Ultrasonic Coating of Poly(D,L-lactic acid)/Poly(lactic-co-glycolic acid) Electrospun Fibers with ZnO Nanoparticles to Increase Angiogenesis in the CAM Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
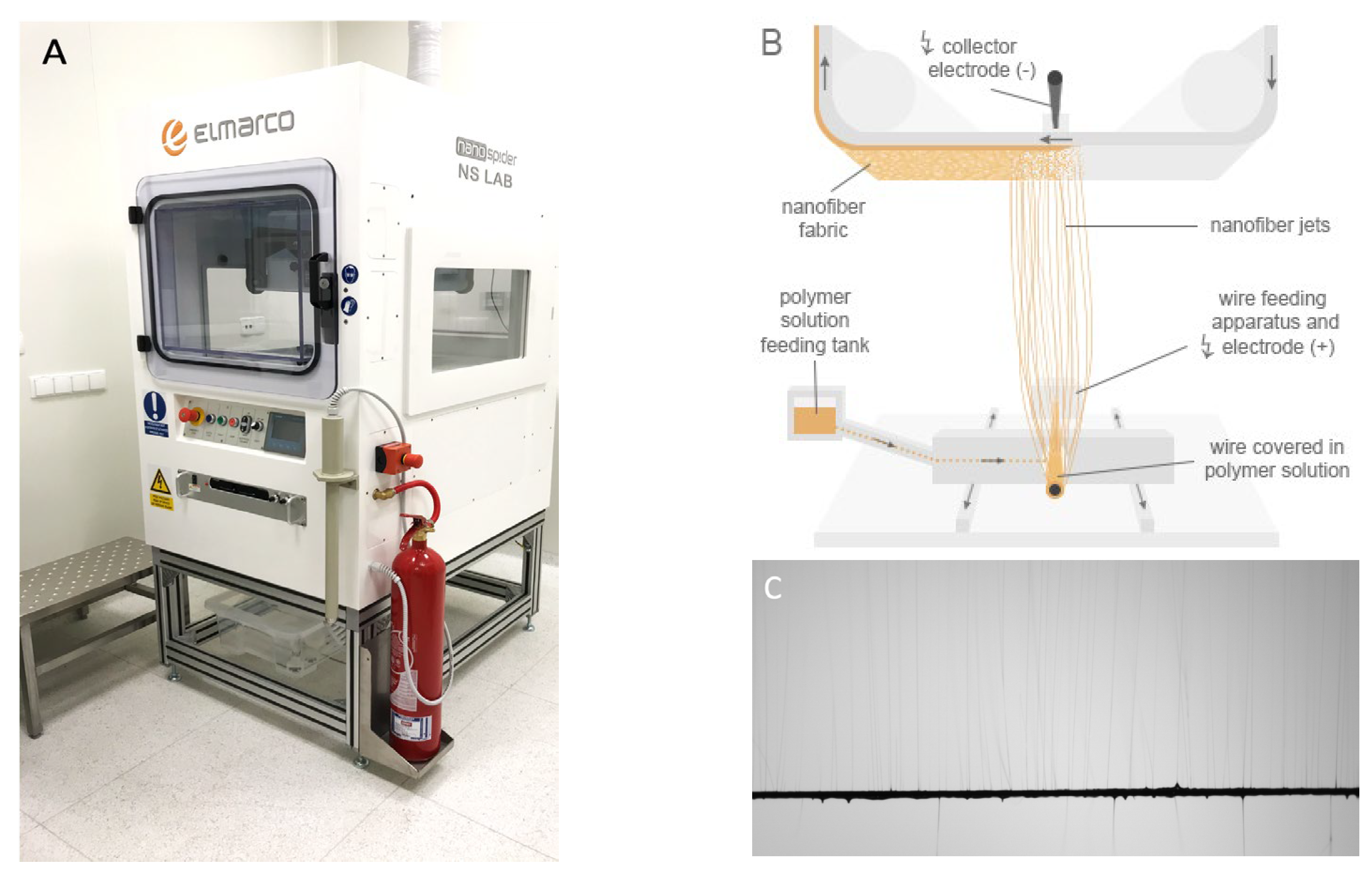
2.2. Electrospinning
2.3. Ultrasonic Coating of Membranes: Sonocoating
2.4. Characterization
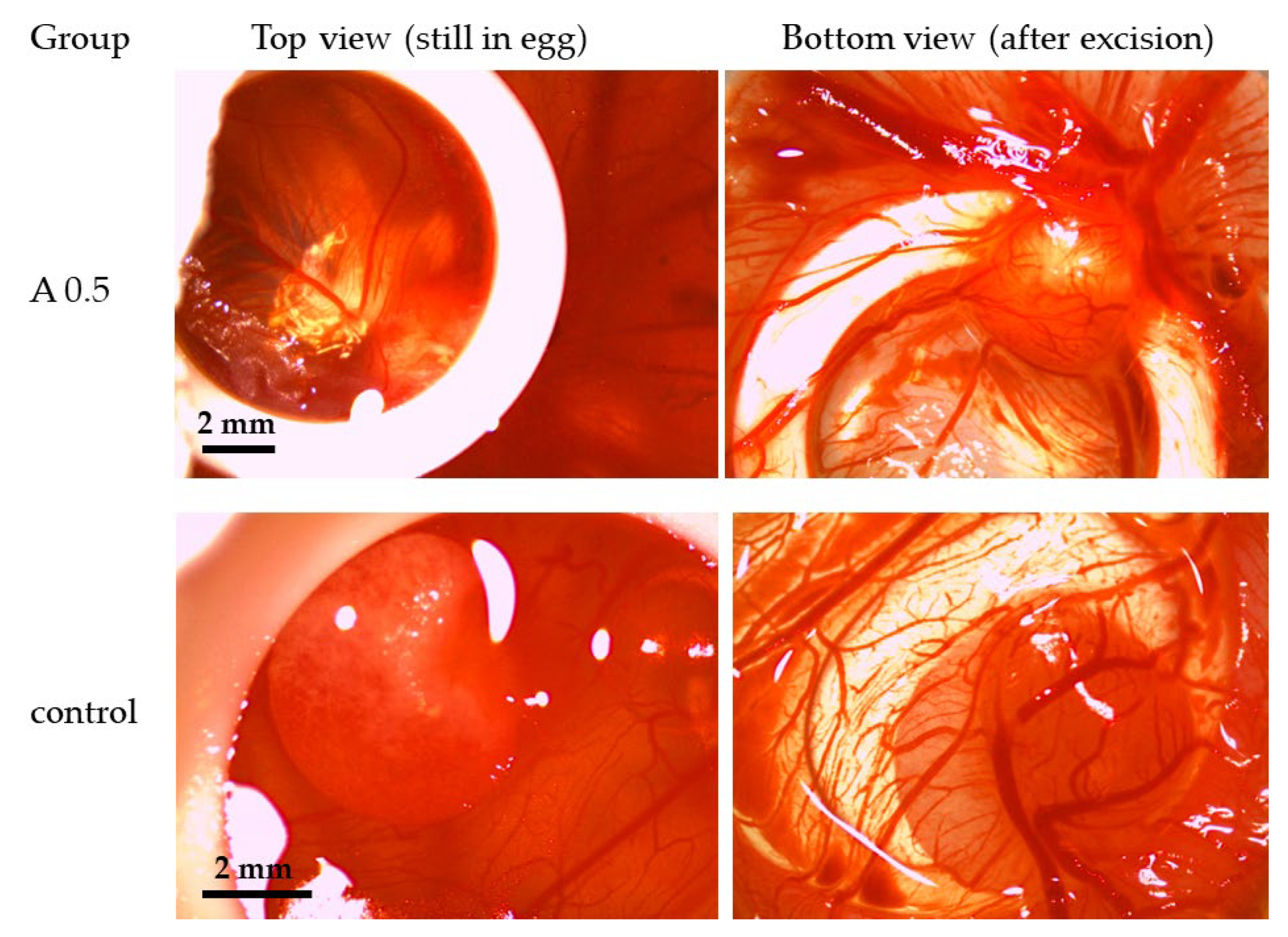
2.5. Chicken Chorioallantoic Membrane (CAM) Assay
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Membranes Characteristics and Morphology
3.2. Water Contact Angle
3.3. Chemical Composition
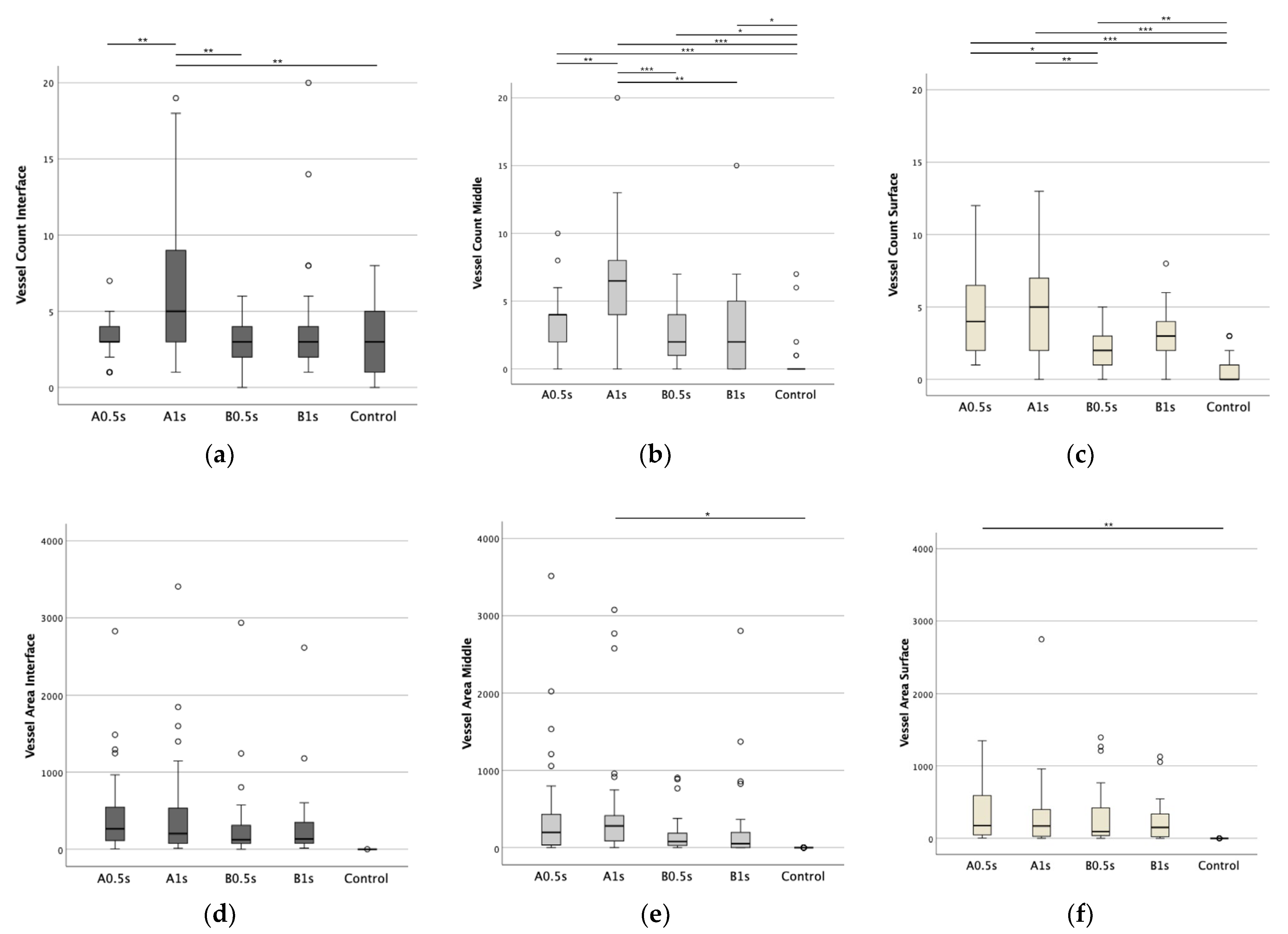
3.4. Impact of ZnO NPs on Angiogenesis in Ovo
3.5. Cell Count in Presence of ZnO NPs
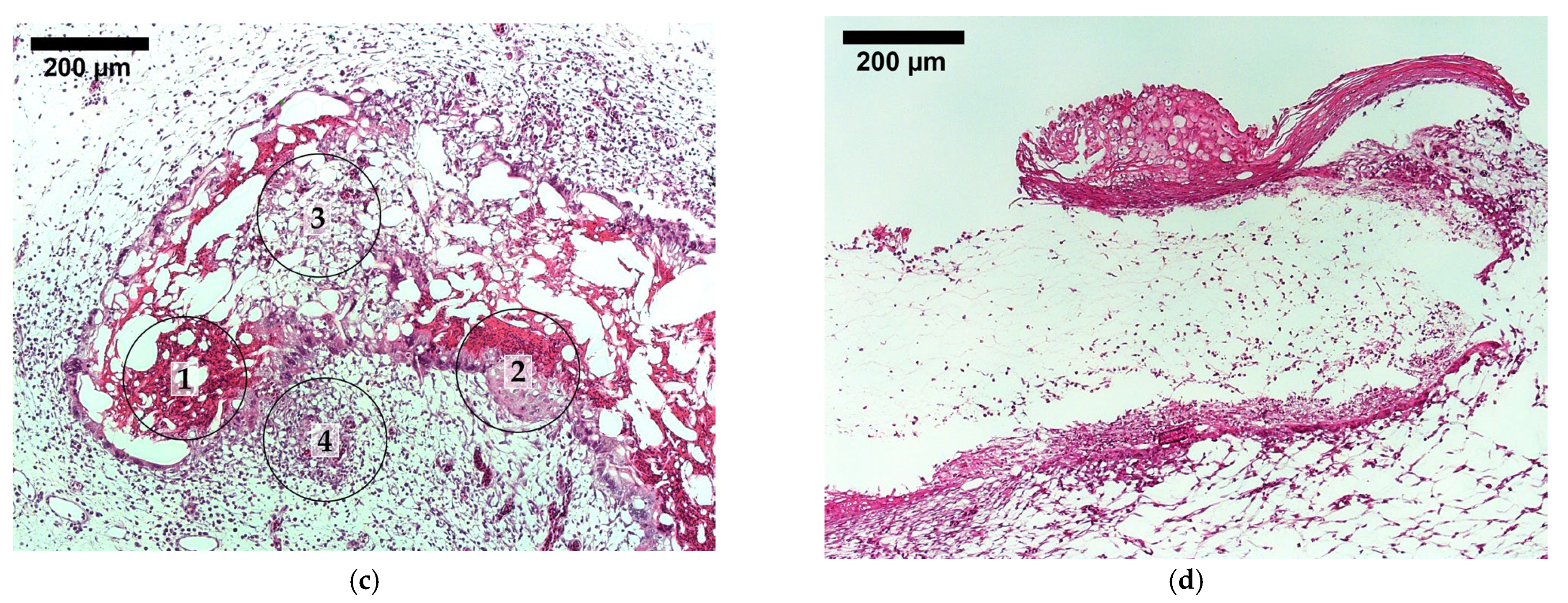
3.6. Tissue Integration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample Name | Type of Nanoparticles | Deposition Time | Amount of ZnO * |
|---|---|---|---|
| A 0.5 | ZnO 25 nm | 0.5 s | 0.1 wt% |
| A 1 | ZnO 25 nm | 1 s | 0.24 wt% |
| A 2 | ZnO 25 nm | 2 s | 0.50 wt% |
| A 4 | ZnO 25 nm | 4 s | 0.61 wt% |
| A 6 | ZnO 25 nm | 6 s | 0.73 wt% |
| A 8 | ZnO 25 nm | 8 s | 0.80 wt% |
| A 10 | ZnO 25 nm | 10 s | 1.01 wt% |
| B 0.5 | ZnO 70 nm | 0.5 s | 0.12 wt% |
| B 1 | ZnO 70 nm | 1 s | 0.18 wt% |
| B 2 | ZnO 70 nm | 2 s | 0.45 wt% |
| B 4 | ZnO 70 nm | 4 s | 0.68 wt% |
| B 6 | ZnO 70 nm | 6 s | 0.82 wt% |
| B 8 | ZnO 70 nm | 8 s | 1.15 wt% |
| B 10 | ZnO 70 nm | 10 s | 1.04 wt% |

References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Rudert, M.; Hutmacher, D.W. Gerüstträgerbasiertes Knochen-Tissue-Engineering. Der Orthopäde 2017, 46, 701–710. [Google Scholar] [CrossRef]
- Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials 2023, 16, 4267. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Goldberg, V.M.; Stevenson, S. Bone graft options: Fact and fancy. Orthopedics 1994, 17, 809–810, 821. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Gao, J.; Chen, S.; Tang, D.; Jiang, L.; Shi, J.; Wang, S. Mechanical Properties and Degradability of Electrospun PCL/PLGA Blended Scaffolds as Vascular Grafts. Trans. Tianjin Univ. 2019, 25, 152–160. [Google Scholar] [CrossRef]
- Xue, A.S.; Koshy, J.C.; Weathers, W.M.; Wolfswinkel, E.M.; Kaufman, Y.; Sharabi, S.E.; Brown, R.H.; Hicks, M.J.; Hollier, L.H. Local foreign-body reaction to commercial biodegradable implants: An in vivo animal study. Craniomaxillofac. Trauma. Reconstr. 2014, 7, 27–34. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, J.; Fortunato, G.; Woźniak, B.; Chodara, A.; Domaschke, S.; Męczyńska-Wielgosz, S.; Kruszewski, M.; Dommann, A.; Łojkowski, W. Polymer Membranes Sonocoated and Electrosprayed with Nano-Hydroxyapatite for Periodontal Tissues Regeneration. Nanomaterials 2019, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, Y.; Li, Z.; Zhou, P.; Mao, Y. Electrospun nanofibrous membrane for biomedical application. SN Appl. Sci. 2022, 4, 172. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, T.; Horvath, J.M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.R.; Clark, E.L. Microscopic observations on the growth of blood capillaries in the living mammal. Am. J. Anat. 1939, 64, 251–301. [Google Scholar] [CrossRef]
- Farris, A.L.; Rindone, A.N.; Grayson, W.L. Oxygen Delivering Biomaterials for Tissue Engineering. J. Mater. Chem. B 2016, 4, 3422–3432. [Google Scholar] [CrossRef] [PubMed]
- Nomi, M.; Atala, A.; Coppi, P.D.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Rahmani, A.; Hashemi-Najafabadi, S.; Eslaminejad, M.B.; Bagheri, F.; Sayahpour, F.A. The effect of modified electrospun PCL-nHA-nZnO scaffolds on osteogenesis and angiogenesis. J. Biomed. Mater. Res. A 2019, 107, 2040–2052. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; Rehman, I.U. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Wang, B.; Lu, G.; Song, K.; Chen, A.; Xing, H.; Wu, J.; Sun, Q.; Li, G.; Cai, M. PLGA-based electrospun nanofibers loaded with dual bioactive agent loaded scaffold as a potential wound dressing material. Colloids Surf. B Biointerfaces 2023, 231, 113570. [Google Scholar] [CrossRef] [PubMed]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Tada-Oikawa, S.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Wu, W.; Yamada, Y.; Mishima, T.; Ichihara, S. Zn(II) released from zinc oxide nano/micro particles suppresses vasculogenesis in human endothelial colony-forming cells. Toxicol. Rep. 2015, 2, 692–701. [Google Scholar] [CrossRef]
- Patra, C.R.; Kim, J.H.; Pramanik, K.; d’Uscio, L.V.; Patra, S.; Pal, K.; Ramchandran, R.; Strano, M.S.; Mukhopadhyay, D. Reactive oxygen species driven angiogenesis by inorganic nanorods. Nano Lett. 2011, 11, 4932–4938. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid. Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Hessien, M.; Taha, A.; Da’na, E. Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites. Materials 2022, 15, 2291. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2019, 13, 14. [Google Scholar] [CrossRef]
- Nan, R.; Liu, S.; Zhai, M.; Zhu, M.; Sun, X.; Chen, Y.; Pang, Q.; Zhang, J. Facile Synthesis of Cu-Doped ZnO Nanoparticles for the Enhanced Photocatalytic Disinfection of Bacteria and Fungi. Molecules 2023, 28, 7232. [Google Scholar] [CrossRef]
- Rosset, A.; Djessas, K.; Goetz, V.; Grillo, S.; Plantard, G. Sol-gel synthesis and solar photocatalytic activity of Ca-alloyed ZnO nanoparticles elaborated using different precursors. RSC Adv. 2020, 10, 25456–25466. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef]
- Alsmadi, M.M.; Al-Nemrawi, N.K.; Obaidat, R.; Abu Alkahsi, A.E.; Korshed, K.M.; Lahlouh, I.K. Insights into the mapping of green synthesis conditions for ZnO nanoparticles and their toxicokinetics. Nanomedicine 2022, 17, 1281–1303. [Google Scholar] [CrossRef]
- Bandeira, M.; Chee, B.S.; Frassini, R.; Nugent, M.; Giovanela, M.; Roesch-Ely, M.; Crespo, J.d.S.; Devine, D.M. Antimicrobial PAA/PAH Electrospun Fiber Containing Green Synthesized Zinc Oxide Nanoparticles for Wound Healing. Materials 2021, 14, 2889. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Verma, A. Amelioration of full thickness dermal wounds by topical application of biofabricated zinc oxide and iron oxide nano-ointment in albino Wistar rats. J. Drug Deliv. Sci. Technol. 2021, 66, 102833. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J. Mater. Chem. B 2021, 9, 1452–1465. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Alvarez Primo, F.; Fregoso, G.; Lalitha Raveendran, N.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Zhou, F.; Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Li, C.M. Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing. Carbohydr. Polym. 2022, 282, 119131. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. In International Review of Cell and Molecular Biology; Elsevier Academic Press Inc.: San Diego, CA, USA, 2008; Volume 270, pp. 181–224. [Google Scholar]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The gelatin sponge-chorioallantoic membrane assay. Nat. Protoc. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int. J. Dev. Biol. 1996, 40, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane as a model for tumor biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Ribatti, D. Two new applications in the study of angiogenesis the CAM assay: Acellular scaffolds and organoids. Microvasc. Res. 2022, 140, 104304. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Greenwood, H.E.; Tyrrell, W.E.; Edwards, R.S.; de Santis, V.; Baark, F.; Firth, G.; Tanc, M.; Terry, S.Y.A.; Herrmann, A.; et al. The chicken chorioallantoic membrane as a low-cost, high-throughput model for cancer imaging. NPJ Imaging 2023, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Woloszyk, A.; Liccardo, D.; Mitsiadis, T. Three-dimensional imaging of the developing vasculature within stem cell-seeded scaffolds cultured in ovo. Front. Physiol. 2016, 7, 194477. [Google Scholar] [CrossRef]
- Woloszyk, A.; Wolint, P.; Becker, A.S.; Boss, A.; Fath, W.; Tian, Y.; Hoerstrup, S.P.; Buschmann, J.; Emmert, M.Y. Novel multimodal MRI and MicroCT imaging approach to quantify angiogenesis and 3D vascular architecture of biomaterials. Sci. Rep. 2019, 9, 19474. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun Membrane Surface Modification by Sonocoating with HA and ZnO:Ag Nanoparticles-Characterization and Evaluation of Osteoblasts and Bacterial Cell Behavior In Vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Method of Manufacturing Bone Implant. E. P. G. E. B. Polish Patent Granted PL226891 (B1), 29 September 2017.
- Waschkies, C.; Kivrak Pfiffner, F.; Wentz, T.; Tian, Y.; Calcagni, M.; Giovanoli, P.; Buschmann, J. SPIO-enhanced MRI as a nondestructive in vivo method to assess vascularization of 3D Degrapol ® scaffolds planted on the Chorioallantoic membrane of the chick embryo in ovo. Matters Sel. 2017. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kämmerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Wolint, P.; Jang, J.-H.; Itani, S.; Jungraithmayr, W.; Waschkies, C.F.; Meier-Bürgisser, G.; Andreoli, S.; Spanaus, K.; Schuepbach, R.A.; et al. High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action; A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay. Cancers 2022, 14, 5122. [Google Scholar] [CrossRef]
- Shahzadi, L.; Chaudhry, A.A.; Aleem, A.R.; Malik, M.H.; Ijaz, K.; Akhtar, H.; Alvi, F.; Khan, A.F.; Rehman, I.U.; Yar, M. Development of K-doped ZnO nanoparticles encapsulated crosslinked chitosan based new membranes to stimulate angiogenesis in tissue engineered skin grafts. Int. J. Biol. Macromol. 2018, 120, 721–728. [Google Scholar] [CrossRef]
- Prieto-Bermejo, R.; Hernández-Hernández, A. The Importance of NADPH Oxidases and Redox Signaling in Angiogenesis. Antioxidants 2017, 6, 32. [Google Scholar] [CrossRef]
- Yang, J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc. Res. 2019, 123, 62–67. [Google Scholar] [CrossRef]
- Xian, D.; Song, J.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Emerging Roles of Redox-Mediated Angiogenesis and Oxidative Stress in Dermatoses. Oxid. Med. Cell Longev. 2019, 2019, 2304018. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species in vascular formation and development. Oxid. Med. Cell Longev. 2013, 2013, 374963. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Tabrizi, M.H.; Ardalan, T.; Yadamani, S.; Safavi, E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019, 44, 30. [Google Scholar]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B 2018, 178, 211–218. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, T.; Ma, J.; Sun, L.; Yang, C.; Meng, L.; Li, Q. Effects of nanoparticle size on the interaction between zinc oxide nanoparticles and bovine serum albumin. J. Biomol. Struct. Dyn. 2020, 38, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, 012044. [Google Scholar] [CrossRef]
- Black, S.M.; DeVol, J.M.; Wedgwood, S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am. J. Physiol. Cell Physiol. 2008, 294, C345–C354. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Grinblat, J.; Hulla, E.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. Ultrasound radiation as a “throwing stones” technique for the production of antibacterial nanocomposite textiles. ACS Appl. Mater. Interfaces 2010, 2, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, B.; Szałaj, U.; Chodara, A.; Mizeracki, J.; Łojkowski, M.; Myszka, D.; Łojkowski, W. Mechanism for sonocoating a polymer surface with nano-hydroxyapatite. Mater. Lett. 2019, 249, 155–159. [Google Scholar] [CrossRef]
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlązak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 669–684. [Google Scholar] [CrossRef]







| Sample Group Name | Size of ZnO NP | Deposition Time | Amount of ZnO NPs * |
|---|---|---|---|
| A 0.5 | 25 nm | 0.5 s | 0.10 wt% |
| A 1 | 25 nm | 1 s | 0.24 wt% |
| B 0.5 | 70 nm | 0.5 s | 0.12 wt% |
| B 1 | 70 nm | 1 s | 0.18 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streich, S.; Higuchi, J.; Opalińska, A.; Wojnarowicz, J.; Giovanoli, P.; Łojkowski, W.; Buschmann, J. Ultrasonic Coating of Poly(D,L-lactic acid)/Poly(lactic-co-glycolic acid) Electrospun Fibers with ZnO Nanoparticles to Increase Angiogenesis in the CAM Assay. Biomedicines 2024, 12, 1155. https://doi.org/10.3390/biomedicines12061155
Streich S, Higuchi J, Opalińska A, Wojnarowicz J, Giovanoli P, Łojkowski W, Buschmann J. Ultrasonic Coating of Poly(D,L-lactic acid)/Poly(lactic-co-glycolic acid) Electrospun Fibers with ZnO Nanoparticles to Increase Angiogenesis in the CAM Assay. Biomedicines. 2024; 12(6):1155. https://doi.org/10.3390/biomedicines12061155
Chicago/Turabian StyleStreich, Selina, Julia Higuchi, Agnieszka Opalińska, Jacek Wojnarowicz, Pietro Giovanoli, Witold Łojkowski, and Johanna Buschmann. 2024. "Ultrasonic Coating of Poly(D,L-lactic acid)/Poly(lactic-co-glycolic acid) Electrospun Fibers with ZnO Nanoparticles to Increase Angiogenesis in the CAM Assay" Biomedicines 12, no. 6: 1155. https://doi.org/10.3390/biomedicines12061155






