Number of Premature Ventricular Complexes Predicts Long-Term Outcomes in Patients with Persistent Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Outcomes
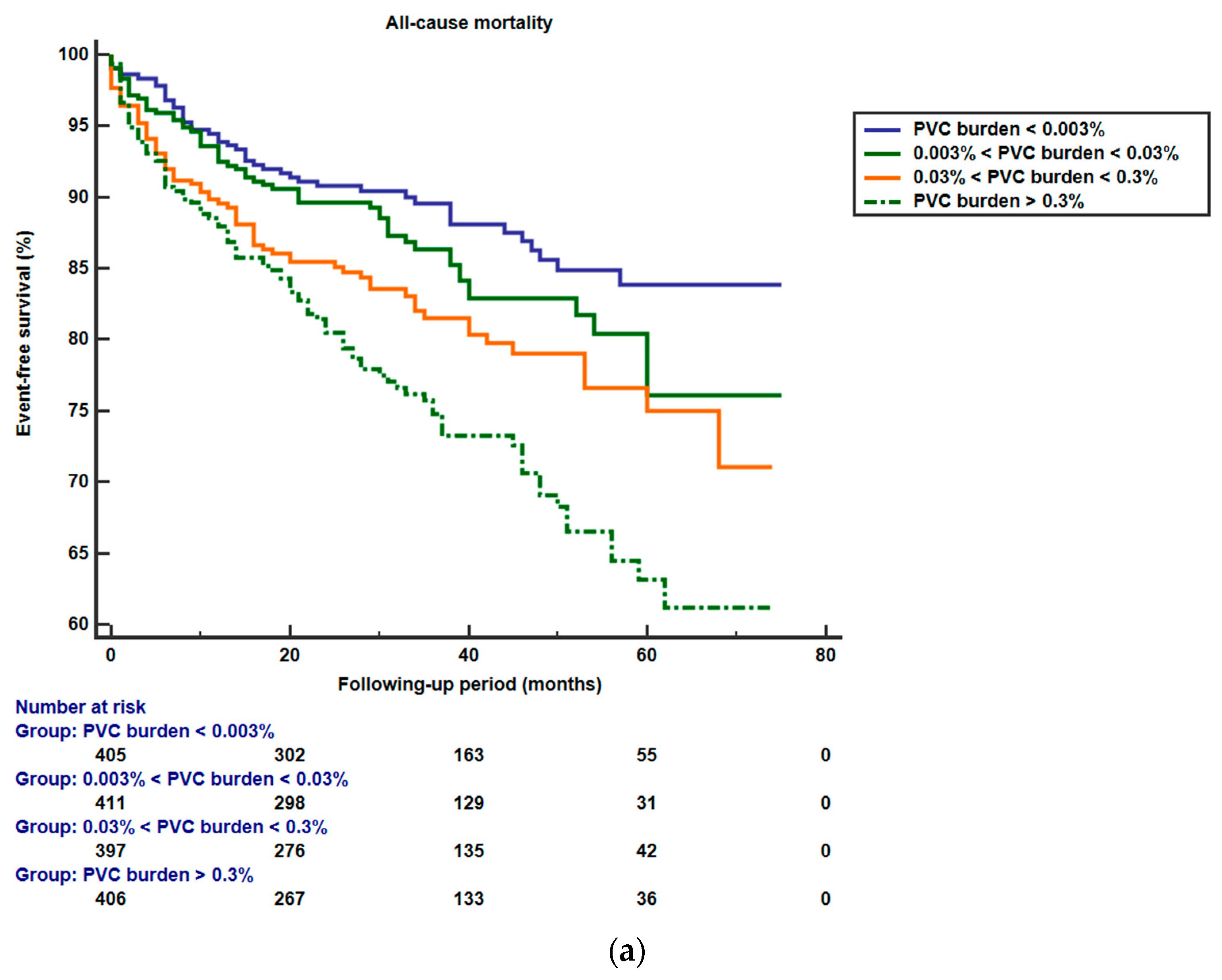
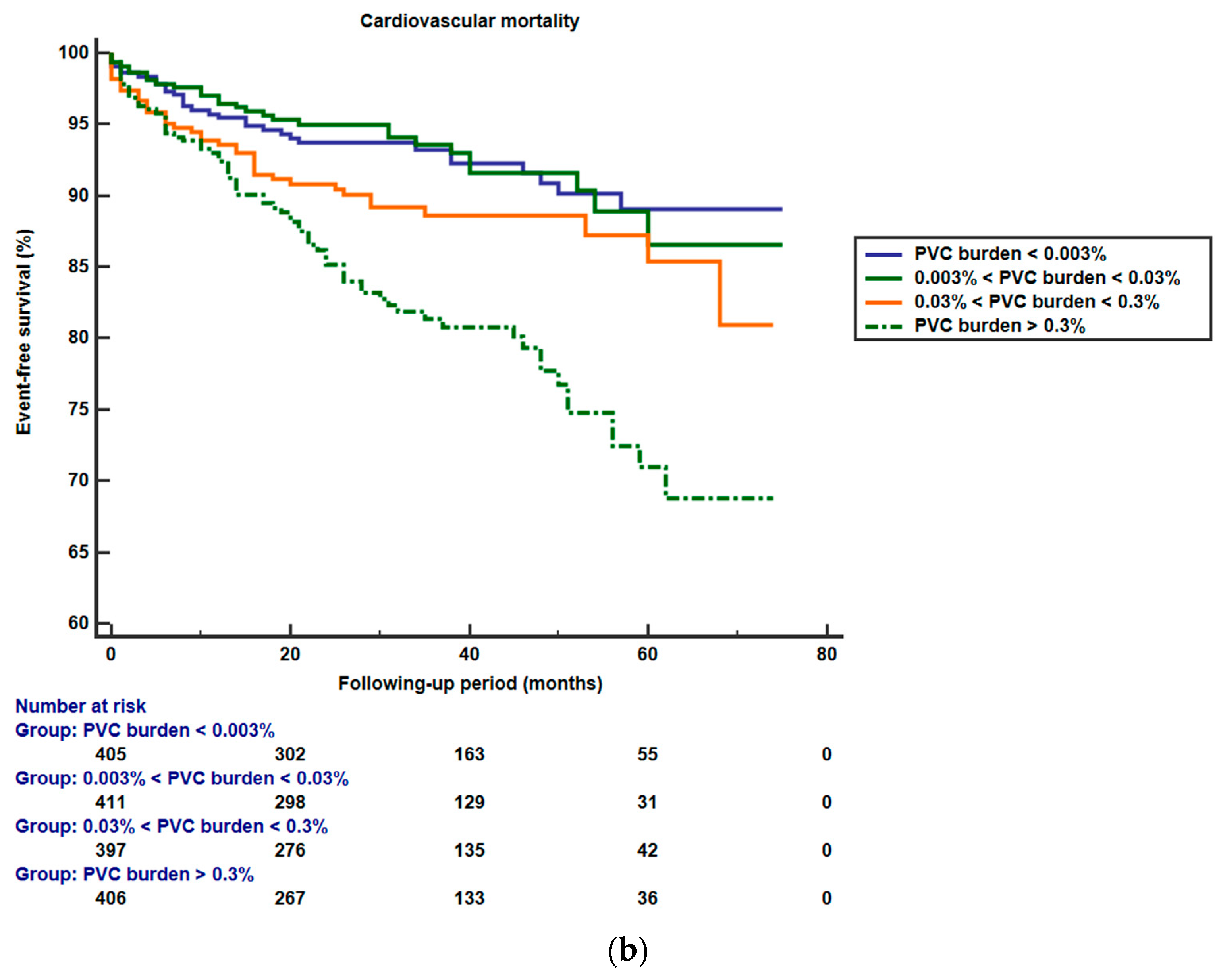
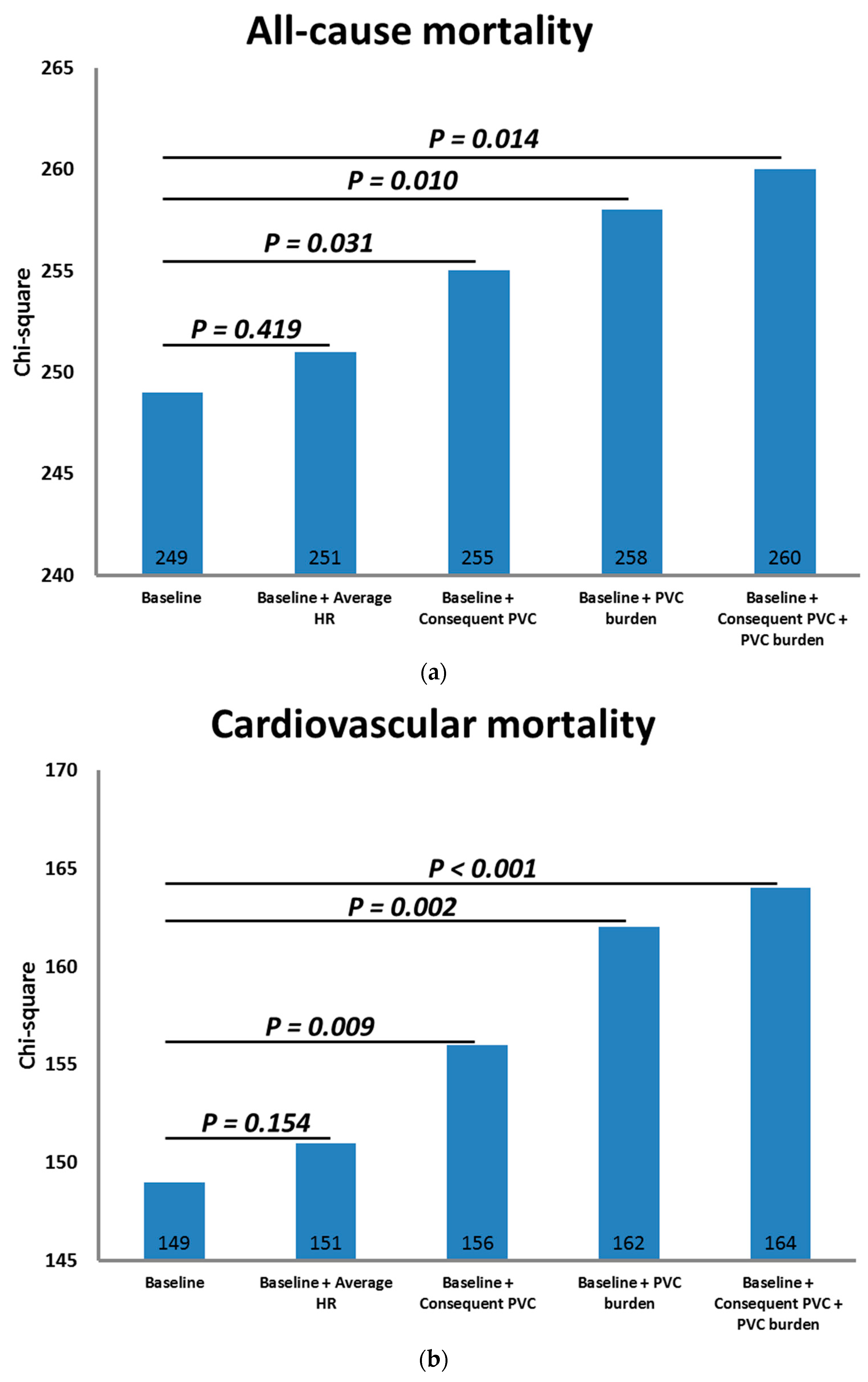
3.2. PVCs and Outcomes
4. Discussions
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, R.J., Jr.; Cascio, W.E.; Schreiner, P.J.; Crow, R.S.; Rautaharju, P.M.; Heiss, G. Prevalence of premature ventricular contractions in a population of African American and white men and women: The Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2002, 143, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.; Cho, S.; Ghayoumi, A.; Yamazaki, T.; Chun, S.; Fearon, W.F.; Froelicher, V.F. Prognostic significance of PVCs and resting heart rate. Ann. Noninvasive Electrocardiol. 2007, 12, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-M.; Lee, G.K.; Klarich, K.W.; Grogan, M. Premature ventricular contraction-induced cardiomyopathy: A treatable condition. Circ. Arrhythmia Electrophysiol. 2012, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.A. Treating patients with ventricular ectopic beats. Heart 2006, 92, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Ataklte, F.; Erqou, S.; Laukkanen, J.; Kaptoge, S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am. J. Cardiol. 2013, 112, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.W.; Dewland, T.A.; Vittinghoff, E.; Mandyam, M.C.; Heckbert, S.R.; Siscovick, D.S.; Stein, P.K.; Psaty, B.M.; Sotoodehnia, N.; Gottdiener, J.S.; et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J. Am. Coll. Cardiol. 2015, 66, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, G.; Levine, M.; Friedmann, P.; Schweitzer, P. The prognostic significance of frequency and morphology of premature ventricular complexes during ambulatory holter monitoring. Ann. Noninvasive Electrocardiol. 2013, 18, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, S.L.; Lin, Y.J.; Lo, L.W.; Chung, F.P.; Chen, Y.Y.; Chao, T.F.; Hu, Y.F.; Tuan, T.C.; Liao, J.N.; et al. Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int. J. Cardiol. 2015, 180, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Ishikawa, S.; Gotoh, T.; Kabutoya, T.; Kayaba, K.; Kajii, E. Cardiac mortality of premature ventricular complexes in healthy people in Japan. J. Cardiol. 2010, 56, 23–26. [Google Scholar] [CrossRef]
- Sajadieh, A.; Nielsen, O.W.; Rasmussen, V.; Hein, H.O.; Frederiksen, B.S.; Davanlou, M.; Hansen, J.F. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am. J. Cardiol. 2006, 97, 1351–1357. [Google Scholar] [CrossRef]
- Chiang, C.E.; Wu, T.J.; Ueng, K.C.; Chao, T.F.; Chang, K.C.; Wang, C.C.; Lin, Y.J.; Yin, W.H.; Kuo, J.Y.; Lin, W.S.; et al. 2016 Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J. Formos. Med. Assoc. Taiwan Yi Zhi 2016, 115, 893–952. [Google Scholar] [CrossRef] [PubMed]
- Behnes, M.; Rusnak, J.; Taton, G.; Schupp, T.; Reiser, L.; Bollow, A.; Reichelt, T.; Engelke, N.; Ellguth, D.; Kuche, P. Atrial fibrillation is associated with increased mortality in patients presenting with ventricular tachyarrhythmias. Sci. Rep. 2019, 9, 14291. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.-Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010, 7, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio Munoz, F.; Syed, F.F.; Noheria, A.; Cha, Y.M.; Friedman, P.A.; Hammill, S.C.; Munger, T.M.; Venkatachalam, K.L.; Shen, W.K.; Packer, D.L.; et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J. Cardiovasc. Electrophysiol. 2011, 22, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Yoshimura, H.; Ohba, Y.; Matsumoto, Y.; Yamamoto, U.; Mohri, M.; Yamamoto, H.; Origuchi, H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J. Am. Coll. Cardiol. 2005, 45, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Huizar, J.F.; Ellenbogen, K.A.; Tan, A.Y.; Kaszala, K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2328–2344. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-T.; Huang, T.-C.; Huang, M.-H.; Hsu, L.-W.; Su, P.-F.; Liu, Y.-W.; Hung, M.-H.; Liu, P.-Y. The burden of ventricular premature complex is associated with cardiovascular mortality. Front. Cardiovasc. Med. 2022, 8, 797976. [Google Scholar] [CrossRef]
- Bogun, F.; Crawford, T.; Reich, S.; Koelling, T.M.; Armstrong, W.; Good, E.; Jongnarangsin, K.; Marine, J.E.; Chugh, A.; Pelosi, F.; et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007, 4, 863–867. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, S.-L.; Lin, Y.-J.; Chen, Y.-Y.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Chao, T.-F.; Chung, F.-P.; Liao, J.-N. An observational study on the effect of premature ventricular complex burden on long-term outcome. Medicine 2017, 96, e5476. [Google Scholar] [CrossRef]
- Singh, S.N.; Fletcher, R.D.; Fisher, S.G.; Singh, B.N.; Lewis, H.D.; Deedwania, P.C.; Massie, B.M.; Colling, C.; Lazzeri, D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. N. Engl. J. Med. 1995, 333, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, V.A.; Liew, R.; Ward, D.E. Catheter ablation of premature ventricular contraction-induced cardiomyopathy. Nat. Clin. Pr. Cardiovasc. Med. 2008, 5, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Lee, Y.-H.; Huang, X.-M.; Asirvatham, S.J.; Shen, W.-K.; Friedman, P.A.; Hodge, D.O.; Slusser, J.P.; Song, Z.-Y.; Packer, D.L. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: A single-center retrospective study. Heart Rhythm. 2014, 11, 187–193. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Yokokawa, M.; Morady, F.; Kim, H.M.; Mathew, S.; Tilz, R.; Kuck, K.-H.; Nagashima, K.; Tedrow, U.; Stevenson, W.G. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin. Electrophysiol. 2015, 1, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Potfay, J.; Kaszala, K.; Tan, A.Y.; Sima, A.P.; Gorcsan III, J.; Ellenbogen, K.A.; Huizar, J.F. Abnormal left ventricular mechanics of ventricular ectopic beats: Insights into origin and coupling interval in premature ventricular contraction–induced cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2015, 8, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Kowlgi, G.N.; Ramirez, R.J.; Balderas-Villalobos, J.; Jovin, D.; Parker, C.; Om, E.; Airapetov, S.; Kaszala, K.; Tan, A.Y. Eccentric hypertrophy in an animal model of mid-and long-term premature ventricular contraction–induced cardiomyopathy. Heart Rhythm O2 2021, 2, 80–88. [Google Scholar] [CrossRef]
- Walters, T.E.; Szilagyi, J.; Alhede, C.; Sievers, R.; Fang, Q.; Olgin, J.; Gerstenfeld, E.P. Dyssynchrony and fibrosis persist after resolution of cardiomyopathy in a swine premature ventricular contraction model. Clin. Electrophysiol. 2020, 6, 1367–1376. [Google Scholar] [CrossRef]
- Gupta, A.; Harrington, M.; Albert, C.M.; Bajaj, N.S.; Hainer, J.; Morgan, V.; Bibbo, C.F.; Bravo, P.E.; Osborne, M.T.; Dorbala, S. Myocardial scar but not ischemia is associated with defibrillator shocks and sudden cardiac death in stable patients with reduced left ventricular ejection fraction. JACC Clin. Electrophysiol. 2018, 4, 1200–1210. [Google Scholar] [CrossRef]
- Modin, D.; Biering-Sørensen, S.R.; Møgelvang, R.; Jensen, J.S.; Biering-Sørensen, T. Prognostic importance of left ventricular mechanical dyssynchrony in predicting cardiovascular death in the general population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2018, 11, e007528. [Google Scholar] [CrossRef]
- Olgun, H.; Yokokawa, M.; Baman, T.; Kim, H.M.; Armstrong, W.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Crawford, T.; Oral, H. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011, 8, 1046–1049. [Google Scholar] [CrossRef]
- Sun, Y.; Blom, N.A.; Yu, Y.; Ma, P.; Wang, Y.; Han, X.; Swenne, C.A.; van der Wall, E.E. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: An echocardiographic evaluation. Int. J. Cardiovasc. Imaging 2003, 19, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, J.S.; Homsi, M.; Shivkumar, K.; Miller, J.M. Coupling interval variability differentiates ventricular ectopic complexes arising in the aortic sinus of valsalva and great cardiac vein from other sources: Mechanistic and arrhythmic risk implications. J. Am. Coll. Cardiol. 2014, 63, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Park, K.-H.; Nam, J.-H.; Lee, J.; Choi, Y.-J.; Kong, E.-J.; Lee, H.-W.; Son, J.-W.; Kim, U.; Park, J.-S. Increased variability of the coupling interval of premature ventricular contractions as a predictor of cardiac mortality in patients with left ventricular dysfunction. Circ. J. 2015, 79, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Badhwar, N.; Vedantham, V.; Tseng, Z.H.; Lee, B.K.; Lee, R.J.; Marcus, G.M.; Olgin, J.E.; Gerstenfeld, E.P.; Scheinman, M.M. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Hamon, D.; Rajendran, P.S.; Chui, R.W.; Ajijola, O.A.; Irie, T.; Talebi, R.; Salavatian, S.; Vaseghi, M.; Bradfield, J.S.; Armour, J.A. Premature ventricular contraction coupling interval variability destabilizes cardiac neuronal and electrophysiological control: Insights from simultaneous cardioneural mapping. Circ. Arrhythmia Electrophysiol. 2017, 10, e004937. [Google Scholar] [CrossRef] [PubMed]
- Scorza, R.; Jonsson, M.; Friberg, L.; Rosenqvist, M.; Frykman, V. Prognostic implication of premature ventricular contractions in patients without structural heart disease. Europace 2023, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Huang, M.H.; Huang, T.C.; Hsu, C.H.; Lin, S.H.; Liu, P.Y. High burden of premature ventricular complex increases the risk of new-onset atrial fibrillation. J. Am. Heart Assoc. 2023, 12, e027674. [Google Scholar] [CrossRef] [PubMed]
- Behnes, M.; Hoffmann, U.; Lang, S.; Weiss, C.; Ahmad-Nejad, P.; Neumaier, M.; Borggrefe, M.; Brueckmann, M. Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin. Res. Cardiol. 2011, 100, 335–342. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Atrial fibrillation: Effects beyond the atrium? Cardiovasc. Res. 2015, 105, 238–247. [Google Scholar] [CrossRef]
- Chishaki, A.S.; Li, F.-J.; Takeshita, A.; Chishaki, H. Different features of ventricular arrhythmias and the RR-interval dynamics in atrial fibrillation related to the patient’s clinical characteristics: An analysis using RR-interval plotting. J. Electrocardiol. 2004, 37, 207–217. [Google Scholar] [CrossRef]
- Rashba, E.J.; NA, E.I. Is there a mechanistic link between atrial fibrillation and vulnerability to ventricular arrhythmias? J. Cardiovasc. Electrophysiol. 2011, 22, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Rordorf, R.; De Ferrari, G.M.; Leonardi, S.; Thomas, L.; Wojdyla, D.M.; Ridefelt, P.; Lawrence, J.H.; De Caterina, R.; Vinereanu, D.; et al. Digoxin and Mortality in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Fonarow, G.C.; Simon, D.N.; Thomas, L.E.; Marzec, L.N.; Pokorney, S.D.; Gersh, B.J.; Go, A.S.; Hylek, E.M.; Kowey, P.R.; et al. Digoxin Use and Subsequent Outcomes Among Patients in a Contemporary Atrial Fibrillation Cohort. J. Am. Coll. Cardiol. 2015, 65, 2691–2698. [Google Scholar] [CrossRef]
| Survivors (n = 1481) | Non-Survivors (n = 286) | p-Value | |
|---|---|---|---|
| Clinical variables | |||
| Age, years | 69.0 ± 12.9 | 77.0 ± 11.0 | <0.001 |
| Men, % | 60.4 | 54.2 | 0.049 |
| CHA2DS2-VASc | 2.9 ± 1.8 | 4.2 ± 1.7 | <0.001 |
| Diabetes mellitus, % | 25.9 | 35.3 | 0.001 |
| Hypertension, % | 57.5 | 65.7 | 0.010 |
| Ischemic stroke, % | 15.6 | 29.7 | <0.001 |
| Vascular disease, % | 11.4 | 18.2 | 0.002 |
| Heart failure, % | 21.4 | 38.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 75.2 ± 33.4 | 60.9 ± 36.6 | <0.001 |
| LVEF, % | 59.4 ± 14.6 | 60.4 ± 15.2 | 0.342 |
| Resting HR, bpm | 81.0 ± 19.7 | 80.7 ± 21.9 | 0.769 |
| Systolic blood pressure, mmHg | 129.1 ± 22.0 | 129.2 ± 24.8 | 0.945 |
| Diastolic blood pressure, mmHg | 73.9 ± 14.9 | 76.6 ± 14.0 | 0.003 |
| Medications | |||
| Beta-blocker, % | 68.4 | 61.7 | 0.029 |
| ACEI or ARB, % | 66.7 | 63.9 | 0.363 |
| Non-dihydropyridine CCB, % | 50.2 | 57.9 | 0.017 |
| Digoxin, % | 37.3 | 46.0 | 0.006 |
| Anti-arrhythmic agent, % | 26.2 | 23.5 | 0.349 |
| Anticoagulant, % | 67.7 | 51.0 | <0.001 |
| 24-h Holter | |||
| 24-h average HR, bpm | 82 ± 19 | 82 ± 21 | 0.983 |
| Total PVC burden (%) * | 0.03 (0.00–0.25) | 0.09 (0.01–0.80) | <0.001 |
| Total PVC numbers * | 29 (2–268) | 108 (6–949) | <0.001 |
| Isolated PVC number * | 27 (1–263) | 100 (6–890) | <0.001 |
| PVC couplet episodes | 4.0 ± 11.0 | 6.8 ± 15.1 | <0.001 |
| PVC triplet episodes | 0.6 ± 3.9 | 0.9 ± 5.6 | 0.33 ± 9 |
| VT episodes | 0.2 ± 1.3 | 3.0 ± 41.5 | 0.009 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p-Value | Hazard Ratio (95% Confidence Interval) | p-Value | |
| Age, per 10 years | 1.83 (1.63–2.05) | <0.001 | 1.65 (1.46–1.86) | <0.001 |
| Hypertension | 1.42 (1.11–1.81) | 0.005 | ||
| Diabetes mellitus | 1.50 (1.18–1.92) | 0.001 | ||
| Heart failure | 2.04 (1.61–2.58) | <0.001 | 1.98 (1.52–2.56) | <0.001 |
| Stroke | 1.92 (1.49–2.48) | < 0.001 | 1.83 (1.41–2.39) | <0.001 |
| Vascular disease | 1.54 (1.14–2.08) | 0.005 | ||
| Glomerular filtration rate, per 10 mL/min/1.73 m2 | 0.87 (0.83–0.90) | <0.001 | 0.93 (0.89–0.97) | <0.001 |
| Use of anticoagulants | 0.46 (0.36–0.58) | <0.001 | 0.46 (0.36–0.58) | <0.001 |
| Use of ACEI/ARB | 0.80 (0.63–1.02) | 0.075 | 0.61 (0.47–0.80) | <0.001 |
| Use of beta-blockers | 0.74 (0.58–0.94) | 0.014 | 0.76 (0.59–0.97) | 0.027 |
| Use of non-dihydropyridine CCB | 1.25 (0.99–1.58) | 0.065 | ||
| Use of digoxin | 1.30 (1.03–1.64) | 0.026 | 1.45 (1.13–1.86) | 0.004 |
| All-Cause Mortality | Cardiovascular Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Hazard Ratio (95% CI) | p-Value | Adjusted Hazard Ratio * (95% CI) | p-Value | Crude Hazard Ratio (95% CI) | p-Value | Adjusted Hazard Ratio † (95% CI) | p-Value | |
| 24 h average HR (per 10 BPM) | 1.05 (0.97–1.13) | 0.22 | 1.03 (0.97–1.09) | 0.377 | 0.95 (0.87–1.03) | 0.200 | 0.94 (0.88–1.03) | 0.949 |
| 24 h PVC burden (%) | 1.11 (1.04–1.17) | 0.001 | 1.10 (1.03–1.17) | 0.004 | 1.15 (1.08–1.22) | <0.001 | 1.14 (1.06–1.22) | <0.001 |
| PVCs < 100/24-h | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 |
| PVCs 100–1000/24-h | 1.59 (1.21–2.10) | 1.37 (1.04–1.81) | 1.48 (1.03–2.14) | 1.22 (0.84–1.77) | ||||
| PVCs > 1000/24-h | 2.23 (1.67–2.97) | 1.87 (1.39–2.51) | 2.71 (1.90–3.85) | 2.18 (1.52–3.11) | ||||
| PVC couplets episode | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.00–1.02) | 0.013 | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.00–1.02) | 0.003 |
| PVC triplets episode | 1.00 (0.99–1.03) | 0.417 | 1.00 (0.98–1.03) | 0.917 | 1.01 (0.98–1.04) | 0.484 | 1.00 (0.97–1.03) | 0.918 |
| VT episode | 1.00 (1.00–1.01) | 0.002 | 1.01 (1.00–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| Presence of consecutive PVCs (couplet, triplet or VT episode) | 1.62 (1.28–2.04) | <0.001 | 1.30 (1.03–1.64) | 0.031 | 1.89 (1.41–2.54) | <0.001 | 1.50 (1.11–2.03) | 0.009 |
| 24 h PVC Number | 24 h PVC Burden | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Age | 0.120 | 0.06 | 0.128 | <0.0001 |
| CHA2DS2-VASc | 0.140 | <0.0001 | 0.144 | <0.0001 |
| eGFR | −0.076 | 0.0002 | −0.076 | 0.0002 |
| LVEF | −0.142 | <0.0001 | −0.099 | <0.0001 |
| 24 h average HR | 0.029 | 0.216 | −0.039 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, K.-C.; Chan, Y.-H.; Wang, C.-L. Number of Premature Ventricular Complexes Predicts Long-Term Outcomes in Patients with Persistent Atrial Fibrillation. Biomedicines 2024, 12, 1149. https://doi.org/10.3390/biomedicines12061149
Yen K-C, Chan Y-H, Wang C-L. Number of Premature Ventricular Complexes Predicts Long-Term Outcomes in Patients with Persistent Atrial Fibrillation. Biomedicines. 2024; 12(6):1149. https://doi.org/10.3390/biomedicines12061149
Chicago/Turabian StyleYen, Kun-Chi, Yi-Hsin Chan, and Chun-Li Wang. 2024. "Number of Premature Ventricular Complexes Predicts Long-Term Outcomes in Patients with Persistent Atrial Fibrillation" Biomedicines 12, no. 6: 1149. https://doi.org/10.3390/biomedicines12061149
APA StyleYen, K.-C., Chan, Y.-H., & Wang, C.-L. (2024). Number of Premature Ventricular Complexes Predicts Long-Term Outcomes in Patients with Persistent Atrial Fibrillation. Biomedicines, 12(6), 1149. https://doi.org/10.3390/biomedicines12061149






