Stellate Ganglion Block Attenuates LPS-Induced Acute Lung Injury by Activating Sirt3 Regulation of Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Design
2.3. Establishment of ALI Rats Model
2.4. Implement of SGB
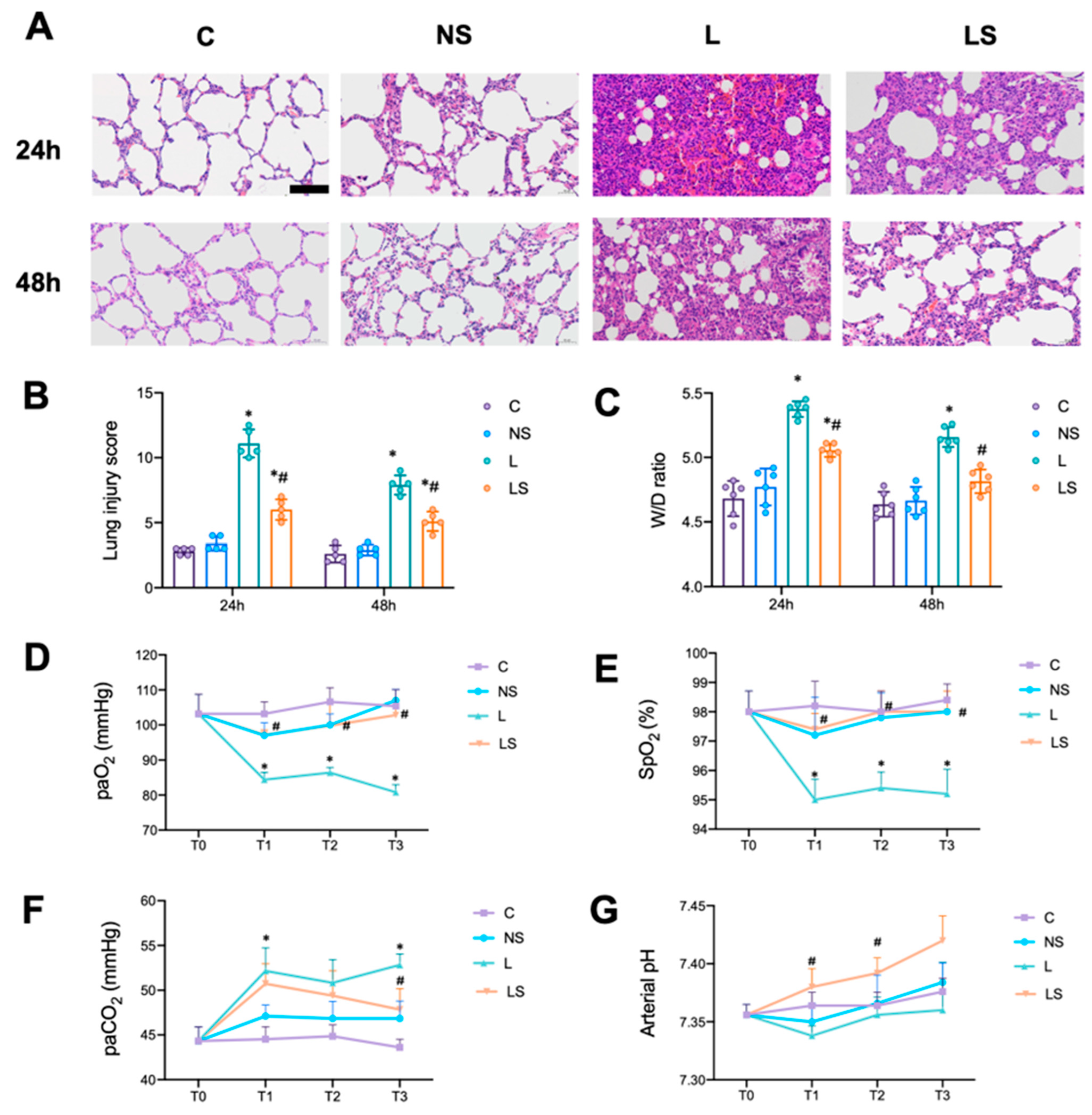
2.5. Lung Histopathology
2.6. Lung Wet/Dry (W/D) Weight Ratio
2.7. Arterial Blood Gas Analysis
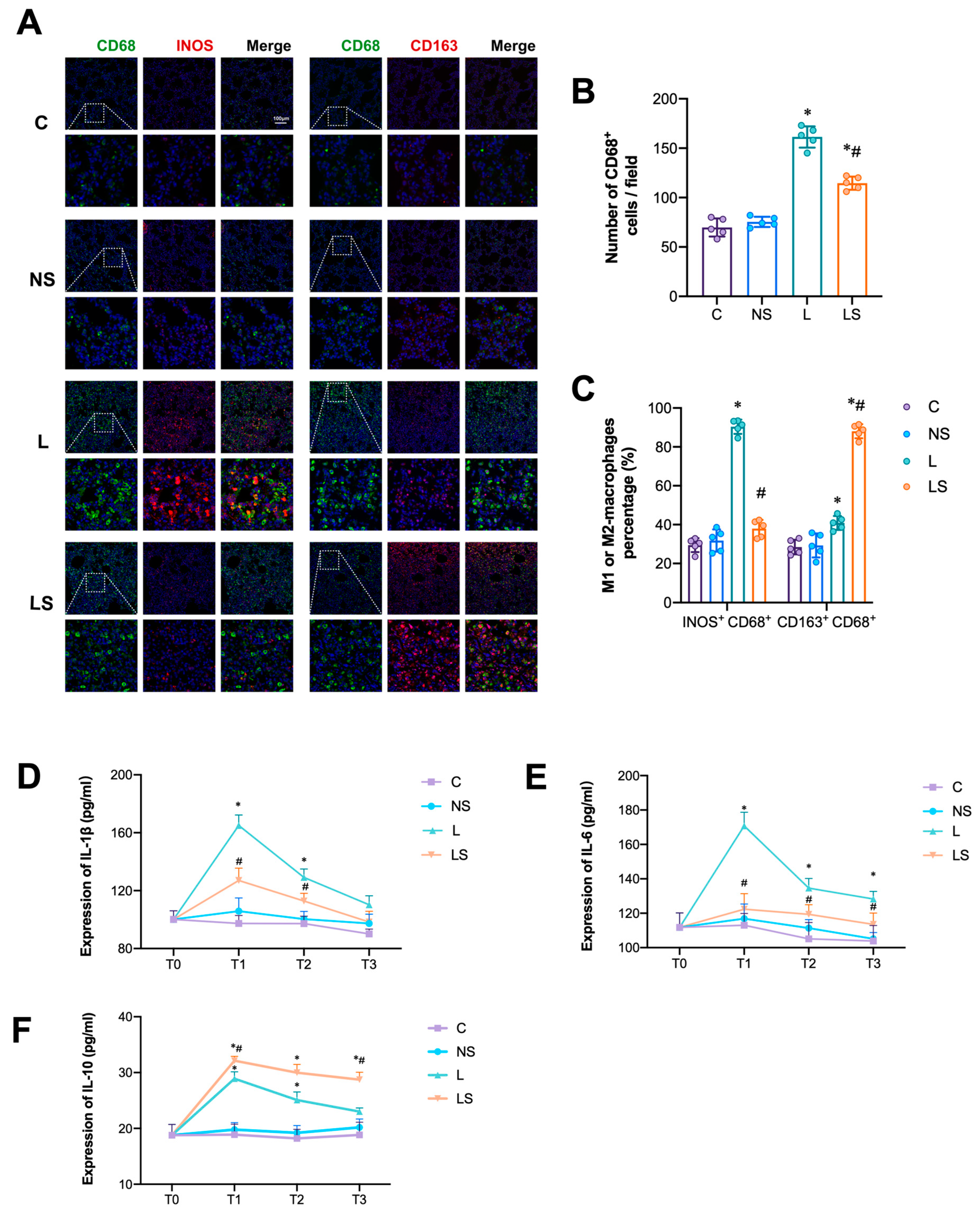
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
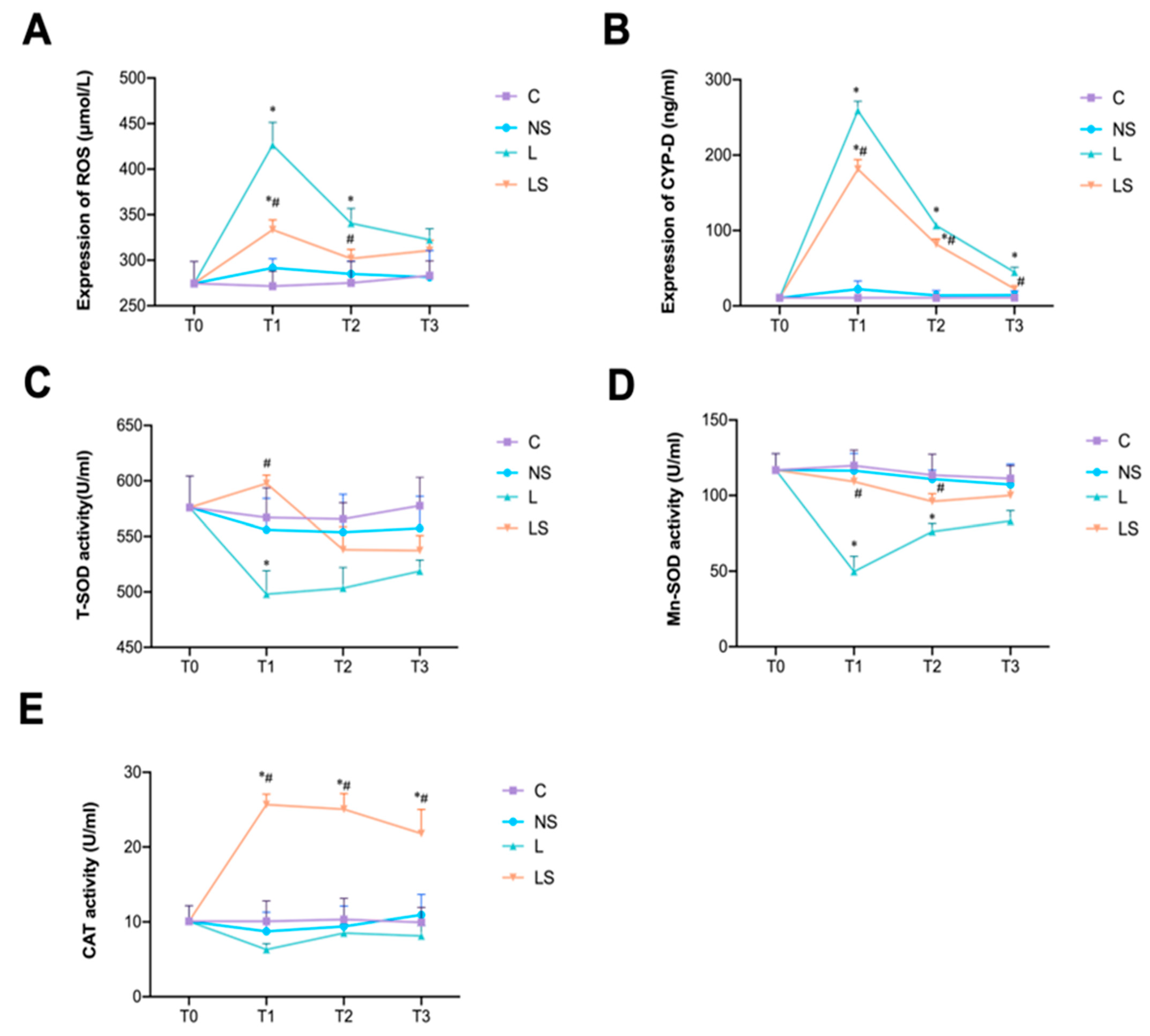
2.9. Oxidative and Antioxidant Indexes Assay
2.10. Immunofluorescence Analysis
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Horner Sign Presented after SGB
3.2. SGB Protected Rats from LPS-Induced ALI
3.3. SGB Inhibited Oxidative Stress
3.4. SGB Inhibited M1-AM Differentiation and Pro-Inflammatory Cytokines Secretion
3.5. Effects of SGB on Sirt3 Signaling-Associated Proteins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef]
- Gofeld, M.; Shankar, H. 56—Peripheral and Visceral Sympathetic Blocks. In Practical Management of Pain, 5th ed.; Benzon, H.T., Rathmell, J.P., Wu, C.L., Turk, D.C., Argoff, C.E., Hurley, R.W., Eds.; Mosby: Philadelphia, PA, USA, 2014; pp. 755–767.e2. [Google Scholar]
- Gunduz, O.H.; Kenis-Coskun, O. Ganglion blocks as a treatment of pain: Current perspectives. J. Pain Res. 2017, 10, 2815–2826. [Google Scholar] [CrossRef]
- Olmsted, K.L.R.; Bartoszek, M.; Mulvaney, S.; McLean, B.; Turabi, A.; Young, R.; Kim, E.; Vandermaas-Peeler, R.; Morgan, J.K.; Constantinescu, O.; et al. Effect of Stellate Ganglion Block Treatment on Posttraumatic Stress Disorder Symptoms: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 130–138. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, T.; Li, W.; Bo, Y. Regulating autonomic nervous system homeostasis improves pulmonary function in rabbits with acute lung injury. BMC Pulm. Med. 2017, 17, 98. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Lang, H.; Hu, X.; Jing, S.; Luo, M.; Xu, G.; Zhou, Z. Effect of a Stellate Ganglion Block on Acute Lung Injury in Septic Rats. Inflammation 2018, 41, 1601–1609. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, N.; Li, Y.; Ma, Q.; Zhou, Y.; Qiao, Z.; Li, S.; Liu, C.; Zhang, L.; Yuan, M.; et al. Stellate ganglion block relieves acute lung injury induced by severe acute pancreatitis via the miR-155-5p/SOCS5/JAK2/STAT3 axis. Eur. J. Med. Res. 2022, 27, 231. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T., IV; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef]
- Kurundkar, D.; Kurundkar, A.R.; Bone, N.B.; Becker, E.J., Jr.; Liu, W.; Chacko, B.; Darley-Usmar, V.; Zmijewski, J.W.; Thannickal, V.J. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 2019, 4, e120722. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Han, K.; Wang, Y.; Wang, L.; Gao, J.; Zhao, S.; Wang, G.; Chen, S.; Luo, A.; et al. Mitochondrial PKM2 deacetylation by procyanidin B2-induced SIRT3 upregulation alleviates lung ischemia/reperfusion injury. Cell Death Dis. 2022, 13, 594. [Google Scholar] [CrossRef]
- Aziz, M.; Brenner, M.; Wang, P. Extracellular CIRP (eCIRP) and inflammation. J. Leukoc. Biol. 2019, 106, 133–146. [Google Scholar] [CrossRef]
- Zhou, M.; Aziz, M.; Yen, H.T.; Ma, G.; Murao, A.; Wang, P. Extracellular CIRP dysregulates macrophage bacterial phagocytosis in sepsis. Cell Mol. Immunol. 2023, 20, 80–93. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, M.; Guo, H.; Liu, H.; Zhang, G.; Xu, C.; Chen, H. Emodin Alleviates Severe Acute Pancreatitis-Associated Acute Lung Injury by Inhibiting the Cold-Inducible RNA-Binding Protein (CIRP)-Mediated Activation of the NLRP3/IL-1β/CXCL1 Signaling. Front. Pharmacol. 2021, 12, 655372. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kong, Q.; Xia, Z.; Zhan, L.; Duan, W.; Song, X. Penehyclidine hydrochloride alleviates lipopolysaccharideinduced acute lung injury in rats: Potential role of caveolin1 expression upregulation. Int. J. Mol. Med. 2019, 43, 2064–2074. [Google Scholar]
- Gulcu, N.; Gonca, E.; Kocoglu, H. A lateral percutaneous technique for stellate ganglion blockade in rats. Anesth. Analg. 2009, 108, 1701–1704. [Google Scholar] [CrossRef]
- Song, C.; Li, H.; Li, Y.; Dai, M.; Zhang, L.; Liu, S.; Tan, H.; Deng, P.; Liu, J.; Mao, Z.; et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp. Cell Res. 2019, 382, 111486. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Suzuki, Y.; Yoshimura, K.; Yasui, H.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Macrophage Mannose Receptor CD206 Predicts Prognosis in Community-acquired Pneumonia. Sci. Rep. 2019, 9, 18750. [Google Scholar] [CrossRef]
- Yang, L.; Xie, M.; Yang, M.; Yu, Y.; Zhu, S.; Hou, W.; Kang, R.; Lotze, M.T.; Billiar, T.R.; Wang, H.; et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014, 5, 4436. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.; Dai, X.; Xu, S.; Li, T.; Zhang, Q.; Zhao, K.S.; Chen, Z. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert. Opin. Ther. Targets 2016, 20, 645–652. [Google Scholar] [CrossRef]
- Wu, J.; Deng, Z.; Sun, M.; Zhang, W.; Yang, Y.; Zeng, Z.; Wu, J.; Zhang, Q.; Liu, Y.; Chen, Z.; et al. Polydatin protects against lipopolysaccharide-induced endothelial barrier disruption via SIRT3 activation. Lab. Investig. 2020, 100, 643–656. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Zhong, P.; Huang, H. Recent progress in the research of cold-inducible RNA-binding protein. Future Sci. OA. 2017, 3, FSO246. [Google Scholar] [CrossRef]
- Qiang, X.; Yang, W.L.; Wu, R.; Zhou, M.; Jacob, A.; Dong, W.; Kuncewitch, M.; Ji, Y.; Yang, H.; Wang, H.; et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 2013, 19, 1489–1495. [Google Scholar] [CrossRef]
- Chen, L.; Ran, D.; Xie, W.; Xu, Q.; Zhou, X. Cold-inducible RNA-binding protein mediates cold air inducible airway mucin production through TLR4/NF-kappaB signaling pathway. Int. Immunopharmacol. 2016, 39, 48–56. [Google Scholar] [CrossRef]
- Juan, Y.; Haiqiao, W.; Xie, W.; Huaping, H.; Zhong, H.; Xiangdong, Z.; Kolosov, V.P.; Perelman, J.M. Cold-inducible RNA-binding protein mediates airway inflammation and mucus hypersecretion through a post-transcriptional regulatory mechanism under cold stress. Int. J. Biochem. Cell Biol. 2016, 78, 335–348. [Google Scholar] [CrossRef]
- Yang, W.L.; Sharma, A.; Wang, Z.; Li, Z.; Fan, J.; Wang, P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci. Rep. 2016, 6, 26571. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Pillars Article: Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef]
- Jie, X.L.; Luo, Z.R.; Yu, J.; Tong, Z.R.; Li, Q.Q.; Wu, J.H.; Tao, Y.; Feng, P.S.; Lan, J.P.; Wang, P. Pi-Pa-Run-Fei-Tang alleviates lung injury by modulating IL-6/JAK2/STAT3/IL-17 and PI3K/AKT/NF-κB signaling pathway and balancing Th17 and Treg in murine model of OVA-induced asthma. J. Ethnopharmacol. 2023, 317, 116719. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhang, H.; Zhuo, Y.; Zhang, L.; Yang, L.; Gao, Q.; Tu, Z.; Shao, R.; Wang, Y.; et al. Xuanfei Baidu Decoction suppresses complement overactivation and ameliorates IgG immune complex-induced acute lung injury by inhibiting JAK2/STAT3/SOCS3 and NF-κB signaling pathway. Phytomedicine 2023, 109, 154551. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 2013, 55, 275–286. [Google Scholar] [CrossRef]
- Liu, W.; Qaed, E.; Zhu, H.G.; Dong, M.X.; Tang, Z. Non-energy mechanism of phosphocreatine on the protection of cell survival. Biomed. Pharmacother. 2021, 141, 111839. [Google Scholar] [CrossRef]
- Lv, D.; Luo, M.; Yan, J.; Yang, X.; Luo, S. Protective Effect of Sirtuin 3 on CLP-Induced Endothelial Dysfunction of Early Sepsis by Inhibiting NF-κB and NLRP3 Signaling Pathways. Inflammation 2021, 44, 1782–1792. [Google Scholar] [CrossRef]


 denotes inhibition.
denotes inhibition.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Ji, J.; Li, R.; Gao, L.; He, X. Stellate Ganglion Block Attenuates LPS-Induced Acute Lung Injury by Activating Sirt3 Regulation of Oxidative Stress and Inflammation. Biomedicines 2024, 12, 1148. https://doi.org/10.3390/biomedicines12061148
Dai S, Ji J, Li R, Gao L, He X. Stellate Ganglion Block Attenuates LPS-Induced Acute Lung Injury by Activating Sirt3 Regulation of Oxidative Stress and Inflammation. Biomedicines. 2024; 12(6):1148. https://doi.org/10.3390/biomedicines12061148
Chicago/Turabian StyleDai, Shiyun, Jun Ji, Rongrong Li, Lu Gao, and Xingying He. 2024. "Stellate Ganglion Block Attenuates LPS-Induced Acute Lung Injury by Activating Sirt3 Regulation of Oxidative Stress and Inflammation" Biomedicines 12, no. 6: 1148. https://doi.org/10.3390/biomedicines12061148
APA StyleDai, S., Ji, J., Li, R., Gao, L., & He, X. (2024). Stellate Ganglion Block Attenuates LPS-Induced Acute Lung Injury by Activating Sirt3 Regulation of Oxidative Stress and Inflammation. Biomedicines, 12(6), 1148. https://doi.org/10.3390/biomedicines12061148






