Activation of CD14+ Monocytes via the IFN-γ Signaling Pathway Is Associated with Immune-Related Adverse Events in Hepatocellular Carcinoma Patients Receiving PD-1 Inhibition Combination Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Evaluation Reference Criteria
2.3. Proteomic Profiling of Soluble Factors in Plasma
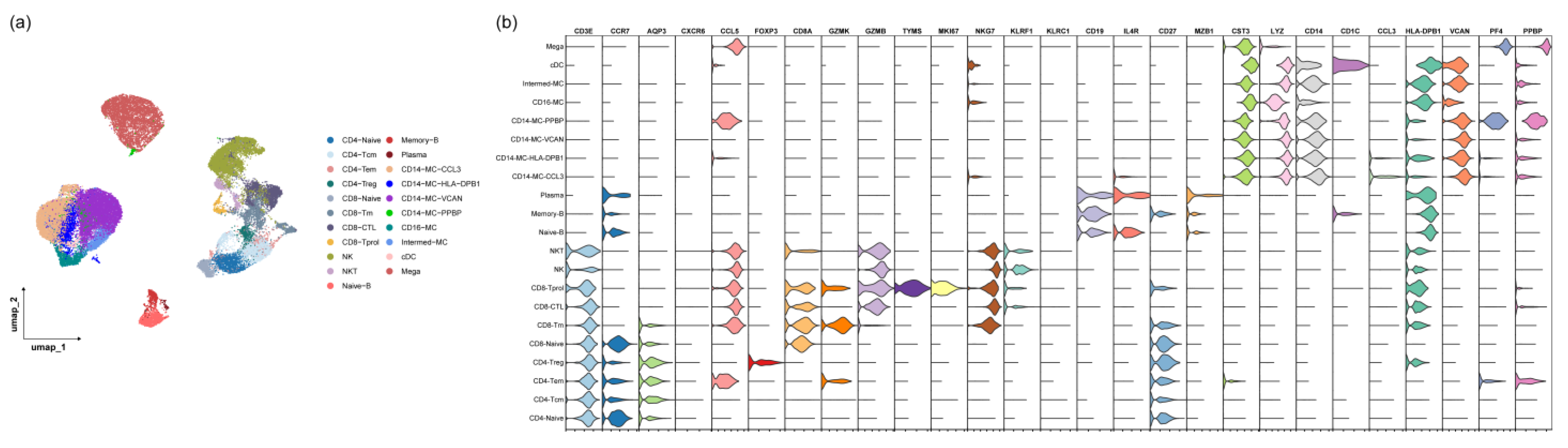
2.4. Single-Cell RNA Preparation and Sequencing
2.5. Single-Cell RNA Sequencing Data Analysis
2.6. Cellular Communication Analysis
2.7. Defining Cell State Scores
2.8. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Analysis of Plasma Proteomics Characteristics of irAEs
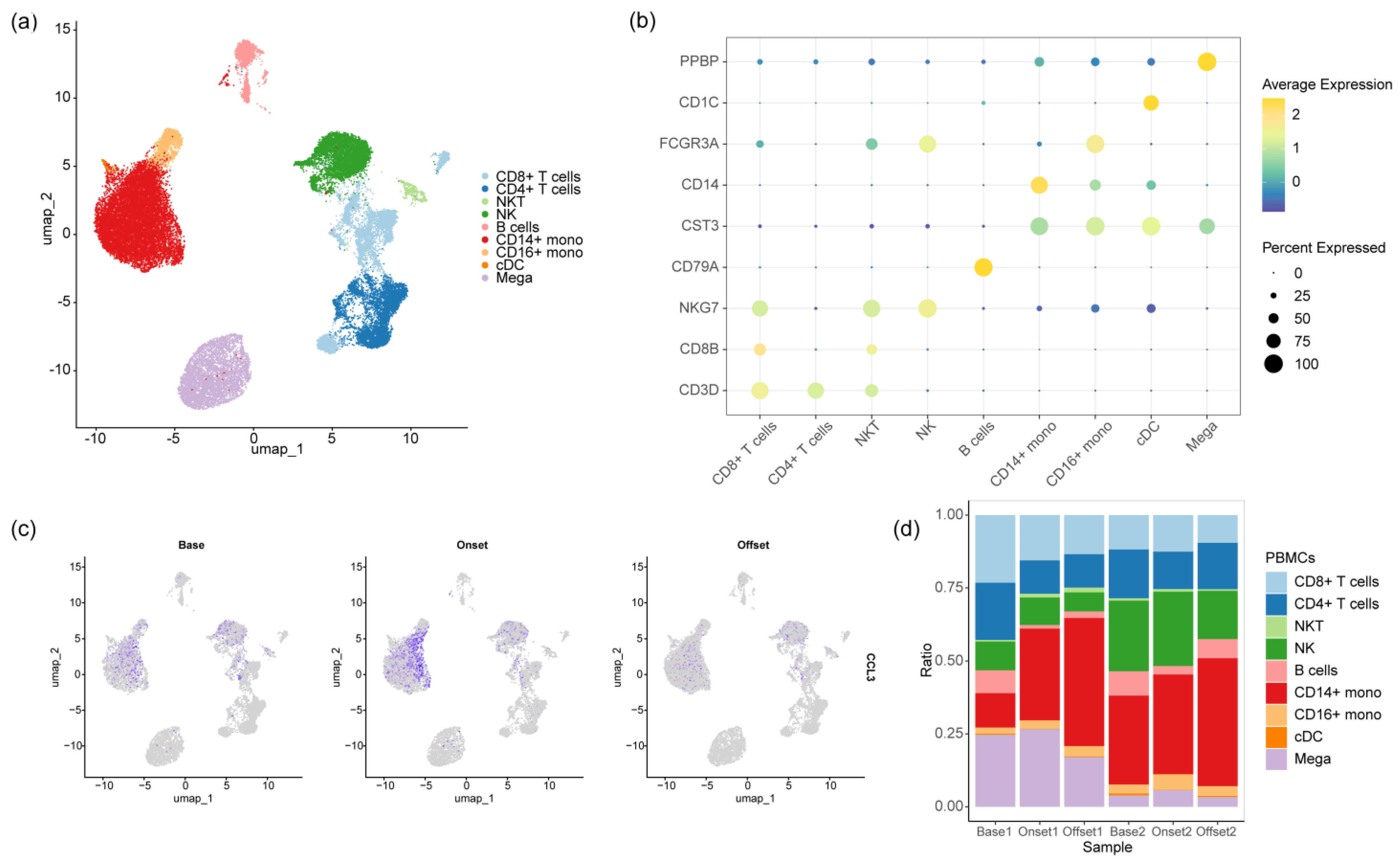
3.3. The Monocyte Proportion Increases during the Progression of irAEs
3.4. CD14-MC-CCL3 during the Occurrence of irAEs
3.5. The IFN-γ Signaling Pathway Promotes Monocyte Activation during the Occurrence of irAEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| All Patients n = 71 | |
|---|---|
| Objective Response Rate Using mRECIST v1.1 | n = 13 (18.3%) |
| Complete response | 1 (1.4%) |
| Partial response | 12 (16.9%) |
| Stable disease | 31 (43.7%) |
| Progressive disease | 20 (28.2%) |
| Unable to determine | 7 (9.9%) |
| Disease control rate, a | 51 (71.8%) |
Appendix B
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Sury, K.; Perazella, M.A.; Shirali, A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol. 2018, 14, 571–588. [Google Scholar] [CrossRef]
- Chuah, S.; Lee, J.; Song, Y.; Kim, H.D.; Wasser, M.; Kaya, N.A.; Bang, K.; Lee, Y.J.; Jeon, S.H.; Suthen, S.; et al. Uncoupling immune trajectories of response and adverse events from anti-PD-1 immunotherapy in hepatocellular carcinoma. J. Hepatol. 2022, 77, 683–694. [Google Scholar] [CrossRef]
- Yofe, I.; Dahan, R.; Amit, I. Single-cell genomic approaches for developing the next generation of immunotherapies. Nat. Med. 2020, 26, 171–177. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. (Engl. Ed.) 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.; Haanen, J.B.; Marmol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013, 31, 616–622. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Juhl, C.B.; Chen, I.M.; Kellermann, L.; Nielsen, O.H. Immune checkpoint Inhibitor-Induced diarrhea and Colitis: Incidence and Management. A systematic review and Meta-analysis. Cancer Treat. Rev. 2022, 109, 102440. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Zhou, Y.; Medik, Y.B.; Patel, B.; Zamler, D.B.; Chen, S.; Chapman, T.; Schneider, S.; Park, E.M.; Babcock, R.L.; Chrisikos, T.T.; et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220, e20221333. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef]
- Hommes, J.W.; Verheijden, R.J.; Suijkerbuijk, K.P.M.; Hamann, D. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front. Oncol. 2020, 10, 585311. [Google Scholar] [CrossRef]
- Gorbachev, A.V.; Kobayashi, H.; Kudo, D.; Tannenbaum, C.S.; Finke, J.H.; Shu, S.; Farber, J.M.; Fairchild, R.L. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J. Immunol. 2007, 178, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 1997, 61, 246–257. [Google Scholar] [CrossRef]
- Qian, C.; An, H.; Yu, Y.; Liu, S.; Cao, X. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood 2007, 109, 3308–3315. [Google Scholar] [CrossRef]
- Menke, J.; Zeller, G.C.; Kikawada, E.; Means, T.K.; Huang, X.R.; Lan, H.Y.; Lu, B.; Farber, J.; Luster, A.D.; Kelley, V.R. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J. Am. Soc. Nephrol. 2008, 19, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef]
- Fa’ak, F.; Buni, M.; Falohun, A.; Lu, H.; Song, J.; Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Awiwi, M.O.; Tahon, N.H.; et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J. Immunother. Cancer 2023, 11, e006814. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, M.F.; Torquato, H.; Salu, B.R.; Oliveira, A.C.D.; Oliva, M.L.V.; Paredes-Gamero, E.J.; Abdulahad, W.H.; Brouwer, E.; de Souza, A.W.S. Monocyte subsets and monocyte-related chemokines in Takayasu arteritis. Sci. Rep. 2023, 13, 2092. [Google Scholar] [CrossRef]
- Ren, X.; Wen, W.; Fan, X.; Hou, W.; Su, B.; Cai, P.; Li, J.; Liu, Y.; Tang, F.; Zhang, F.; et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell 2021, 184, 1895–1913.e19. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Potapov, I.; Chillara, S.; Del Sol, A. Leveraging systems biology for predicting modulators of inflammation in patients with COVID-19. Sci. Adv. 2021, 7, eabe5735. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Li, Y.; Yin, Q.; Li, H.; Li, J.; Zhou, B.; Meng, J.; Lian, H.; Wu, M.; et al. Versican Promotes Cardiomyocyte Proliferation and Cardiac Repair. Circulation 2024, 149, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.C.; Hao, D.; Jain, P.; Li, Y.; Cai, Q.; Yao, Y.; Nie, L.; Liu, Y.; Jin, J.; Wang, W.; et al. TIGIT is the central player in T-cell suppression associated with CAR T-cell relapse in mantle cell lymphoma. Mol. Cancer 2022, 21, 185. [Google Scholar] [CrossRef]
- Muller, W.A. New mechanisms and pathways for monocyte recruitment. J. Exp. Med. 2001, 194, F47–F51. [Google Scholar] [CrossRef]
- Imhof, B.A.; Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004, 4, 432–444. [Google Scholar] [CrossRef]
- Song, J.; Farris, D.; Ariza, P.; Moorjani, S.; Varghese, M.; Blin, M.; Chen, J.; Tyrrell, D.; Zhang, M.; Singer, K.; et al. Age-associated adipose tissue inflammation promotes monocyte chemotaxis and enhances atherosclerosis. Aging Cell 2023, 22, e13783. [Google Scholar] [CrossRef]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.A.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef]
- Postea, O.; Vasina, E.M.; Cauwenberghs, S.; Projahn, D.; Liehn, E.A.; Lievens, D.; Theelen, W.; Kramp, B.K.; Butoi, E.D.; Soehnlein, O.; et al. Contribution of platelet CX3CR1 to platelet-monocyte complex formation and vascular recruitment during hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1186–1193. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, H.; Luo, L.; Zhang, L.; Xiong, A.; Wang, J.; Wang, Q.; Liu, Y.; Liu, S.; Xiong, Y.; et al. Single cell characteristics of patients with vaccine-related adverse reactions following inactivated COVID-19 vaccination. Hum. Vaccin. Immunother. 2023, 19, 2246542. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Juhl, M.; Peng, Q.; Fink, T.; Porsborg, S.R. Selection and validation of reference genes for qPCR analysis of differentiation and maturation of THP-1 cells into M1 macrophage-like cells. Immunol. Cell Biol. 2022, 100, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Panek, C.A.; Ramos, M.V.; Mejias, M.P.; Abrey-Recalde, M.J.; Fernandez-Brando, R.J.; Gori, M.S.; Salamone, G.V.; Palermo, M.S. Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation. Cell. Mol. Immunol. 2015, 12, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Döcke, W.D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.D.; Kox, W. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat. Med. 1997, 3, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, H.P.; Arat, S.; Gao, J.; Wen, J.; Xia, S.; Kalabat, D.; Oziolor, E.; Virgen-Slane, R.; Affolter, T.; Ji, C. T cells and monocyte-derived myeloid cells mediate immunotherapy-related hepatitis in a mouse model. J. Hepatol. 2021, 75, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, J.Y.; Xing, X.; Huang, H.H.; Xia, P.; Dai, X.P.; Hu, W.; Zhang, C.; Song, J.W.; Fan, X.; et al. Global transcriptomic characterization of T cells in individuals with chronic HIV-1 infection. Cell Discov. 2022, 8, 29. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Sawada, M.; Goto, K.; Morimoto-Okazawa, A.; Haruna, M.; Yamamoto, K.; Yamamoto, Y.; Nakagawa, S.; Hiramatsu, K.; Matsuzaki, S.; Kobayashi, E.; et al. PD-1+ Tim3+ tumor-infiltrating CD8 T cells sustain the potential for IFN-γ production, but lose cytotoxic activity in ovarian cancer. Int. Immunol. 2020, 32, 397–405. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | No irAEs (n = 37) | irAEs (n = 34) | p |
|---|---|---|---|
| Age (years) | 55.51 ± 12.24 | 59.32 ± 10.18 | 0.157 |
| Sex | 0.876 | ||
| Male | 30 (81.1%) | 29 (85.3%) | |
| Female | 7 (18.9%) | 5 (14.7%) | |
| Basics of hepatitis | 0.680 | ||
| Viral hepatitis B | 30 (81.1%) | 27 (79.4%) | |
| Viral hepatitis C | 3 (8.1%) | 1 (2.9%) | |
| Alcoholic hepatitis | 3 (8.1%) | 4 (11.8%) | |
| Without basics of hepatitis | 1 (2.7%) | 2 (5.9%) | |
| BCLC stage | 0.390 | ||
| B | 12 (32.4%) | 7 (20.6%) | |
| C (PVTT) | 15 (40.5%) | 19 (55.9%) | |
| C (M) | 10 (27.1%) | 8 (23.5%) | |
| Child–Pugh stage | 0.710 | ||
| A | 19 (51.4%) | 15 (44.1%) | |
| B | 18 (48.6%) | 19 (55.9%) | |
| AFP | 1 | ||
| <400 ng/mL | 26 (70.3%) | 23 (67.6%) | |
| ≥400 ng/mL | 11 (29.7%) | 11 (32.4%) | |
| MELD | 9.5 (7, 11.5) | 8.5 (7, 11.5) | |
| Immunotherapy | 0.287 | ||
| Sintilimab | 14 (37.8%) | 19 (55.9%) | |
| Camrelizumab | 14 (37.8%) | 11 (32.4%) | |
| Tislelizumab | 7 (18.9%) | 2 (5.9%) | |
| Toripalimab | 2 (5.4%) | 2 (5.9%) | |
| Combination targeted treatment | <0.01 | ||
| Lenvatinib | 25 (67.6%) | 32 (94.1%) | |
| Sorafenib | 10 (27.0%) | 2 (5.9%) | |
| Bevacizumab | 2 (5.4%) | 0 | |
| Combined local–regional treatment | 0.410 | ||
| TACE | 8 (21.6%) | 3 (8.8%) | |
| Tumor ablation | 6 (16.2%) | 4 (11.8%) | |
| Radiation therapy | 1 (2.7%) | 1 (2.9%) | |
| No | 22 (59.5%) | 26 (76.5%) | |
| Objective response rate, a | 7 (18.9%) | 6 (17.6%) | 1 |
| Disease control rate, b | 24 (64.9%) | 20 (58.8%) | 0.780 |
| Patients with irAEs (n = 34) | ||
|---|---|---|
| Any Grade | Grade 3–4 | |
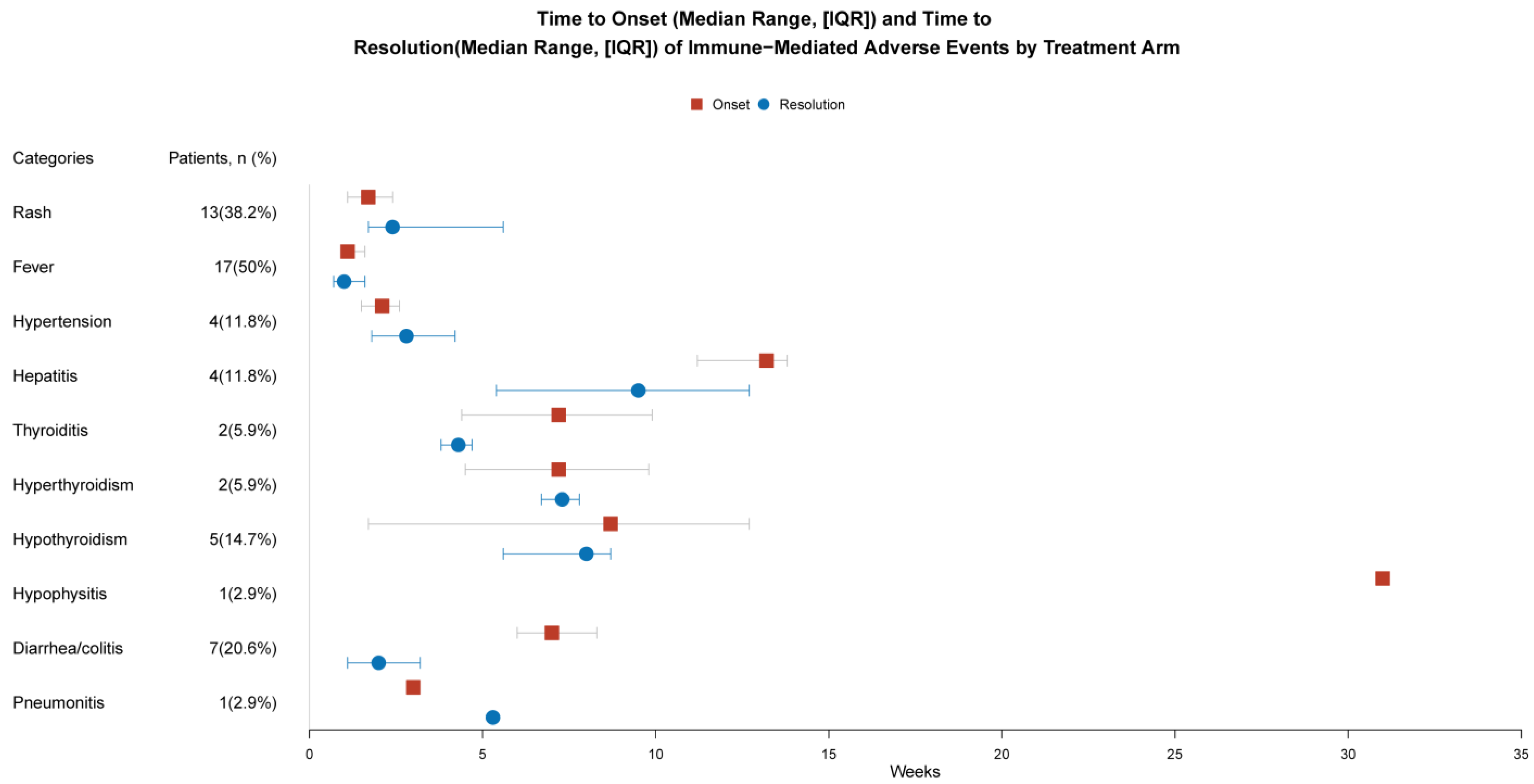
| Rash | 13 (38.2%) | 4 (11.8%) |
| Fever | 17 (50%) | 2 (5.9%) |
| Hypertension | 4 (11.8%) | 0 |
| Hepatitis | 4 (11.8%) | 3 (8.8%) |
| Thyroiditis | 2 (5.9%) | 0 |
| Hyperthyroidism | 2 (5.9%) | 0 |
| Hypothyroidism | 5 (14.7%) | 0 |
| Hypophysitis | 1 (2.9%) | 0 |
| Diarrhea/colitis | 7 (20.6%) | 1 (2.9%) |
| Pneumonitis | 1 (2.9%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Pan, S.; Tian, J.; Yu, Y.; Wang, S.; Qiu, Q.; Shen, Y.; Yang, L.; Liu, X.; Luan, J.; et al. Activation of CD14+ Monocytes via the IFN-γ Signaling Pathway Is Associated with Immune-Related Adverse Events in Hepatocellular Carcinoma Patients Receiving PD-1 Inhibition Combination Therapy. Biomedicines 2024, 12, 1140. https://doi.org/10.3390/biomedicines12061140
Song Y, Pan S, Tian J, Yu Y, Wang S, Qiu Q, Shen Y, Yang L, Liu X, Luan J, et al. Activation of CD14+ Monocytes via the IFN-γ Signaling Pathway Is Associated with Immune-Related Adverse Events in Hepatocellular Carcinoma Patients Receiving PD-1 Inhibition Combination Therapy. Biomedicines. 2024; 12(6):1140. https://doi.org/10.3390/biomedicines12061140
Chicago/Turabian StyleSong, Yaoru, Shida Pan, Jiahe Tian, Yingying Yu, Siyu Wang, Qin Qiu, Yingjuan Shen, Luo Yang, Xiaomeng Liu, Junqing Luan, and et al. 2024. "Activation of CD14+ Monocytes via the IFN-γ Signaling Pathway Is Associated with Immune-Related Adverse Events in Hepatocellular Carcinoma Patients Receiving PD-1 Inhibition Combination Therapy" Biomedicines 12, no. 6: 1140. https://doi.org/10.3390/biomedicines12061140
APA StyleSong, Y., Pan, S., Tian, J., Yu, Y., Wang, S., Qiu, Q., Shen, Y., Yang, L., Liu, X., Luan, J., Wang, Y., Wang, J., Fan, X., Meng, F., & Wang, F.-S. (2024). Activation of CD14+ Monocytes via the IFN-γ Signaling Pathway Is Associated with Immune-Related Adverse Events in Hepatocellular Carcinoma Patients Receiving PD-1 Inhibition Combination Therapy. Biomedicines, 12(6), 1140. https://doi.org/10.3390/biomedicines12061140





