Continuous Monitoring of Advanced Hemodynamic Parameters during Hemodialysis Demonstrated Early Variations in Patients Experiencing Intradialytic Hypotension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Overview
2.2. The PPG-Based Monitor
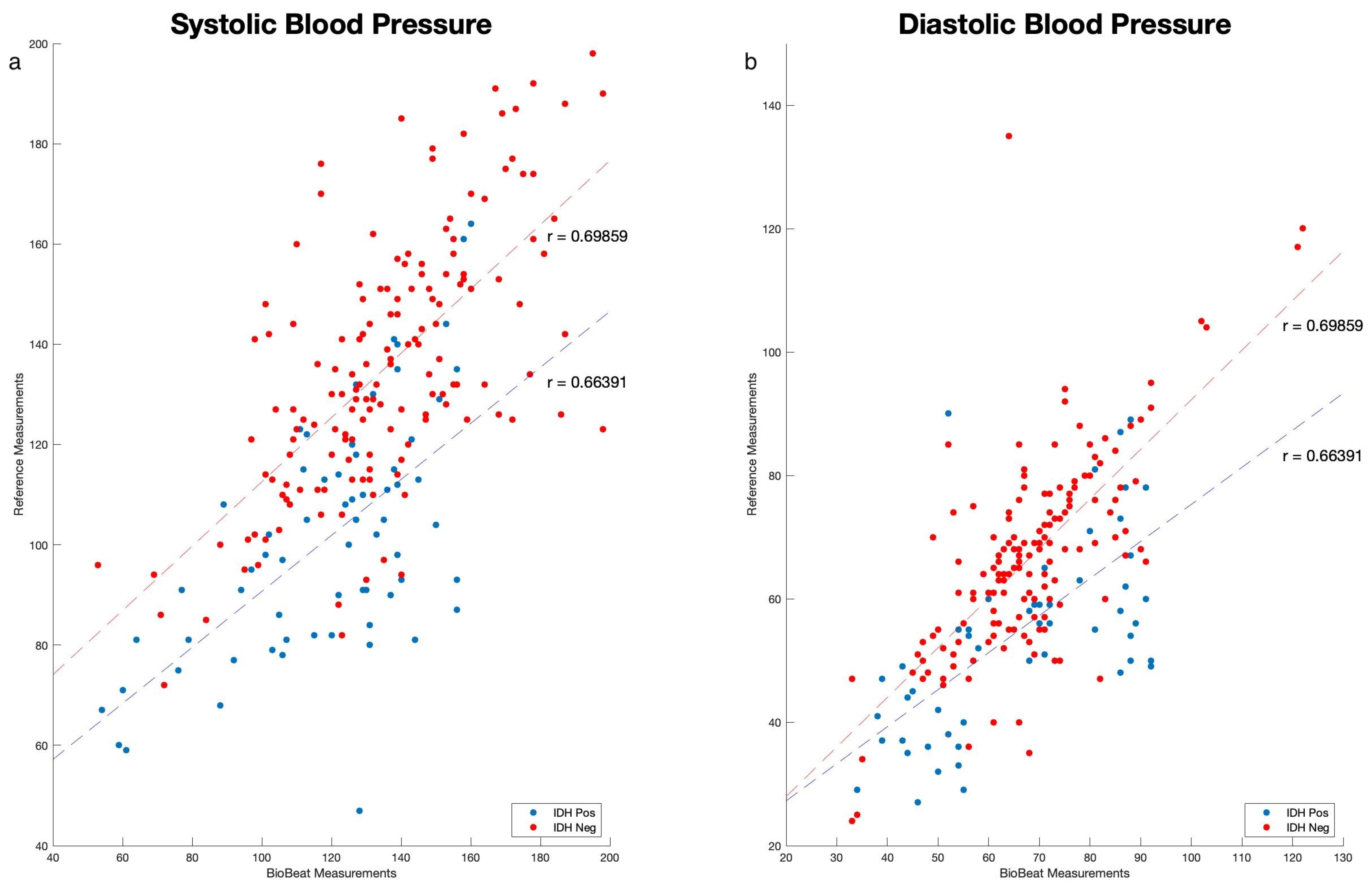
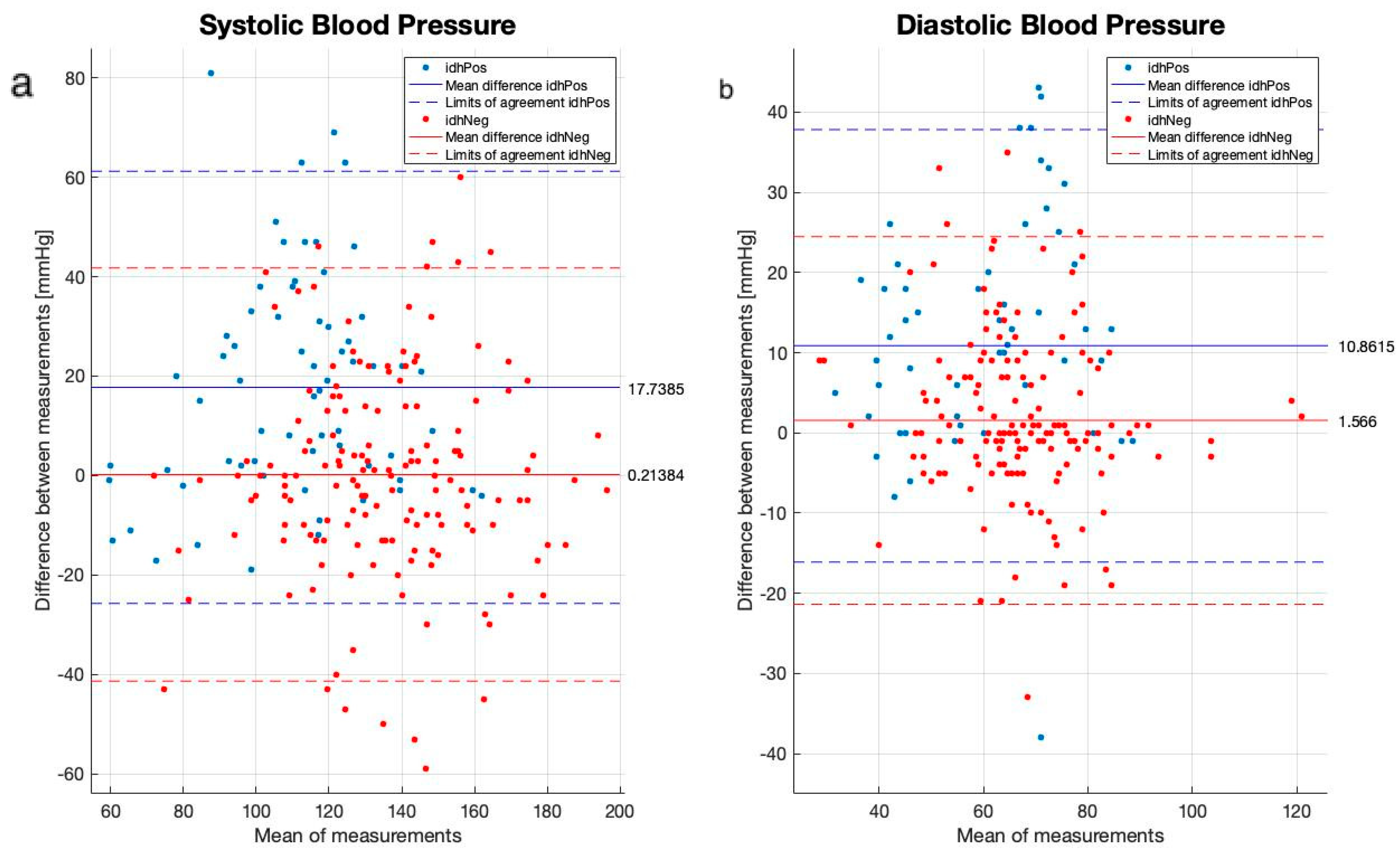
2.3. Data Collection and Statistical Analysis
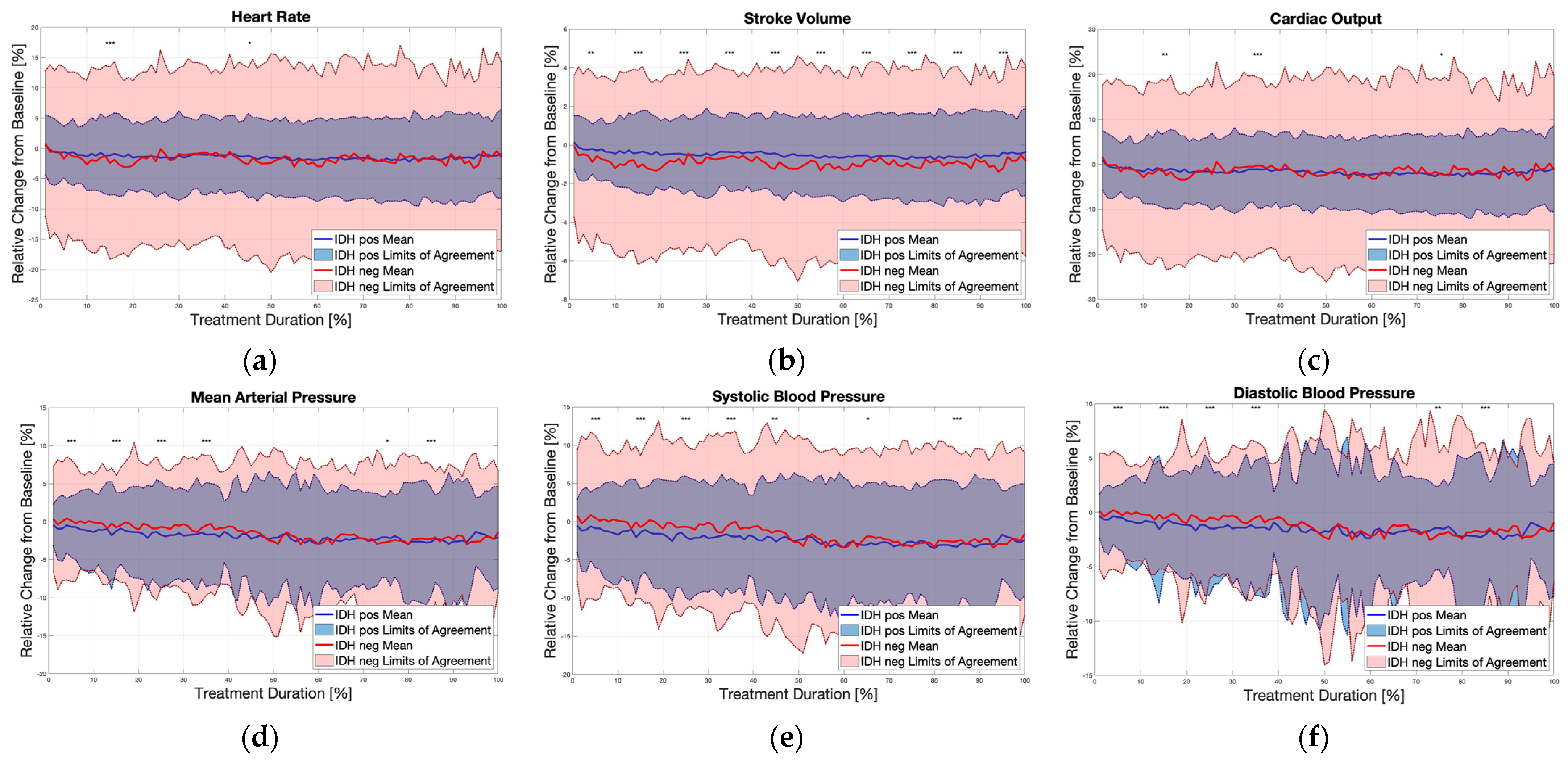
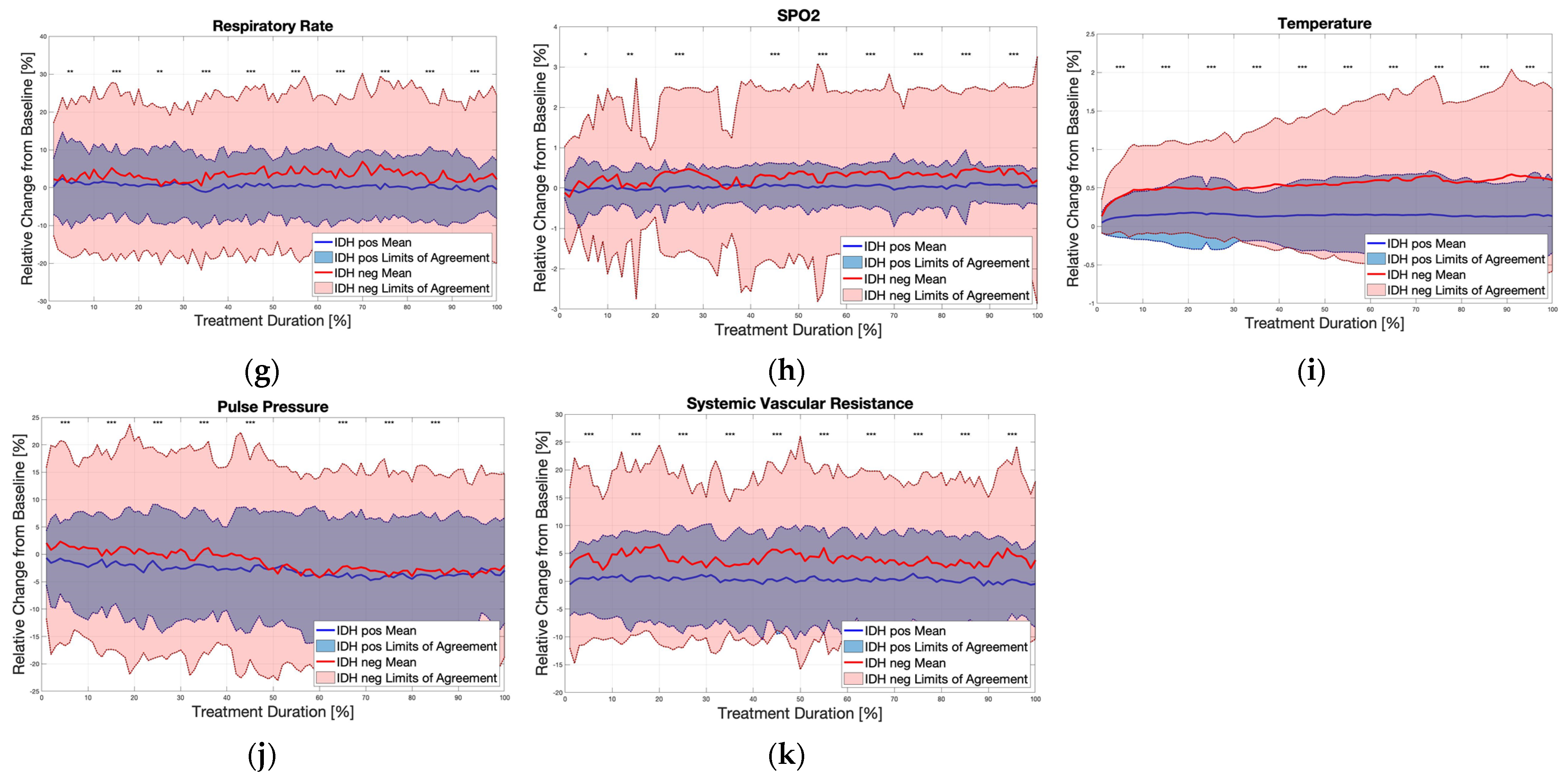
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Himmelfarb, J. Hemodialysis complications. Am. J. Kidney Dis. 2005, 45, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Xue, H.; Lynch, K.E.; Curhan, G.C.; Brunelli, S.M. Association of mortality risk with various definitions of intradialytic hypotension. J. Am. Soc. Nephrol. 2015, 26, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.; Basci, A.; Pizzarelli, F.; Canaud, B.; Haage, P.; Fouque, D.; Konner, K.; Martin-Malo, A.; Pedrini, L.; Tattersall, J.; et al. EBPG guideline on haemodynamic instability. Nephrol. Dial. Transplant. 2007, 22, ii22–ii44. [Google Scholar] [CrossRef] [PubMed]
- Tislér, A.; Akócsi, K.; Borbás, B.; Fazakas, L.; Ferenczi, S.; Görögh, S.; Kulcsár, I.; Nagy, L.; Sámik, J.; Szegedi, J.; et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol. Dial. Transplant. 2003, 18, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Odudu, A. Hemodialysis-associated cardiomyopathy: A newly defined disease entity. Semin. Dial. 2014, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Burton, J.O.; Selby, N.M.; Leccisotti, L.; Korsheed, S.; Baker, C.S.; Camici, P.G. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin. J. Am. Soc. Nephrol. 2008, 3, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, P.; Santoro, A. Dialysis-induced hypotension: A fresh look at pathophysiology. Blood Purif. 1993, 11, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.J.; Joubert, J.; O’Grady, D.; Nathanson, B.H.; Chait, Y.; Levin, N.W. Comparison of stroke volume measurements during hemodialysis using bioimpedance cardiography and echocardiography. Hemodial. Int. 2018, 22, 201–208. [Google Scholar] [CrossRef]
- Lee, A.J.; Cohn, J.H.; Ranasinghe, J.S. Cardiac output assessed by invasive and minimally invasive techniques. Anesthesiol. Res. Pract. 2011, 2011, 475151. [Google Scholar] [CrossRef]
- Levin, N.W.; de Abreu, M.H.F.G.; Borges, L.E.; Tavares Filho, H.A.; Sarwar, R.; Gupta, S.; Hafeez, T.; Lev, S.; Williams, C. Hemodynamic response to fluid removal during hemodialysis: Categorization of causes of intradialytic hypotension. Nephrol. Dial. Transplant. 2018, 33, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Nachman, D.; Constantini, K.; Poris, G.; Wagnert-Avraham, L.; Gertz, S.D.; Littman, R.; Kabakov, E.; Eisenkraft, A.; Gepner, Y. Wireless, noninvasive, wearable device for continuous remote monitoring of hemodynamic parameters in a swine model of controlled hemorrhagic shock. Sci. Rep. 2020, 10, 17684. [Google Scholar] [CrossRef] [PubMed]
- Eisenkraft, A.; Maor, Y.; Constantini, K.; Goldstein, N.; Nachman, D.; Levy, R.; Halberthal, M.; Horowitz, N.A.; Golan, R.; Rosenberg, E.; et al. Continuous Remote Patient Monitoring Shows Early Cardiovascular Changes in COVID-19 Patients. J. Clin. Med. 2021, 10, 4218. [Google Scholar] [CrossRef]
- Nachman, D.; Eisenkraft, A.; Kolben, Y.; Carmon, E.; Hazan, E.; Goldstein, N.; Ben Ishay, A.; Hershkovitz, M.; Fons, M.; Merin, R.; et al. Diurnal cardio-respiratory changes in ambulatory individuals deciphered using a multi-parameter wearable device. Digit. Health 2023, 9, 20552076231218885. [Google Scholar] [CrossRef] [PubMed]
- Eisenkraft, A.; Goldstein, N.; Merin, R.; Fons, M.; Ishay, A.B.; Nachman, D.; Gepner, Y. Developing a real-time detection tool and an early warning score using a continuous wearable multi-parameter monitor. Front. Physiol. 2023, 14, 1138647. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, I.; Merin, R.; Gluzman, Y.; Grinshpan, R.; Shtivelman, A.; Eisenkraft, A.; Rubinshtein, R. Assessing the use of a noninvasive monitoring system providing multiple cardio-pulmonary parameters following revascularization in STEMI patients. Digit. Health 2023, 9, 20552076231179014. [Google Scholar] [CrossRef] [PubMed]
- Dagan, M.; Kolben, Y.; Goldstein, N.; Ben Ishay, A.; Fons, M.; Merin, R.; Eisenkraft, A.; Amir, O.; Asleh, R.; Ben-Yehuda, A.; et al. Advanced Hemodynamic Monitoring Allows Recognition of Early Response Patterns to Diuresis in Congestive Heart Failure Patients. J. Clin. Med. 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Dialysis hypotension: A hemodynamic analysis. Kidney Int. 1991, 39, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.F. Attending rounds: A patient with intradialytic hypotension. Clin. J. Am. Soc. Nephrol. 2014, 9, 798–803. [Google Scholar] [CrossRef]
- Chou, J.A.; Kalantar-Zadeh, K.; Mathew, A.T. A brief review of intradialytic hypotension with a focus on survival. Semin. Dial. 2017, 30, 473–480. [Google Scholar] [CrossRef]
- Chou, J.A.; Streja, E.; Nguyen, D.V.; Rhee, C.M.; Obi, Y.; Inrig, J.K.; Amin, A.; Kovesdy, C.P.; Sim, J.J.; Kalantar-Zadeh, K. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol. Dial. Transplant. 2018, 33, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sars, B.; van der Sande, F.M.; Kooman, J.P. Intradialytic Hypotension: Mechanisms and Outcome. Blood Purif. 2020, 49, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Ertuglu, L.A.; Afsar, B.; Ozdogan, E.; Siriopol, D.; Covic, A.; Basile, C.; Ortiz, A. An update review of intradialytic hypotension: Concept, risk factors, clinical implications and management. Clin. Kidney J. 2020, 13, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Nachman, D.; Gilan, A.; Goldstein, N.; Constantini, K.; Littman, R.; Eisenkraft, A.; Grossman, E.; Gepner, Y. Twenty-Four-Hour Ambulatory Blood Pressure Measurement Using a Novel Noninvasive, Cuffless, Wireless Device. Am. J. Hypertens. 2021, 34, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Nachman, D.; Gepner, Y.; Goldstein, N.; Kabakov, E.; Ishay, A.B.; Littman, R.; Azmon, Y.; Jaffe, E.; Eisenkraft, A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020, 10, 16116. [Google Scholar] [CrossRef]
- Massaro, A.F. Approach to the Patient with Shock. In Harrison’s Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=3095§ionid=265425332 (accessed on 11 June 2023).
- United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2023. Available online: https://usrds-adr.niddk.nih.gov/2023 (accessed on 19 May 2024).
- Canaud, B.; Kooman, J.; Davenport, A.; Campo, D.; Carreel, E.; Morena-Carrere, M.; Cristol, J.P. Digital health technology to support care and improve outcomes of chronic kidney disease patients: As a case illustration, the Withings toolkit health sensing tools. Front. Nephrol. 2023, 3, 1148565. [Google Scholar] [CrossRef]
- Hazan, E.; Azarya, I.; Dvir, D.; Gonen, V.; Ekhilevitch, G.; Merin, R.; Eisenkraft, A.; Carmon, E. Assessing Workflow, Satisfaction, and Potential Cost Reduction when Using a Cuffless Ambulatory Blood Pressure Monitor. J. Fam. Med. Community Health 2022, 9, 1189. [Google Scholar]

| non-IDH (n = 76) | IDH (n = 22) | p-Value | |
|---|---|---|---|
| Age (years) | 65.5 9.41 | 67.0 13.7 | 0.13 |
| Female (%) | 36.4 | 32.9 | 0.76 |
| Medications | |||
| Diuretics (%) | 18.2 | 44.7 | 0.026 |
| ACEi/ARB/MRA (%) | 18.2 | 23.7 | 0.022 |
| CCB (%) | 40.9 | 35.5 | 0.028 |
| β-Blockers (%) | 40.9 | 51.3 | 0.59 |
| α-Blockers (%) | 13.6 | 34.2 | 0.64 |
| Medical Background | |||
| Heart failure (%) | 36.4 | 39.5 | 0.39 |
| Hypertension (%) | 95.5 | 72.4 | 0.066 |
| Diabetes mellitus (%) | 77.3 | 50.0 | 0.79 |
| Peripheral vascular disease (%) | 59.1 | 15.8 | <0.001 |
| Pulmonary hypertension (%) | 45.5 | 21.1 | 0.021 |
| non-IDH (n = 76) | IDH (n = 22) | p-Value | |
|---|---|---|---|
| Pre-dialysis weight (kg) | 96.0 ± 8.02 | 75.3 ± 12.6 | <0.001 |
| Post-dialysis weight (kg) | 93.3 ± 7.78 | 73.7 ± 12.6 | <0.001 |
| Weight difference (kg) | −1.71 ± 0.91 | −2.12 ± 0.70 | 0.059 |
| Dialysis duration (hours) | 4.10 ± 0.35 | 3.85 ± 0.31 | 0.004 |
| Heart rate (beats/min) | 87 ± 12 | 83 ± 14 | 0.18 |
| Oxygen saturation (%) | 98.0 ± 0.7 | 97.7 ± 1.9 | 0.59 |
| Respiratory rate (breaths/min) | 12 ± 1 | 12 ± 2 | 0.39 |
| Temperature (°C) | 36.0 ± 0.1 | 36.0 ± 0.2 | 0.12 |
| Cardiac Output (L/min) | 7.37 ± 1.1 | 7.22 ± 1.7 | 0.41 |
| Stroke volume (mL) | 85.1 ± 7.8 | 86.4 ± 9.2 | 0.56 |
| Systolic blood pressure (mmHg) | 123 ± 34 | 136 ± 32 | 0.20 |
| Diastolic blood pressure (mmHg) | 64 ± 19 | 67 ± 16 | 0.34 |
| Mean arterial pressure (mmHg) | 84 ± 23 | 90 ± 20 | 0.27 |
| Pulse pressure (mmHg) | 59 ± 22 | 69 ± 23 | 0.20 |
| Systemic vascular resistance (dyn·s·cm−5) | 947 ± 340 | 1040 ± 283 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolben, Y.; Gork, I.; Peled, D.; Amitay, S.; Moshel, P.; Goldstein, N.; Ben Ishay, A.; Fons, M.; Tabi, M.; Eisenkraft, A.; et al. Continuous Monitoring of Advanced Hemodynamic Parameters during Hemodialysis Demonstrated Early Variations in Patients Experiencing Intradialytic Hypotension. Biomedicines 2024, 12, 1177. https://doi.org/10.3390/biomedicines12061177
Kolben Y, Gork I, Peled D, Amitay S, Moshel P, Goldstein N, Ben Ishay A, Fons M, Tabi M, Eisenkraft A, et al. Continuous Monitoring of Advanced Hemodynamic Parameters during Hemodialysis Demonstrated Early Variations in Patients Experiencing Intradialytic Hypotension. Biomedicines. 2024; 12(6):1177. https://doi.org/10.3390/biomedicines12061177
Chicago/Turabian StyleKolben, Yotam, Ittamar Gork, David Peled, Shani Amitay, Peleg Moshel, Nir Goldstein, Arik Ben Ishay, Meir Fons, Michael Tabi, Arik Eisenkraft, and et al. 2024. "Continuous Monitoring of Advanced Hemodynamic Parameters during Hemodialysis Demonstrated Early Variations in Patients Experiencing Intradialytic Hypotension" Biomedicines 12, no. 6: 1177. https://doi.org/10.3390/biomedicines12061177
APA StyleKolben, Y., Gork, I., Peled, D., Amitay, S., Moshel, P., Goldstein, N., Ben Ishay, A., Fons, M., Tabi, M., Eisenkraft, A., Gepner, Y., & Nachman, D. (2024). Continuous Monitoring of Advanced Hemodynamic Parameters during Hemodialysis Demonstrated Early Variations in Patients Experiencing Intradialytic Hypotension. Biomedicines, 12(6), 1177. https://doi.org/10.3390/biomedicines12061177






