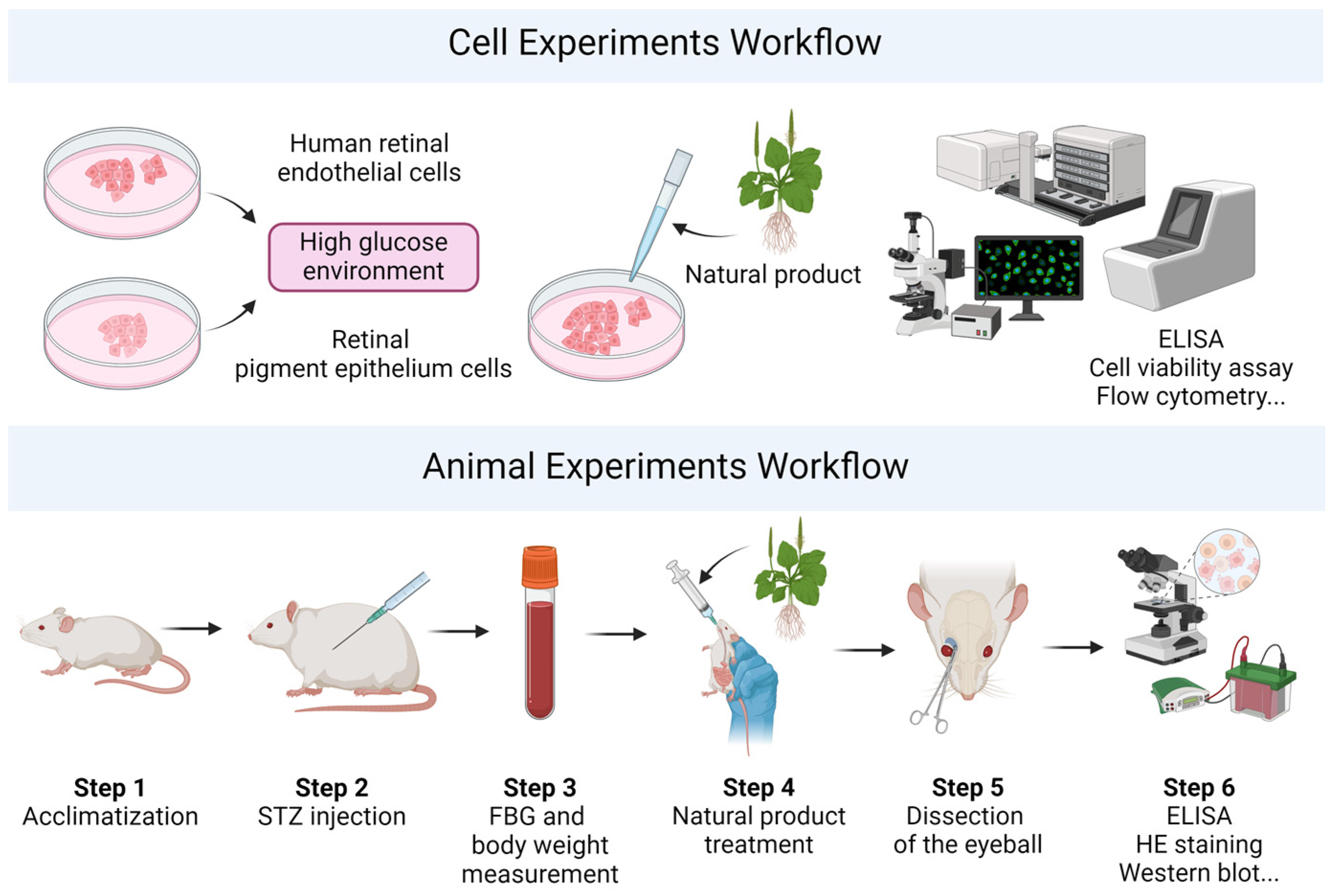
4. Experimental Models for Diabetes Research
4.1. Cell Models
4.2. Animal Models
4.2.1. Chemical-Induced Models
4.2.2. Genetic Models
4.2.3. Diet-Induced Models
4.2.4. Neovascularization Models
5. Natural Products in the Treatment of Diabetic Retinopathy
5.1. Key Natural Products in DR Treatment
5.1.1. Astragaloside-IV
5.1.2. Resveratrol
5.1.3. Astaxanthin
5.1.4. Quercetin
5.1.5. Berberine
5.2. Other Potential Natural Products in DR Treatment
5.2.1. Saponins
5.2.2. Phenols
5.2.3. Terpenoids
5.2.4. Flavonoids
5.2.5. Saccharide
5.2.6. Quinone
5.2.7. Steroid
5.2.8. Vitamin D
5.2.9. Natural Products with Various Bioactive Agents
6. Mechanisms of Action, Toxicity, and Drug–Drug Interactions of Natural Products
6.1. Bioavailability and Ocular Metabolic Pathways of Natural Products
6.2. Toxicity and Side Effects
6.3. Drug–Drug Interactions
7. Limitations and Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Huang, J.; Sun, Y.; Xu, W.; Qian, H. Emerging Role of Extracellular Vesicles in Diabetic Retinopathy. Theranostics 2024, 14, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. The Effect of Hyperlipidemia on the Course of Diabetic Retinopathy-Literature Review. J. Clin. Med. 2022, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhong, W. HbA1c Variability as an Independent Risk Factor for Microvascular Complications in Type 1 Diabetes. J. Diabetes Sci. Technol. 2024, 18, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Majsterek, I. Relationship between Biochemical Pathways and Non-Coding RNAs Involved in the Progression of Diabetic Retinopathy. J. Clin. Med. 2024, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Gangwani, R.A.; Lian, J.X.; McGhee, S.M.; Wong, D.; Li, K.K. Diabetic Retinopathy Screening: Global and Local Perspective. Hong Kong Med. J. 2016, 22, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; et al. Fruit Intake and Incident Diabetic Retinopathy with Type 2 Diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.P.; Madeira, M.H.; Messias, A.L.; Martinho, A.C.-V.; Santos, T.; Sousa, D.C.; Figueira, J.; Cunha-Vaz, J. Different Retinopathy Phenotypes in Type 2 Diabetes Predict Retinopathy Progression. Acta Diabetol. 2021, 58, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.-T.; Zhang, J.-F. Inflammation in Diabetic Retinopathy: Possible Roles in Pathogenesis and Potential Implications for Therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Xie, Y.; Du, A.; Chen, M.; Lai, S.; Wei, X.; Ji, L.; Wang, C. Panax Notoginseng Saponins Alleviate Diabetic Retinopathy by Inhibiting Retinal Inflammation: Association with the NF-κB Signaling Pathway. J. Ethnopharmacol. 2024, 319, 117135. [Google Scholar] [CrossRef] [PubMed]
- Herdade, A.S.; Silva, I.M.; Calado, Â.; Saldanha, C.; Nguyen, N.-H.; Hou, I.; Castanho, M.; Roy, S. Effects of Diabetes on Microcirculation and Leukostasis in Retinal and Non-Ocular Tissues: Implications for Diabetic Retinopathy. Biomolecules 2020, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Sun, W.-J.; An, X.-D.; Zhang, Y.-H.; Zhao, X.-F.; Sun, Y.-T.; Yang, C.-Q.; Kang, X.-M.; Jiang, L.-L.; Ji, H.-Y.; Lian, F.-M. The Ideal Treatment Timing for Diabetic Retinopathy: The Molecular Pathological Mechanisms Underlying Early-Stage Diabetic Retinopathy Are a Matter of Concern. Front. Endocrinol. 2023, 14, 1270145. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and Angiogenic Biomarkers in Diabetic Retinopathy. Biochem. Med. 2020, 30, 030502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, L.; Huang, R.; Wu, P.; He, L. Comparison of Inflammatory Cytokines Levels in the Aqueous Humor with Diabetic Retinopathy. Int. Ophthalmol. 2020, 40, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yao, J.; Chen, C.; Wang, J.; Zhou, A. Fetuin-B Overexpression Promotes Inflammation in Diabetic Retinopathy Through Activating Microglia and the NF-κB Signaling Pathway. Curr. Eye Res. 2024, 49, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Pavlou, S.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. IL-33 Regulates Müller Cell-Mediated Retinal Inflammation and Neurodegeneration in Diabetic Retinopathy. Dis. Models Mech. 2023, 16, dmm050174. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Bek, T. Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; Monickaraj, F.; Legendre, C.; Cabrera, A.P.; Llaci, L.; Bilagody, C.; McGuire, P.; Das, A. Transcriptomics Analysis of Pericytes from Retinas of Diabetic Animals Reveals Novel Genes and Molecular Pathways Relevant to Blood-Retinal Barrier Alterations in Diabetic Retinopathy. Exp. Eye Res. 2020, 195, 108043. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Tan, B.; Yu, D.-Y.; Balaratnasingam, C. Differentiating Microaneurysm Pathophysiology in Diabetic Retinopathy Through Objective Analysis of Capillary Nonperfusion, Inflammation, and Pericytes. Diabetes 2022, 71, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Vähätupa, M.; Järvinen, T.A.H.; Uusitalo-Järvinen, H. Exploration of Oxygen-Induced Retinopathy Model to Discover New Therapeutic Drug Targets in Retinopathies. Front. Pharmacol. 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Xu, H.; Zhang, C.; Yang, Q.; Zhang, L.; Zhang, J. Time-Dependent Changes in Hypoxia- and Gliosis-Related Factors in Experimental Diabetic Retinopathy. Eye 2019, 33, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Li, Y.; Chen, Z.; Xiong, Y.; Zhou, T.; Tao, W.; Xu, F.; Yang, H.; Ylä-Herttuala, S.; et al. MicroRNA-15b Targets VEGF and Inhibits Angiogenesis in Proliferative Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2020, 105, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, S.; Houle, F.; Landry, J.; Huot, J. P38 MAP Kinase Activation by Vascular Endothelial Growth Factor Mediates Actin Reorganization and Cell Migration in Human Endothelial Cells. Oncogene 1997, 15, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Sozen-Delil, F.I.; Cekic, O.; Haklar, G. Serum and Vitreous Vascular Endothelial Growth Factor Levels in Diabetic Retinopathy. Int. Ophthalmol. 2023, 43, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Cheng, Z.; Yu, X.; HorYue, T.; Haiyong, C.; Yibin, F. Berberine Improves Insulin-Induced Diabetic Retinopathy through Exclusively Suppressing Akt/mTOR-Mediated HIF-1α/VEGF Activation in Retina Endothelial Cells. Int. J. Biol. Sci. 2021, 17, 4316. [Google Scholar]
- Wang, L.; Li, S.; Wang, L.; Lin, K.; Du, J.; Miao, W.; Zhang, L. Uncovering the Protective Mechanism of Taohong Siwu Decoction against Diabetic Retinopathy via HIF-1 Signaling Pathway Based on Network Analysis and Experimental Validation. BMC Complement. Med. Ther. 2020, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zheng, Y.; Sun, Y.; Lai, M.; Qiu, J.; Gui, F.; Zeng, Q.; Liu, F. Suppressing Long Noncoding RNA OGRU Ameliorates Diabetic Retinopathy by Inhibition of Oxidative Stress and Inflammation via miR-320/USP14 Axis. Free Radic. Biol. Med. 2021, 169, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Guzman, D.C.; Olguín, H.J.; García, E.H.; Peraza, A.V.; de la Cruz, D.Z.; Soto, M.P. Mechanisms Involved in the Development of Diabetic Retinopathy Induced by Oxidative Stress. Redox Rep. 2017, 22, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, G.E.; Heck, T.G. Inflammation, Oxidative Stress and Altered Heat Shock Response in Type 2 Diabetes: The Basis for New Pharmacological and Non-Pharmacological Interventions. Arch. Physiol. Biochem. 2022, 128, 411–425. [Google Scholar] [CrossRef]
- Satari, M.; Aghadavod, E.; Mobini, M.; Asemi, Z. Association between miRNAs Expression and Signaling Pathways of Oxidative Stress in Diabetic Retinopathy. J. Cell Physiol. 2019, 234, 8522–8532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, Y.; Zhu, L.; Wang, Z.; Fang, C.; Jiang, C.; Pan, K.; Zhang, J.; Yin, Z. Arjunolic Acid from Cyclocarya paliurus Ameliorates Diabetic Retinopathy through AMPK/mTOR/HO-1 Regulated Autophagy Pathway. J. Ethnopharmacol. 2022, 284, 114772. [Google Scholar] [CrossRef] [PubMed]
- Karam-Palos, S.; Andrés-Blasco, I.; Campos-Borges, C.; Zanón-Moreno, V.; Gallego-Martínez, A.; Alegre-Ituarte, V.; García-Medina, J.J.; Pastor-Idoate, S.; Sellés-Navarro, I.; Vila-Arteaga, J.; et al. Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients. J. Clin. Med. 2023, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Blasco, I.; Gallego-Martínez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arévalo, J.F.; Pinazo-Durán, M.D. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic Molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. Int. J. Mol. Sci. 2023, 24, 8227. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Bai, W.; Yang, L. Astaxanthin Inhibits Oxidative Stress and Apoptosis in Diabetic Retinopathy. Acta Histochem. 2023, 125, 152069. [Google Scholar] [CrossRef] [PubMed]
- Nonarath, H.J.; Hall, A.E.; SenthilKumar, G.; Abroe, B.; Eells, J.T.; Liedhegner, E.S. 670nm Photobiomodulation Modulates Bioenergetics and Oxidative Stress, in Rat Müller Cells Challenged with High Glucose. PLoS ONE 2021, 16, e0260968. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Cho, S.; Park, J.; Jin, Y.; Kim, H.M.; Jee, D. Cost-Effectiveness of the Anti-Vascular Endothelial Growth Factor Intravitreal Injection and Panretinal Photocoagulation for Patients with Proliferative Diabetic Retinopathy in South Korea. BMC Health Serv. Res. 2023, 23, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zehden, J.A.; Mortensen, X.M.; Reddy, A.; Zhang, A.Y. Systemic and Ocular Adverse Events with Intravitreal Anti-VEGF Therapy Used in the Treatment of Diabetic Retinopathy: A Review. Curr. Diabetes Rep. 2022, 22, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment Techniques and Clinical Guidelines for Photocoagulation of Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology 1987, 94, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Maugeri, A.; Lotery, A.; Longo, A.; Bonfiglio, V.; Russo, A.; Avitabile, T.; Pulvirenti, A.; Furino, C.; Cennamo, G.; et al. Intravitreal Anti-Vascular Endothelial Growth Factors, Panretinal Photocoagulation and Combined Treatment for Proliferative Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis. Acta Ophthalmol. 2021, 99, e795–e805. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Kim, S.-G.; Lee, S.K. 4-Hexylresorcinol-in Duced Angiogenesis Potential in Human Endothelial Cells. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Kim, D.; Zhu, Y.K.; Oh, D.-C.; Huang, R.Z.; Wang, H.-S.; Liang, D.; Lee, S.K. Glechomanamides A–C, Germacrane Sesquiterpenoids with an Unusual Δ8-7,12-Lactam Moiety from Salvia scapiformis and Their Antiangiogenic Activity. J. Nat. Prod. 2019, 82, 3056–3064. [Google Scholar] [CrossRef]
- Liao, Z.-Y.; Liang, I.-C.; Li, H.-J.; Wu, C.-C.; Lo, H.-M.; Chang, D.-C.; Hung, C.-F. Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. Int. J. Mol. Sci. 2020, 21, 5541. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gong, R.; Hu, S.; Cai, L.; Chen, L. Gambogic Acid Ameliorates Diabetes-Induced Proliferative Retinopathy through Inhibition of the HIF-1α/VEGF Expression via Targeting PI3K/AKT Pathway. Life Sci. 2018, 192, 293–303. [Google Scholar] [CrossRef]
- Rymaszewski, Z.; Abplanalpl, M.A. Human Retinal Vascular Cells Differ from Umbilical Cells in Synthetic Functions and Their Response to GIucose. Proc. Soc. Exp. Biol. Med. 1992, 199, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-M.; Fan, B.; Li, Y.-L.; Zuo, Z.-Y.; Li, G.-Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, M.; Wang, Y.; Ma, H.; Zhou, Y.; Jiang, X.; Liu, Y. Updates on RPE Cell Damage in Diabetic Retinopathy (Review). Mol. Med. Rep. 2023, 28, 185. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, X.; Zhang, D.; Han, W. Astragaloside-IV Alleviates High Glucose-Induced Ferroptosis in Retinal Pigment Epithelial Cells by Disrupting the Expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 2022, 13, 8238–8253. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Kim, N.H.; Lee, Y.M.; Kim, J.S. Effect of Magnolol on TGF-Β1 and Fibronectin Expression in Human Retinal Pigment Epithelial Cells under Diabetic Conditions. Eur. J. Pharmacol. 2007, 562, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front. Immunol. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Sergeys, J.; Etienne, I.; Van Hove, I.; Lefevere, E.; Stalmans, I.; Feyen, J.H.M.; Moons, L.; Van Bergen, T. Longitudinal In Vivo Characterization of the Streptozotocin-Induced Diabetic Mouse Model: Focus on Early Inner Retinal Responses. Investig. Ophthalmol. Vis. Sci. 2019, 60, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Cervia, D. Diabetic Retinopathy: A Matter of Retinal Ganglion Cell Homeostasis. Neural Regen. Res. 2020, 15, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Moleiro, A.F.; Conceição, G.; Leite-Moreira, A.F.; Rocha-Sousa, A. A Critical Analysis of the Available In Vitro and Ex Vivo Methods to Study Retinal Angiogenesis. J. Ophthalmol. 2017, 2017, 3034953. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-D.; Zhang, C.-Y.; Zhang, J.-T.; Gu, L.-M.; Xu, G.-T.; Zhang, J.-F. Epigenetic Modifications and Metabolic Memory in Diabetic Retinopathy: Beyond the Surface. Neural Regen. Res. 2023, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.; Yazdanyar, A. Animal Models of Diabetic Retinopathy. Ann. Transl. Med. 2021, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- McLetchie, N.G. Alloxan Diabetes: A Discovery, Albeit a Minor One. J. R. Coll. Physicians Edinb. 2002, 32, 134–142. [Google Scholar] [PubMed]
- Sadikan, M.Z.; Abdul Nasir, N.A.; Lambuk, L.; Mohamud, R.; Reshidan, N.H.; Low, E.; Singar, S.A.; Mohmad Sabere, A.S.; Iezhitsa, I.; Agarwal, R. Diabetic Retinopathy: A Comprehensive Update on in Vivo, in Vitro and Ex Vivo Experimental Models. BMC Ophthalmol. 2023, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Polewik, K.; Kosek, M.; Jamrozik, D.; Matuszek, I.; Smędowski, A.; Lewin-Kowalik, J.; Pietrucha-Dutczak, M. Rodent Models of Diabetic Retinopathy as a Useful Research Tool to Study Neurovascular Cross-Talk. Biology 2023, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Feit-Leichman, R.A.; Kinouchi, R.; Takeda, M.; Fan, Z.; Mohr, S.; Kern, T.S.; Chen, D.F. Vascular Damage in a Mouse Model of Diabetic Retinopathy: Relation to Neuronal and Glial Changes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ji, J.; Bian, J.; Fu, Y.; Ge, Y.; Yuan, Z. Tacrolimus (FK506) Prevents Early Retinal Neovascularization in Streptozotocin-Induced Diabetic Mice. Int. Immunopharmacol. 2012, 14, 606–612. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, S.; Gao, S.; Wang, Y.; Li, N.; Shen, X. Interleukin-17A Attenuates Photoreceptor Cell Apoptosis in Streptozotocin-Induced Diabetic Mouse Model. Bioengineered 2022, 13, 14175–14187. [Google Scholar] [CrossRef] [PubMed]
- Sadikan, M.Z.; Nasir, N.A.A.; Iezhitsa, I.; Agarwal, R. Antioxidant and Anti-Apoptotic Effects of Tocotrienol-Rich Fraction against Streptozotocin-Induced Diabetic Retinopathy in Rats. Biomed. Pharmacother. 2022, 153, 113533. [Google Scholar] [CrossRef] [PubMed]
- Sadikan, M.Z.; Abdul Nasir, N.A.; Bakar, N.S.; Iezhitsa, I.; Agarwal, R. Tocotrienol-Rich Fraction Reduces Retinal Inflammation and Angiogenesis in Rats with Streptozotocin-Induced Diabetes. BMC Complement. Med. Ther. 2023, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Abdul Ghani, N.A.; Abdul Nasir, N.A.; Lambuk, L.; Sadikan, M.Z.; Agarwal, R.; Ramli, N. The Effect of Palm Oil-Derived Tocotrienol-Rich Fraction in Preserving Normal Retinal Vascular Diameter in Streptozotocin-Induced Diabetic Rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.N.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Leiter, E.H. The NOD Mouse: A Model for Analyzing the Interplay Between Heredity and Environment in Development of Autoimmune Disease. ILAR J. 1993, 35, 4–14. [Google Scholar] [CrossRef]
- Li, C.-R.; Sun, S.-G. VEGF Expression and Cell Apoptosis in NOD Mouse Retina. Int. J. Ophthalmol. 2010, 3, 224–227. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The Db/Db Mouse: A Useful Model for the Study of Diabetic Retinal Neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K. BTBR Ob/Ob Mouse Model of Type 2 Diabetes Exhibits Early Loss of Retinal Function and Retinal Inflammation Followed by Late Vascular Changes. Diabetologia 2018, 61, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Retinopathy in a Diet-Induced Type 2 Diabetic Rat Model and Role of Epigenetic Modifications. Diabetes 2020, 69, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E.H. Quantification of Oxygen-Induced Retinopathy in the Mouse: A Model of Vessel Loss, Vessel Regrowth and Pathological Angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yuan, S.; Liu, X.; Mao, P.; Zhao, C.; Huang, Q.; Zhang, R.; Fang, Y.; Song, Q.; Yuan, D.; et al. Protective Effects of Astragaloside IV on Db/Db Mice with Diabetic Retinopathy. PLoS ONE 2014, 9, e112207. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Xu, M.; Fu, C.; Huang, Y.; Wang, T.-H.; Zuo, Z.-F.; Liu, X.-Z. A Mechanistic Exploratory Study on the Therapeutic Efficacy of Astragaloside IV Against Diabetic Retinopathy Revealed by Network Pharmacology. Front. Pharmacol. 2022, 13, 903485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol Exhibits an Effect on Attenuating Retina Inflammatory Condition and Damage of Diabetic Retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H. Effects of Trans-Resveratrol on Type 1 Diabetes-Induced up-Regulation of Apoptosis and Mitogen-Activated Protein Kinase Signaling in Retinal Pigment Epithelium of Dark Agouti Rats. Eur. J. Pharmacol. 2021, 904, 174167. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol Improves Diabetic Retinopathy Possibly through Oxidative Stress—Nuclear Factor κB—Apoptosis Pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, Y.-S.; Kang, S.-S.; Cho, G.-J.; Choi, W.-S. Resveratrol Inhibits Neuronal Apoptosis and Elevated Ca2+/Calmodulin-Dependent Protein Kinase II Activity in Diabetic Mouse Retina. Diabetes 2010, 59, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, T.; Deng, T.; Wang, Z. Protective Effects of Astaxanthin against Diabetic Retinal Vessels and Pro-Inflammatory Cytokine Synthesis. Int. J. Clin. Exp. Med. 2019, 12, 4725–4734. [Google Scholar]
- Ola, M.S.; Ahmed, M.M.; Shams, S.; Al-Rejaie, S.S. Neuroprotective Effects of Quercetin in Diabetic Rat Retina. Saudi J. Biol. Sci. 2017, 24, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal Neuroprotective Effects of Quercetin in Streptozotocin-Induced Diabetic Rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Ge, H.; Liu, H.; Kern, T.S.; Du, L.; Guan, L.; Su, S.; Liu, P. Leukocytes from Diabetic Patients Kill Retinal Endothelial Cells: Effects of Berberine. Mol. Vis. 2013, 19, 2092. [Google Scholar] [PubMed]
- Na, L.; Xu, M.; Chen, J.-L.; Chen, G.-J.; Sun, J.; Zhang, Q.; Li, J.-Q.; Guo, X.-L.; Zuo, Z.-F.; Liu, X.-Z.; et al. 4D-DIA Quantitative Proteomics Revealed the Core Mechanism of Diabetic Retinopathy after Berberine Treatment. Eur. J. Pharmacol. 2023, 958, 175947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, X.; Liu, K. The Modulation of cAMP/PKA Pathway by Asiaticoside Ameliorates High Glucose-Induced Inflammation and Apoptosis of Retinal Pigment Epithelial Cells. J. Bioenerg. Biomembr. 2022, 54, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Hu, X.-Q.; Ouyang, J.; Dai, B.; Xu, Y. The Effect of Astragalin on the VEGF Production of Cultured Müller Cells under High Glucose Conditions. Bio-Med. Mater. Eng. 2012, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, V.; D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Rossi, S.; Giunta, S. Carnosol Attenuates High Glucose Damage in Human Retinal Endothelial Cells through Regulation of ERK/Nrf2/HO-1 Pathway. J. Asian Nat. Prod. Res. 2023, 25, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Hu, Y.; Huang, C.; Wei, N. Nimbolide Ameliorates the Streptozotocin-Induced Diabetic Retinopathy in Rats through the Inhibition of TLR4/NF-κB Signaling Pathway. Saudi J. Biol. Sci. 2021, 28, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, J.; Cheng, Y.; Dong, X.; Zhang, M.; Pei, C. Palbinone Alleviates Diabetic Retinopathy in STZ-induced Rats by Inhibiting NLRP3 Inflammatory Activity. J. Biochem. Mol. Toxicol. 2020, 34, e22489. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.P.; Mamidala, E.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Evaluation of the Andrographolides Role and Its Indoleamine 2,3-Dioxygenase Inhibitory Potential and Attendant Molecular Mechanism against STZ-Induced Diabetic Rats. Saudi J. Biol. Sci. 2020, 27, 713–719. [Google Scholar] [CrossRef]
- Zeng, X.; Deng, Y.; Yuan, M.; He, Q.; Wu, Y.; Li, S. Study on the Antioxidant Effect of Tanshinone IIA on Diabetic Retinopathy and Its Mechanism Based on Integrated Pharmacology. Evid.-Based Complement. Altern. Med. 2022, 2022, 9990937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, J.; Shi, S.; Qin, X.; Zhang, K.; Xu, J. Kaempferol Protects Retinal Ganglion Ceils from High-Glucose-Induced Injury by Regulating Vasohibin-1. Neurosci. Lett. 2020, 716, 134633. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Zhao, C.; Peng, Q.; Xie, P.; Liu, Q.H. Kaempferol Inhibited VEGF and PGF Expression and in Vitro Angiogenesis of HRECs under Diabetic-like Environment. Braz. J. Med. Biol. Res. 2017, 50, e5396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Gao, X.; Yuan, Z.; Ma, T.; Li, G.; Zhang, X. Tilianin Protects Diabetic Retina through the Modulation of Nrf2/TXNIP/NLRP3 Inflammasome Pathways. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Geng, Q.; Li, T.; Mohammad Firdous, S.; Zhou, X. Morin Attenuates STZ-Induced Diabetic Retinopathy in Experimental Animals. Saudi J. Biol. Sci. 2020, 27, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, Q.; Wang, X.; Liu, H.; Wang, Y. 7,8-Dihydroxyflavone Ameliorates High-Glucose Induced Diabetic Apoptosis in Human Retinal Pigment Epithelial Cells by Activating TrkB. Biochem. Biophys. Res. Commun. 2018, 495, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nagase, T.; Katsura, Y.; Takahashi, H.; Natsugari, H.; Oshitari, T.; Kosano, H. In Vitro Studies on Nobiletin Isolated from Citrus Plants and the Bioactive Metabolites, Inhibitory Action against Gelatinase Enzymatic Activity and the Molecular Mechanisms in Human Retinal Müller Cell Line. Biomed. Pharmacother. 2017, 93, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.P.; Sankeshi, V.; Naik, R.R.; Thirupathi, P.; Das, B.; Raju, T.N. The Inhibitory Effect of Isoflavones Isolated from Caesalpinia pulcherrima on Aldose Reductase in STZ Induced Diabetic Rats. Chem.-Biol. Interact. 2015, 237, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Moon, M.K.; Kim, J.S. Epicatechin Breaks Preformed Glycated Serum Albumin and Reverses the Retinal Accumulation of Advanced Glycation End Products. Eur. J. Pharmacol. 2015, 748, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Wang, Q.; Liang, H.; Jiang, X. Fucoidan Protects ARPE-19 Cells from Oxidative Stress via Normalization of Reactive Oxygen Species Generation through the Ca2+-Dependent ERK Signaling Pathway. Mol. Med. Rep. 2015, 11, 3746–3752. [Google Scholar] [CrossRef]
- Yang, W.; Yu, X.; Zhang, Q.; Lu, Q.; Wang, J.; Cui, W.; Zheng, Y.; Wang, X.; Luo, D. Attenuation of Streptozotocin-Induced Diabetic Retinopathy with Low Molecular Weight Fucoidan via Inhibition of Vascular Endothelial Growth Factor. Exp. Eye Res. 2013, 115, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Wang, W.; Zhang, H.; Ma, N.; Fu, W.; Zhao, M.; Gao, X.; Hao, X.; Zhang, Z. Aloe-Emodin Suppresses Hypoxia-Induced Retinal Angiogenesis via Inhibition of HIF-1α/VEGF Pathway. Int. J. Biol. Sci. 2016, 12, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Gao, X. Diosgenin Protects Retinal Pigment Epithelial Cells from Inflammatory Damage and Oxidative Stress Induced by High Glucose by Activating AMPK/Nrf2/HO-1 Pathway. Immun. Inflam. Dis. 2022, 10, e698. [Google Scholar] [CrossRef]
- Liao, W.-L.; Huang, C.-P.; Wang, H.-P.; Lei, Y.-J.; Lin, H.-J.; Huang, Y.-C. Diosgenin, a Natural Steroidal Sapogenin, Alleviates the Progression of Diabetic Retinopathy in Diabetic Mice. In Vivo 2023, 37, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Seo, J.; Lee, D.H.; Kim, Y.-H.; Kim, J.-H.; Wie, M.-B.; Byun, J.-K.; Yun, J.-H. Ulmus Davidiana 60% Edible Ethanolic Extract for Prevention of Pericyte Apoptosis in Diabetic Retinopathy. Front. Endocrinol. 2023, 14, 1138676. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Choung, S.-Y. Protective Effects of Resveratrol and Its Analogs on Age-Related Macular Degeneration In Vitro. Arch. Pharm. Res. 2016, 39, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lv, L.; Feng, H.; Gu, W. Unlocking the Potential: Quercetin and Its Natural Derivatives as Promising Therapeutics for Sepsis. Biomedicines 2024, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Laky, M.; Arslan, M.; Zhu, X.; Rausch-Fan, X.; Moritz, A.; Sculean, A.; Laky, B.; Ramseier, C.A.; Stähli, A.; Eick, S. Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 735. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, N.; Ignatova, M.; Manolova, N.; Rashkov, I.; Toshkova, R.; Georgieva, A. Nanoparticles Based on Complex of Berberine Chloride and Polymethacrylic or Polyacrylic Acid with Antioxidant and in Vitro Antitumor Activities. Int. J. Pharm. 2020, 584, 119426. [Google Scholar] [CrossRef] [PubMed]
- Ratanakomol, T.; Roytrakul, S.; Wikan, N.; Smith, D.R. Berberine Inhibits Dengue Virus through Dual Mechanisms. Molecules 2021, 26, 5501. [Google Scholar] [CrossRef]
- Wat, N.; Wong, R.L.; Wong, I.Y. Associations between Diabetic Retinopathy and Systemic Risk Factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A Review of Pharmacokinetic and Pharmacological Properties of Asiaticoside, a Major Active Constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302, 115865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Mu, X.; Deng, J.; Wu, X.; He, W.; Liu, Y.; Gu, R.; Han, F.; Nie, X. Asiaticoside-Nitric Oxide Promoting Diabetic Wound Healing through the miRNA-21-5p/TGF-Β1/SMAD7/TIMP3 Signaling Pathway. J. Ethnopharmacol. 2024, 319, 117266. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, Y.; Wu, K.; Liu, M.; Zhang, J.; Zou, F.; Ou-Yang, J. Astragalus Polysaccharide Reduces Hepatic Endoplasmic Reticulum Stress and Restores Glucose Homeostasis in a Diabetic KKAy Mouse Model. Acta Pharmacol. Sin. 2007, 28, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, K.; Mao, X.; Wu, Y.; Ouyang, J. Astragalus Polysaccharide Improves Insulin Sensitivity in KKAy Mice: Regulation of PKB/GLUT4 Signaling in Skeletal Muscle. J. Ethnopharmacol. 2010, 127, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, F.; Wang, N.; Wu, Y.; Zou, F.; Wu, K.; Liu, M.; Ouyang, J. Hypoglycemic Effect of Polysaccharide Enriched Extract of Astragalus membranaceus in Diet Induced Insulin Resistant C57BL/6J Mice and Its Potential Mechanism. Phytomedicine 2009, 16, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, M.; Sun, Q.; Guo, Z.; Lin, Y.; Li, S.; Huang, Y.; Li, Y.; Fu, Q. Magnolol Attenuates Macrophage Pyroptosis Triggered by Streptococcus equi Subsp. zooepidemicus. Int. Immunopharmacol. 2024, 131, 111922. [Google Scholar] [CrossRef] [PubMed]
- Szałabska-Rąpała, K.; Zych, M.; Borymska, W.; Londzin, P.; Dudek, S.; Kaczmarczyk-Żebrowska, I. Beneficial Effect of Honokiol and Magnolol on Polyol Pathway and Oxidative Stress Parameters in the Testes of Diabetic Rats. Biomed. Pharmacother. 2024, 172, 116265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Hu, Y.; Wei, S.; Adu-Frimpong, M.; Sun, C.; Qi, G. Improving Cellular Uptake and Synergetic Anti-Tumor Effects of Magnolol and Brucea javanica Oil through Self-Microemulsion. Drug Dev. Ind. Pharm. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qiu, S.; Xie, L.; Liu, C.; Sun, S. Nimbolide Suppresses Non-Small Cell Lung Cancer Cell Invasion and Migration via Manipulation of DUSP4 Expression and ERK1/2 Signaling. Biomed. Pharmacother. 2017, 92, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S.; Nivetha, R.; Palrasu, M.; Mishra, R. Nimbolide, a Neem Limonoid, Is a Promising Candidate for the Anticancer Drug Arsenal. J. Med. Chem. 2021, 64, 3560–3577. [Google Scholar] [CrossRef]
- Munipally, P.K.; Agraharm, S.G.; Valavala, V.K.; Gundae, S.; Turlapati, N.R. Evaluation of Indoleamine 2,3-Dioxygenase Expression and Kynurenine Pathway Metabolites Levels in Serum Samples of Diabetic Retinopathy Patients. Arch. Physiol. Biochem. 2011, 117, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, D.; Gajendran, B.; Hu, Q.; Zhang, J.; Wang, S.; Han, M.; Xu, Y.; Shen, X. Tanshinone IIA Ameliorates Experimental Diabetic Cardiomyopathy by Inhibiting Endoplasmic Reticulum Stress in Cardiomyocytes via SIRT1. Phytother. Res. 2023, 37, 3543–3558. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Chen, Q.; Wang, X.; Ouyang, X.; Yang, M.; Dong, Y.; Li, J.; Li, Y.; Luo, S.; Liu, Y.; et al. The Combination of Tanshinone IIA and Astragaloside IV Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting the STING Pathway. Chin. Med. 2024, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Liu, J.; Zhou, M.; Wang, H.; Zhu, H.; Jiang, M.; Bo, Q.; Sun, X. Chrysin Alleviates DNA Damage to Improve Disturbed Immune Homeostasis and Pro-Angiogenic Environment in Laser-Induced Choroidal Neovascularization. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119657. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, C.; Wan, L.; Peng, L.; Wen, S.; Fang, W.; Chen, H.; Wang, K.; Yang, X.; Huang, J.; et al. (-)-Epicatechin Ameliorates Type 2 Diabetes Mellitus by Reshaping the Gut Microbiota and Gut-Liver Axis in GK Rats. Food Chem. 2024, 447, 138916. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in Physiological Functions and Mechanisms of (-)-Epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Dai, H.; Jiang, S.; Yu, L. Advanced Glycation End Products in Diabetic Retinopathy and Phytochemical Therapy. Front. Nutr. 2022, 9, 1037186. [Google Scholar] [CrossRef] [PubMed]
- Luoa, H.; Jia, X.; Zhanga, M.; Renb, Y.; Tana, R.; Jianga, H.; Wua, X. Aloe-Emodin: Progress in Pharmacological Activity, Safety, and Pharmaceutical Formulation Applications. Mini Rev. Med. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Srinivasan, B.P.; Srivastava, S.; Gaur, S.; Saxena, R. Effects of Trigonella foenum-graecum (L.) on Retinal Oxidative Stress, and Proinflammatory and Angiogenic Molecular Biomarkers in Streptozotocin-Induced Diabetic Rats. Mol. Cell Biochem. 2014, 388, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and Mineral Supplements in the Primary Prevention of Cardiovascular Disease and Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, C.; Liu, D. Glycated Hemoglobin A1C and Vitamin D and Their Association with Diabetic Retinopathy Severity. Nutr. Diabetes 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D Deficiency Is Associated with Retinopathy in Children and Adolescents with Type 1 Diabetes. Diabetes Care 2011, 34, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Sahli, M.W.; Nie, J.; LaMonte, M.J.; Lutsey, P.L.; Klein, B.E.K.; Mares, J.A.; Meyers, K.J.; Andrews, C.A.; Klein, R. Adequate Vitamin D Status Is Associated with the Reduced Odds of Prevalent Diabetic Retinopathy in African Americans and Caucasians. Cardiovasc. Diabetol. 2016, 15, 128. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Seo, C.-S.; Ha, H.; Jung, D.; Lee, H.; Lee, N.-H.; Lee, J.-A.; Kim, J.-H.; Lee, Y.-K.; Son, J.-K.; et al. Protective Effects of Ulmus Davidiana Var. Japonica against OVA-Induced Murine Asthma Model via Upregulation of Heme Oxygenase-1. J. Ethnopharmacol. 2010, 130, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Park, Y.M.; Lu, Y.; Chang, H.W.; Na, M.; Lee, S.H. Inhibition of Prostaglandin D2 Production by Trihydroxy Fatty Acids Isolated from Ulmus Davidiana Var. Japonica. Phytother. Res. 2013, 27, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Lim, Y.; Kim, J.H.; Heo, W.; Lee, K.Y.; Shin, H.J.; Kim, J.K.; Lee, J.H.; Kim, Y.J. Root Bark of Ulmus Davidiana Var. Japonica Restrains Acute Alcohol-Induced Hepatic Steatosis Onset in Mice by Inhibiting ROS Accumulation. PLoS ONE 2017, 12, e0188381. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Jang, E.H.; Bang, C.; Kim, G.L.; Yoon, S.Y.; Lee, D.H.; Koo, J.; Na, J.H.; Lee, S.; Kim, J.-H. Bakuchiol, Main Component of Root Bark of Ulmus Davidiana Var. Japonica, Inhibits TGF-β-Induced in Vitro EMT and In Vivo Metastasis. Arch. Biochem. Biophys. 2021, 709, 108969. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Harrison, E.H. Mechanisms of Selective Delivery of Xanthophylls to Retinal Pigment Epithelial Cells by Human Lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Peng, T.; Zhao, X.; Li, S.; Farhan, M.; Zheng, W. Recent Trends in Drug-Delivery Systems for the Treatment of Diabetic Retinopathy and Associated Fibrosis. Adv. Drug Deliv. Rev. 2021, 173, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No Effects of Quercetin from Onion Skin Extract on Serum Leptin and Adiponectin Concentrations in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Tang, W.; Huang, Z.; Wang, X.; Gui, S.; Gao, X.; Xiao, D.; Tao, L.; Jiang, Z.; Wang, X. Ultrasmall Coordination Polymer Nanodots Fe-Quer Nanozymes for Preventing and Delaying the Development and Progression of Diabetic Retinopathy. Adv. Funct. Mater. 2023, 33, 2300261. [Google Scholar] [CrossRef]
- Toragall, V.; Muzaffar, J.C.; Baskaran, V. Lutein Loaded Double-Layered Polymer Nanocarrier Modulate H2O2 and CoCl2 Induced Oxidative and Hypoxia Damage and Angiogenic Markers in ARPE-19 Cells. Int. J. Biol. Macromol. 2023, 240, 124378. [Google Scholar] [CrossRef]
- Ramsay, E.; Hagström, M.; Vellonen, K.-S.; Boman, S.; Toropainen, E.; Del Amo, E.M.; Kidron, H.; Urtti, A.; Ruponen, M. Role of Retinal Pigment Epithelium Permeability in Drug Transfer between Posterior Eye Segment and Systemic Blood Circulation. Eur. J. Pharm. Biopharm. 2019, 143, 18–23. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Rabbit as an Animal Model for Intravitreal Pharmacokinetics: Clinical Predictability and Quality of the Published Data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Maji, A.K.; Mishra, S.K.; Ishfaq, P.M.; Devkota, H.P.; Silva, A.S.; Das, N. Goldenseal (Hydrastis canadensis L.) and Its Active Constituents: A Critical Review of Their Efficacy and Toxicological Issues. Pharmacol. Res. 2020, 160, 105085. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. The Therapeutic Effects of Berberine against Different Diseases: A Review on the Involvement of the Endoplasmic Reticulum Stress. Phytother. Res. 2022, 36, 3215–3231. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Mishra, S.K.; Mithal, A. Vitamin D Toxicity Resulting from Overzealous Correction of Vitamin D Deficiency. Clin. Endocrinol. 2015, 83, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Davies, J.S. A Review of the Growing Risk of Vitamin D Toxicity from Inappropriate Practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.S.; Ames-Sibin, A.P.; Bonetti, C.I.; Leal, L.E.; Peralta, R.M.; de Sá-Nakanishi, A.B.; Comar, J.F.; Bracht, A.; Bracht, L. The Short-Term Effects of Berberine in the Liver: Narrow Margins between Benefits and Toxicity. Toxicol. Lett. 2022, 368, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Li, W.Q.; Xia, S.; Guo, L.; Miao, Y.; Zhang, B.-K. Metabolic Activation of the Toxic Natural Products From Herbal and Dietary Supplements Leading to Toxicities. Front. Pharmacol. 2021, 12, 758468. [Google Scholar] [CrossRef]
- Nencini, C.; Galluzzi, P.; Pippi, F.; Menchiari, A.; Micheli, L. Hepatotoxicity of Teucrium chamaedrys L. Decoction: Role of Difference in the Harvesting Area and Preparation Method. Indian J. Pharmacol. 2014, 46, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; García-Cortés, M.; Medina-Caliz, I.; Hernandez, N.; Parana, R.; Mendizabal, M.; Schinoni, M.I.; Ridruejo, E.; Nunes, V.; Peralta, M.; et al. Herbal and Dietary Supplements-Induced Liver Injury in Latin America: Experience From the LATINDILI Network. Clin. Gastroenterol. Hepatol. 2022, 20, e548–e563. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Fujii, I.; Zhao, J.; Shinohara, M.; Matsukura, M. A Novel Polysaccharide Compound Derived from Algae Extracts Protects Retinal Pigment Epithelial Cells from High Glucose-Induced Oxidative Damage In Vitro. Biol. Pharm. Bull. 2012, 35, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; He, R.; Su, X.; Li, Z.; Xie, X. Effect of Chinese Herbal Compounds on Ocular Fundus Signs and Vision in Conventional Treated-Persons with Non-Proliferative Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 977971. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.-J.; Munoz, J.; Gurley, B.J.; Markowitz, J.S. An Ex Vivo Approach to Botanical-Drug Interactions: A Proof of Concept Study. J. Ethnopharmacol. 2015, 163, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, S.; Weng, Y.; Song, F.; Zhou, S.; Bai, M.; Zhou, H.; Zeng, S.; Jiang, H. Interaction of Six Protoberberine Alkaloids with Human Organic Cation Transporters 1, 2 and 3. Xenobiotica 2016, 46, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, H.; Xie, L.; Liu, X. Effect of Baicalin and Berberine on Transport of Nimodipine on Primary-Cultured, Rat Brain Microvascular Endothelial Cells. Acta Pharmacol. Sin. 2007, 28, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.-D.; Guan, H.-D.; Zhao, X.; Xie, Q.; Cai, F.-J.; Xie, Z.-J.; Dang, R.; Li, M.-L.; Wang, C.-H. Protoberberine Alkaloids: A Review of the Gastroprotective Effects, Pharmacokinetics, and Toxicity. Phytomedicine 2024, 126, 155444. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Gautam, M.; Gairola, S.; Jadhav, S.; Patwardhan, B. Effect of Botanical Immunomodulators on Human CYP3A4 Inhibition: Implications for Concurrent Use as Adjuvants in Cancer Therapy. Integr. Cancer Ther. 2014, 13, 167–175. [Google Scholar] [CrossRef]
- Singh, A.; Zhao, K.; Bell, C.; Shah, A.J. Effect of Berberine on in Vitro Metabolism of Sulfonylureas: A Herb-Drug Interactions Study. Rapid Commun. Mass Spectrom. 2020, 34 (Suppl. S4), e8651. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xu, Z.; Xu, X.; Jin, J.; Zhao, Y.; Wang, T.; Li, Y.; Ma, Y. Organic Cation Transporter and Multidrug and Toxin Extrusion 1 Co-Mediated Interaction between Metformin and Berberine. Eur. J. Pharm. Sci. 2019, 127, 282–290. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; He, Q.; Long, Z.; Zhu, X.; Xiang, W.; Wu, Y.; Lin, S. Exploring the Pharmacological Mechanism of Liuwei Dihuang Decoction for Diabetic Retinopathy: A Systematic Biological Strategy-Based Research. Evid. -Based Complement. Altern. Med. 2021, 2021, 5544518. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-D.; Li, M.-X.; Zhang, W.-H.; Zhang, S.-W.; Gong, Y.-B. Effectiveness and Safety of Traditional Chinese Medicine for Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. World J. Diabetes 2023, 14, 1422–1449. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The Structural Modification of Natural Products for Novel Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef]


| Category | Compound Name | Natural Product | Cell/ Animal Model | Efficacy | Mechanism | References |
|---|---|---|---|---|---|---|
| Key Natural Products | Astragaloside-IV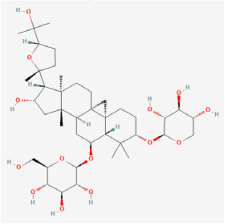 | Astragalus mambranaceus | Human retinal pigment epithelium cells (ARPE-19 cells)/db/db mouse/STZ rats | 1. Decreased the rate of ferroptosis by inhibiting expression of miR-138-5p 2. Decreased GSH content, mitochondria size, and ridge 3. Increased expression of glutathione peroxidase 4, glutamate cysteine ligase, and glutamate cysteine ligase catalytic subunit 4. Inhibited aldose reductase (AR) activation and the overexpression of NF-kB and ERK1/2 phosphorylation 5. Improved the amplitude in pattern electroretinogram and reduced apoptosis 6. Increased retinal thickness, alleviated DR-induced histopathological changes, and elevated blood glucose levels 7. Elevated DR-depressed protein levels of PI3K and AKT | 1. miR-138-5p/Sirt1/Nrf2 2. PI3K/Akt | [55,84,85] |
Resveratrol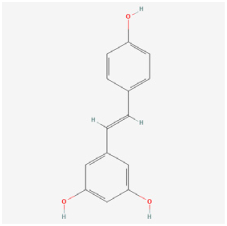 | Grape, peanuts, berries | Rat retinal endothelial cells (RRECs)/STZ rats/STZ mice | 1. Revocered the insulin level and PON1 expression and activity 2. Regulated retinal vascular permeability and mRNA expression of paraoxonase 1 (PON1), VEGF, bFGF, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) 3. Inhibited retinal apoptosis by regulating Ox-LDL, caspase-3, and PON1 activity 4. Reduced inflammatory factors of IL-1β, IL-6, TNF-α, VEGF, Interferon-γ, and MCP-1 5. Mediated alterations in gene and protein expressions related to apoptosis 6. Enhanced SOD activity and reduced the 8-isoprostane level and GSSG/GSH ratio 7. Improved retinal layer disorganization and attenuated retinal thickness reduction 8. Blocked the increase in CaMKII and phosphor-CaMKII protein levels | 1. PON1 2. NF-κB | [86,87,88,89] | |
Astaxanthin (ASX)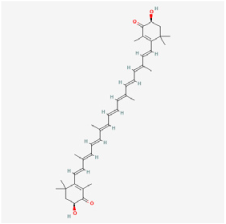 | Haematococcus pluvialis, shrimps | STZ rats | 1. Alleviated the decrease in retinal thickness and ganglion cell layer cell loss 2. Downregulated inflammatory factors of TNF-α, IL-1β, IL-6, MIC-1, and IL-10 3. Exerted antioxidant effects by regulating GSH, GPx, and total antioxidant capacity, malonic dialdehyde (MDA), and ROS levels 4. Fostered the expressions of HO-1 and NQO1 5. Exerted anti-apoptosis effects by regulating caspase-3, Bcl-2, and BAX expressions 6. Decreased the endothelial cell/pericyte ratio and the number of acellular capillaries | Nrf2/Keap 1 | [40,90] | |
Quercetin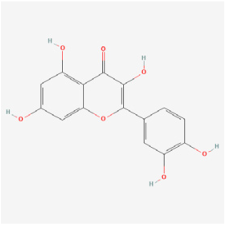 | Licorice | STZ rats | 1. Decreased the expression of BDNF, NGF, TrkB receptor, synaptophysin, and p-Akt 2. Attenuated apoptosis by decreasing caspase-3 and cytochrome c levels, and increasing Bcl-2 expression 3. Increased GSH levels 4. Decreased pro-inflammatory cytokines of TNF-α and IL-1β 5. Increased retinal thickness and cell count in the ganglion cell layer | BNDF/TrkB/Akt/synaptophysin | [91,92] | |
Berberine | Rhizoma Coptidis | HRECs/STZ mice/STZ rats/db/db mice | 1. Increased retinal layer thickness 2. Suppressed VEGF and HIF-1α expressions 3. Suppressed neovascularization 4. Reduced oxidative stress 5. Inhibited leukocyte-mediated killing of HRECs | 1. Akt/mTOR/HIF-1α/VEGF 2. NF-κB | [31,93,94] | |
| Saponins | Asiaticoside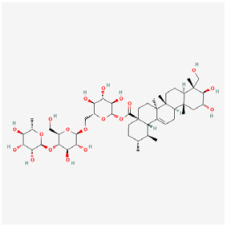 | Centella asiatica | ARPE-19 cells | 1. Restored the cell survival rate 2. Suppressed inflammation cytokines (TNF-α, IL-1β, and IL-6) 3. Regulated apoptosis levels (Bcl-2, Bax, caspase-3, and caspase-9) 4. Activated cAMP and PKA | cAMP/PKA | [95] |
Astragalin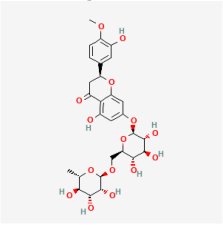 | Astragalus mambranaceus | Rat retinal Müller cells | 1. Decreased VEGF expression | VEGF | [96] | |
| Phenols | Carnosol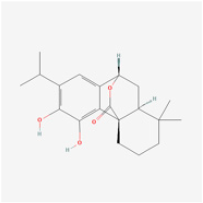 | Rosemary | Human retinal endothelial cells (HRECs) | 1. Increased cell viability and attenuated apoptosis 2. Induced HO-1 expression via Nrf2 activation | ERK1/2/Nrf2/HO-1 | [97] |
Magnolol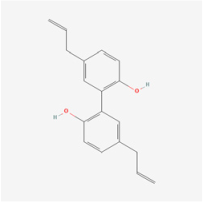 | Magoolia officinalis | ARPE-19 cells | 1. Prevented TGF-β1 and fibronectin expression 2. Inhibited ERK/MAPK/Akt activity | ERK/MAPK/Akt | [56] | |
| Terpenoids | Nimbolide | Neem plants | STZ rats | 1. Suppressed inflammatory factors (TNF-α, IL-1β, and IL-6) 2. Suppressed MCP-1, VEGF, and MMP-9 levels 3. Improved antioxidant levels (SOD, GSH, SOD/CAT, and GSH/GSSG) 4. Suppressed TLR4 and NF-kB expressions | TLR4/NF-kB | [98] |
Arjunolic acid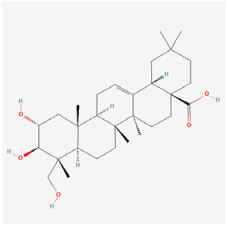 | Cyclocarya paliurus | ARPE-19 cells/STZ rats | 1. Prevented weight loss and increased the retinal thickness and nuclei counts 2. Inhibited oxidative stress, inflammation, and apoptosis 3. Upregulated the HO-1 protein level 4. Activated the AMPK/mTOR/HO-1/autophagy pathway | AMPK/mTOR/HO-1/autophagy | [37] | |
Palbinone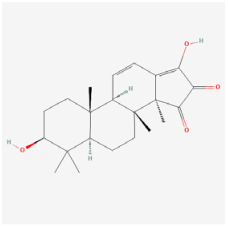 | Paeonia suffruticosa | STZ rats | 1. Improved morphometric and pathological changes of retina tissues 2. Enhanced antioxidant activities (SOD, catalase, and GPx) 3. Alleviated vascular permeability via inhibiting NLRP3 inflammasome formation 4. Reduced pro-inflammatory cytokines (IL-18, and IL-1β) | Nrf2/HO-1 | [99] | |
Andrographolide | Andrographis paniculate (Burm. F.) Nees | STZ rats | 1. Halted the sustained hyperglycemia and reversed the body weight and blood glucose level 2. Boosted cellular antioxidant defense by restoring the GSH level 3. Inhibited IDO and consequential changes in Kynurenine metabolites | - | [100] | |
Glechomanamide B | Salvia | Human microvascular endothelial cells (HMVECs) | 1. Decreased the expressions of VEGF-R2, GLUT1, HK2, and ANGPT2 2. Inhibited tube formation induced by VEGF and BMP4 | VEGF | [49] | |
Tanshinone IIA | Salvia miltiorrhiza bunge | STZ rats | 1. Exerted anti-inflammatory and antioxidation effects by regulating IL-1β, IL-6, TNF-α, SOD, GSH-PX, and MDA expressions 2. Regulated cell proliferation, apoptosis, and neovascularization | - | [101] | |
| Flavonoids | Kaempferol | Kaempferia galanga L. | Retinal ganglion cells (RGCs)/HRECs | 1. Attenuated apoptosis, caspase-3 activity, and LDH leakage 2. Promoted cell viability and decreased ROS levels 3. Inhibited cell proliferation, migration, and tube formation 4. Suppressed the expression of VEGF and PGF 5. Suppressed PI3L expression and ERK1/2, Src, and Akt1 activation | 1. ERK/VASH1 2. Src/Akt1/ERK1/2 | [102,103] |
Tilianin | Traversia baccharoides Hook.f. | STZ rats | 1. Reduced fasting glucose status, HbA1c levels, and augmented serum insulin status 2. Increased Nrf2 and HO-1 expressions and decreased MDA levels 3. Increased SOD, CAT, and GPx levels 4. Decreased expression of TXNIP, NLRP3, ASC, caspase-1, and IL-1β 5. Ameliorated retinal morphological and morphometric changes | Nrf2/TXNIP/NLRP3 | [104] | |
Chrysin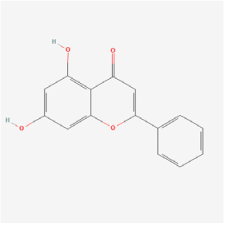 | Oroxylum indicum (Linn.) Bentham ex Kurz | Choroid endothelial cells (RF/6A cells) | 1. Inhibited cell migration via inhibiting AKT and ERK phosphorylation 2. Decreased MMP-2 expression 3. Inhibited HIF-1α and VEGF expressions | VEGF | [50] | |
Morin | Moraceae family plants | STZ rats | 1. Decreased blood glucose levels 2. Improved endogenous antioxidant enzymes’ activity (GPx, CAT, and SOD) 3. Reduced TNF-α, IL-β, and VEGF expressions 4. Increased retina thickness and cell count in the ganglion cell layer | - | [105] | |
Gambogic acid | Gamboges resin | RF/6A cells/STZ mice | 1. Suppressed cell proliferation, migration, and tube formation 2. Decreased HIF-1α and VEGF expressions 3. Attenuated retinal neovascularization | 1. HIF-1α/VEGF 2. PI3K/Akt | [51] | |
7,8-Dihydroxyflavone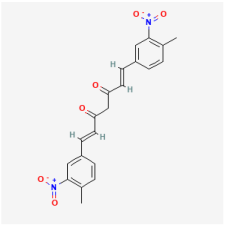 | Citrus, grains, tea | ARPE-19 cells | 1. Ameliorated apoptosis by inhibiting caspase-9 activity and phosphorylating TrkB protein | TrkB | [106] | |
Nobiletin | Citrus plants | Human retinal Müller cells (MIO-M1 cells) | 1. Attenuated MMP-9 enzymatic activity 2. Regulated MMP-9 and tissue inhibitor of metalloproteinase-1 expression | PI3K/Akt | [107] | |
Isoflavones | Caesalpinia pulcherrima | STZ rats | 1. Inhibited AR activity 2. Decreased thiobarbituric acid-reactive substances and protein carbonyl levels 3. Restored GSH levels 4. Increased antioxidant enzymes’ activities (SOD, GPx, and catalase) | - | [108] | |
Epicatechin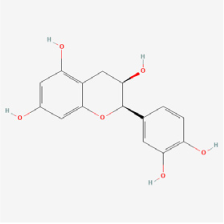 | Green tea, coconut | AGE-injected rats/glycated bovine serum albumin | 1. Reduced AGEs accumulation 2. Inhibited retinal apoptosis | - | [109] | |
| Saccharides | Fucoidan | Brown algae | ARPE-19 cells/brain microvascular endothelial cells/STZ mice | 1. Inhibited apoptosis and ROS generation 2. Inhibited high-glucose-mediated Ca2+ influx 3. Inhibited ERK phosphorylation 4. Inhibited cell proliferation, angiogenic vessels, and VEGF expression 5. Inhibited HIF-1α expression | 1. Ca2+-dependent ERK 2. HIF-1α/VEGF | [110,111] |
| Quinones | Aloe-emodin | Radix et Rhizoma Rhei | ARPE-19 cells/OIR rats | 1. Inhibited the secretion of VEGFA in the ARPE-19 cells under hypoxia conditions 2. Decreased the mRNA and protein expressions of VEGFA and PHD-2 in the ARPE-19 cells 3. Inhibited hypoxia-induced retinal neovascularization in vivo | HIF-a/VEGF | [112] |
| Steroids | Diosgenin | Fenugreek seeds, Wild yam roots | ARPE-19 cells/db/db mice | 1. Increased the viability of ARPE-19 cells 2. Inhibited apoptosis of ARPE-19 cells 3. Reduced the inflammatory response and oxidative stress of ARPE-19 cells 4. Activated the AMPK/Nrf2/HO-1 pathway 5. Increased HDL levels and decreased LDL levels 6. Improved the thickness of the retinal layer 7. Decreased retinal cell apoptosis | AMPK/Nrf2/HO-1 | [113,114] |
| Multiple Bioactive Agents | Ginsenoside Rg1, Ginsenoside Rb1 | Panax notoginseng sponins | MIO-M1 cells/STZ rats | 1. Increased retinal inner nuclear layer thickness, reduced acellular capillaries, and attenuated BRB disruption by upregulated claudin-1 and occluding 2. Abrogated microglial cell activation, and reversed leukocyte adhesion by downregulated intercellular molecule-1 and vascular cell adhesion molecule-1 3. Reduced pro-inflammatory factors (TNF-α, IL-6, and IL-1β), and inhibited expressions of p-IKK, p-IκB, p-p65, and nuclear translocation of p65 | NF-κB | [14] |
  | ||||||
| 60% Edible ethanolic extract of U. davidiana, catechin 7-O-β-D-apiofuranoside  | Ulmus dacidiana | Human placental pericytes/HMVECs | 1. Prevented pericyte apoptosis by blocking the activities of p38 and JNK 2. Increased ZO-1 expression and reduced endothelial permeability by preventing pericyte apoptosis | - | [115] |
| Target | Effects | Compound Name | Natural Product | Category | Reference |
|---|---|---|---|---|---|
| Inflammation | Reduced inflammatory factors (IL-1β, IL-6, TNF-α, Interferon-γ, and MCP-1) | Resveratrol | Grape, peanuts, berries | Phenols | [86,87,88,89] |
| 1. Downregulated inflammatory factors (TNF-α, IL-1β, IL-6, MIC-1, and IL-10) 2. Downregulated NF- kB level | Astaxanthin | Haematococcus pluvialis, shrimps | Terpenoids | [40,90] | |
| Suppressed inflammatory cytokines (TNF-α and IL-1β) | Quercetin | Licorice | Flavonoids | [91,92] | |
| Suppressed inflammatory cytokines (TNF-α, IL-1β, and IL-6) | Asiaticoside | Centella asiatica | Saponins | [95] | |
| 1. Suppressed inflammatory factors (TNF-α, IL-1β, and IL-6) 2. Suppressed TLR4 and NF-kB expressions | Nimbolide | Neem plants | Terpenoids | [98] | |
| Suppressed inflammatory cytokines (TNF-α, IL-1β, and IL-6) | Argunolic acid | Cyclocarya paliurus | Terpenoids | [37] | |
| 1. Reduced pro-inflammatory cytokines (IL-18 and IL-1β) 2. Inhibited NLRP3 inflammasome formation | Palbinone | Paeonia suffruticosa | Terpenoids | [99] | |
| Suppressed inflammatory cytokines (TNF-α, IL-1β, and IL-6) | Tanshinone IIA | Salvia miltiorrhiza bunge | Terpenoids | [101] | |
| Downregulated the expression of inflammasome components (NLRP3 and ASC) along with their downstream targets (caspase-1 and IL-1β) | Tilianin | Traversia baccharoides Hook.f. | Flavonoids | [104] | |
| Suppressed inflammatory cytokines (TNF-α and IL-1β) | Morin | Moraceae family plants | Flavonoids | [105] | |
| 1. Suppressed inflammatory cytokines (TNF-α, IL-1β, and IL-6) 2. Downregulated the expressions of COX-2 and p65 | Diosgenin | Fenugreek seeds, wild yam roots | Steroids | [113,114] | |
| 1. Suppressed pro-inflammatory factors (TNF-α, IL-6, and IL-1β) 2. Inhibited the activation of the NF-kB signaling pathway | Ginsenoside Rg1, Ginsenoside Rb1 | Panax notoginseng sponins | - | [14] | |
| Neovascularization/VEGF | 1. Suppressed VEGF and HIF-1α expressions 2. Suppressed neovascularization by inactivation of cell proliferation and migration | Berberine | Rhizoma Coptidis | Alkaloids | [31,93,94] |
| Reduced VEGF expression | Astragalin | Astragalus mambranaceus | Saponins | [96] | |
| Inhibited tube formation induced by VEGFR2 and BMP4 | Glechomanamides B | Salvia | Terpenoids | [49] | |
| Reduced VEGF expression | Tanshinone IIA | Salvia miltiorrhiza bunge | Terpenoids | [101] | |
| 1. Inhibited cell proliferation, migration, and tube formation 2. Suppressed the expressions of VEGF and PGF | Kaempferol | Kaempferia galanga L. | Flavonoids | [102,103] | |
| 1. Inhibited cell migration 2. Inhibited HIF-1α and VEGF expressions | Chrysin | Oroxylum indicum (Linn.) Bentham ex Kurz | Flavonoids | [50] | |
| 1. Suppressed cell proliferation, migration, and tube formation 2. Inhibited HIF-1α and VEGF expressions 3. Decreased angiogenesis-related expressions of FGF2 and PAI-1 | Gambogic acid | Gamboges resin | Flavonoids | [51] | |
| 1. Inhibited cell proliferation 2. Inhibited HIF-1α and VEGF expressions | Fucoidan | Brown algae | Saccharides | [110,111] | |
| 1. Inhibited the secretion of VEGFA 2. Decreased the mRNA expressions of VEGFA and PHD-2 3. Inhibited hypoxia-induced retinal neovascularization | Aloe-emodin | Radix et Rhizoma Rhei | Quinones | [112] | |
| Oxidative stress | Reduced lipid peroxidation level and ROS content | Astragaloside-IV | Astragalus mambranaceus | Saponins | [55,84,85] |
| 1. Enhanced SOD activity and reduced the 8-isoprostane level and GSSG/GSH ratio in both blood and retina tissue 2. Decreased oxidative stress marker 4-HNE | Resveratrol | Grape, peanuts, berries | Phenols | [86,87,88,89] | |
| 1. Regulated GSH, GPx, MDA, and ROS levels 2. Fostered the expressions of HO-1, NQO1, and IL-10 and restrained the expression of NF-kB | Astaxanthin | Haematococcus pluvialis, shrimps | Terpenoids | [40,90] | |
| Increased GSH and antioxidant enzymes’ activities (SOD and CAT) | Quercetin | Licorice | Flavonoids | [91,92] | |
| Reduced oxidative stress | Berberine | Rhizoma Coptidis | Alkaloids | [31,93,94] | |
| 1. Decreased ROS production 2. Increased HO-1 activity | Carnosol | Rosemary | Phenols | [97] | |
| Decreased MDA and lipid peroxidation levels | Magnolol | Magoolia officinalis | Phenols | [56] | |
| Improved antioxidants’ status by regulating SOD, GSH, SOD/CAT ratio, and GSH/GSSG ratio | Nimbolide | Neem plants | Terpenoids | [98] | |
| 1. Upregulated the HO-1 protein level 2. Downregulated ROS and MDA levels | Argunolic acid | Cyclocarya paliurus | Terpenoids | [37] | |
| 1. Enhanced antioxidant activities (SOD, CAT, and GPx) 2. Enhanced the HO-1 expression | Palbinone | Paeonia suffruticosa | Terpenoids | [99] | |
| 1. Restored the GSH level 2. Inhibited IDO and consequential changes in Kynurenine metabolites | Andrographolide | Andrographis paniculate (Burm. F.) Nees | Terpenoids | [100] | |
| Decreased ROS levels | Kaempferol | Hippophae rhamnoides L. | Flavonoids | [102,103] | |
| 1. Increased Nrf2 and HO-1 expressions and decreased MDA levels 2. Increased SOD, CAT, and GPX levels and decreased MDA levels | Tilianin | Traversia baccharoides Hook.f. | Flavonoids | [104] | |
| 1. Improved endogenous antioxidant enzymes’ activity (GPx, CAT, and SOD) 2. Decreased lipid peroxidase levels | Morin | Moraceae family plants | Flavonoids | [105] | |
| 1. Restored GSH activity 2. Decreased thiobarbituric acid-reactive substances and protein carbonyl levels 3. Inhibited AR activity 4. Increased antioxidant enzymes’ activities (SOD, GPx, and CAT) | Isoflavones | Caesalpinia pulcherrima | Flavonoids | [108] | |
| Inhibited ROS generation through Ca2+-dependent ERK1/2 signaling pathway | Fucoidan | Brown algae | Saccharides | [110,111] | |
| Increased SOD and GPX levels and decreased MDA levels | Diosgenin | Fenugreek seeds, wild yam roots | Steroids | [112] | |
| Apoptosis | Decreased cell apoptosis | Astragaloside-IV | Astragalus mambranaceus | Saponins | [55,84,85] |
| 1. Mediated alternations in gene and protein expressions related to apoptosis (p53, Bax, Bcl-2, caspase-3, caspase-9, p38aMAPK, c-Jun N-terminal kinase-1, and extracellular signal-regulated kinase-1) 2. Blocked the increase in CaMKII and phosphor-CaMKII protein levels | Resveratrol | Grape, peanuts, berries | Phenol | [86,87,88,89] | |
| 1. Inhibited retinal pericytes apoptosis 2. Regulated apoptosis-related proteins (Bcl-2, BAX, and caspase-3) | Astaxanthin | Haematococcus pluvialis, shrimps | Terpenoids | [40,90] | |
| 1. Attenuated apoptosis by decreasing caspase-3 and cytochrome c levels, and increasing Bcl-2 expression 2. Decreased the expression of BDNF, NGF, TrkB receptor, synaptophysin, and p-Akt 3. Suppressed NF-kB activity | Quercetin | Licorice | Flavonoids | [91,92] | |
| regulated apoptosis-related proteins (Bcl-2, Bax, caspase-3, and caspase-9) | Asiaticoside | Centella asiatica | Saponins | [95] | |
| Attenuated cell apoptosis and caspase-3 activity | Kaempferol | Kaempferia galanga L. | Flavonoids | [102,103] | |
| Ameliorated apoptosis by inhibiting caspase-9 activity | 7,8-Dihydroxyflavone | Citrus, grains, tea | Flavonoids | [106] | |
| Inhibited retinal cell apoptosis | Epicatechin | Green tea, coconut | Flavonoids | [109] | |
| 1. Suppressed cell apoptosis 2. Regulated apoptosis-related proteins (Bcl-2, BAX, and caspase-3) | Diosgenin | Fenugreek seeds, wild yam roots | Steroids | [113,114] | |
| 1. Prevented pericyte apoptosis by blocking the activities of p38 and JNK 2. Prevented apoptosis-associated protein (capsase-3) | U60E, C7A | Ulmus dacidiana | - | [115] | |
| Ferroptosis | Decreased the rate of ferroptosis by inhibiting the expression of miR-138-5p | Astragaloside-IV | Astragalus mambranaceus | Saponins | [55,84,85] |
| Cell phosphorylation | Regulated ERK1/2 phosphorylation | Astragaloside-IV | Astragalus mambranaceus | Saponins | [55,84,85] |
| Regulated ERK1/2 phosphorylation | Kaempferol | Kaempferia galanga L. | Flavonoids | [102,103] | |
| Regulated AKT and ERK phosphorylation | Chrysin | Oroxylum indicum (Linn.) Bentham ex Kurz | Flavonoids | [50] | |
| AGEs accumulation | Decreased AGEs production | Resveratrol | Grape, peanuts, berries | Phenols | [86,87,88,89] |
| Decreased AGEs production | Astaxanthin | Haematococcus pluvialis, shrimps | Terpenoids | [40,90] | |
| Reduced AGEs burden | Epicatechin | Green tea | Flavonoids | [109] | |
| MMP | Decreased MMP-2 expression | Chrysin | Oroxylum indicum (Linn.) Bentham ex Kurz | Flavonoids | [50] |
| Attenuated MMP-9 enzymatic activity | Nobiletin | Citrus plants | Flavonoids | [107] | |
| Autophagy | Activated the AMPK/mTOR/HO-1-regulated autophagy pathway | Arjunolic acid | Cyclocarya paliurus | Terpenoids | [37] |
| Leukocyte adhesion | Inhibited leukocyte adhesion and decreased ICAM-1 expression | Berberine | Rhizoma Coptidis | Alkaloids | [31,93,94] |
| Downregulated the protein expression of ICAM-1 and VCAM-1 | Ginsenoside Rg1, Ginsenoside Rb1 | Panax notoginseng sponins | - | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, Y.; Yan, N. The Role of Natural Products in Diabetic Retinopathy. Biomedicines 2024, 12, 1138. https://doi.org/10.3390/biomedicines12061138
Zhao Y, Chen Y, Yan N. The Role of Natural Products in Diabetic Retinopathy. Biomedicines. 2024; 12(6):1138. https://doi.org/10.3390/biomedicines12061138
Chicago/Turabian StyleZhao, Yuxuan, Yi Chen, and Naihong Yan. 2024. "The Role of Natural Products in Diabetic Retinopathy" Biomedicines 12, no. 6: 1138. https://doi.org/10.3390/biomedicines12061138
APA StyleZhao, Y., Chen, Y., & Yan, N. (2024). The Role of Natural Products in Diabetic Retinopathy. Biomedicines, 12(6), 1138. https://doi.org/10.3390/biomedicines12061138








