M2 Macrophages Guide Periosteal Stromal Cell Recruitment and Initiate Bone Injury Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Cranial PSCs from Miniature Pigs
2.2. Extraction of Monocytes from Miniature Pigs
2.3. Collection of M2-Type Macrophage-Derived Medium (M2DM)
2.4. Critical Cranial Bone Defect Model in Miniature Pigs
2.5. Healing Model of Skull Defect in C57BL/6 Mice
2.6. Cell Culture and Cell Differentiation Detection
2.7. Fluorescence-Activated Cell Sorting (FACS) for Macrophages
2.8. ALP Staining and Quantitative Analysis
2.9. ARS Staining and Quantitative Analysis
2.10. Colony Formation Test
2.11. Cell Proliferation Detection by CCK8
2.12. Dorsal Subcutaneous Cell Transplantation in Nude Mice
2.13. Micro-Computed Tomography (Micro-CT)
2.14. Scratch Wound-Healing Assay
2.15. Detection of Immunohistochemistry (IHC)
2.16. Single-Cell Sequencing Analysis
2.17. Statistical Analyses
3. Results
3.1. Cranial Bone Injury Enhanced the Osteogenic Activity of PSCs
3.2. The Injury Reversed the Difference in the Osteogenic Ability of the PSCs in Different Regions
3.3. Characterization of Periosteal Tissue Single-Cell Atlas
3.4. Cell–Cell Interaction Identification and Analysis of MØs in Cranial Injury
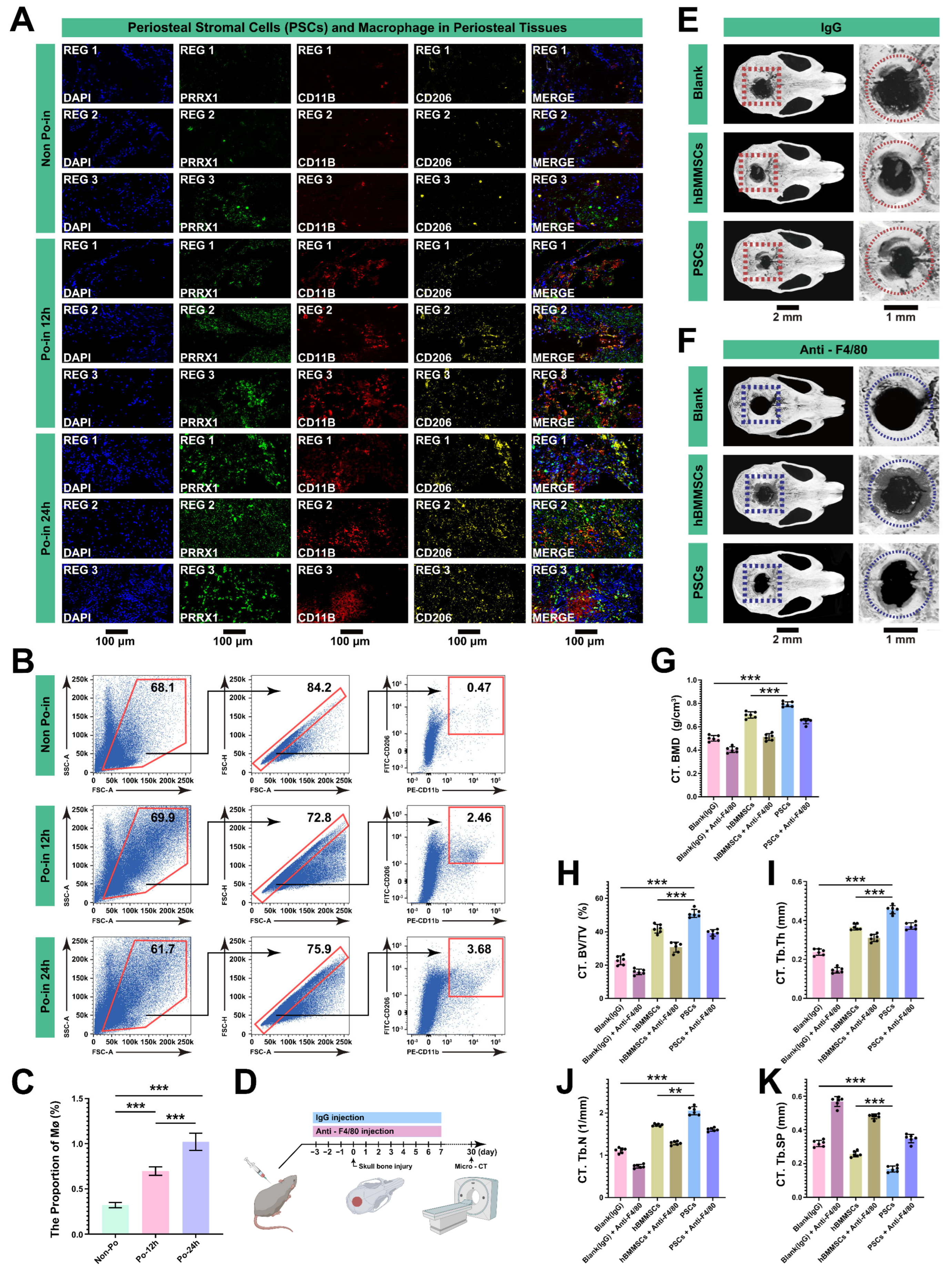
3.5. MØs Are Crucial in the Initial Stages of Bone Reconstruction after Cranial Injury
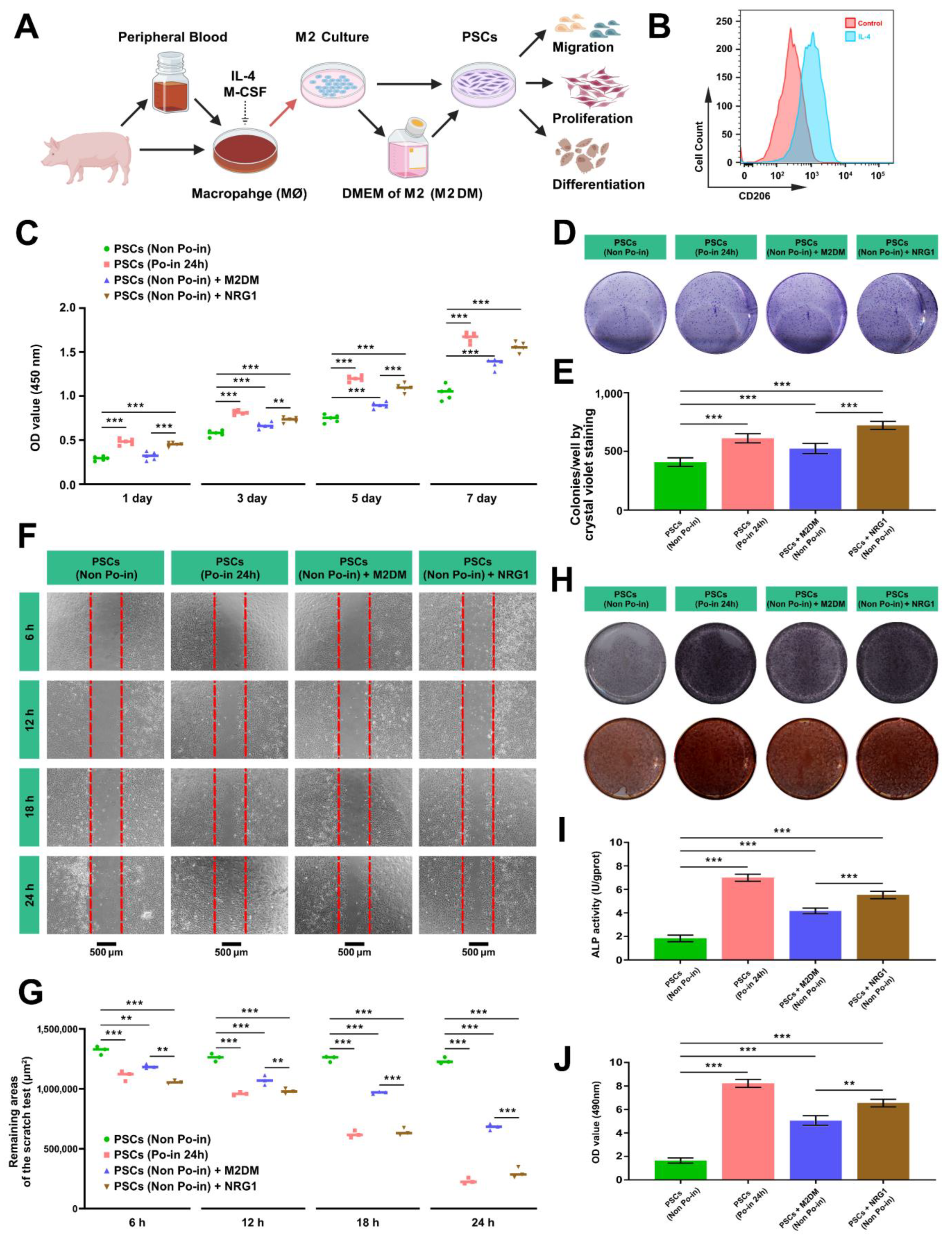
3.6. Both M2DM and NRG1 Promote the Proliferation, Migration, and Osteogenic Activity of Cranial PSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliver, J.D.; Madhoun, W.; Graham, E.M.; Hendrycks, R.; Renouard, M.; Hu, M.S. Stem Cells Regenerating the Craniofacial Skeleton: Current State-of-the-Art and Future Directions. J. Clin. Med. 2020, 9, 3307. [Google Scholar] [CrossRef]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Newton, P.T.; Li, L.; Zhou, B.; Schweingruber, C.; Hovorakova, M.; Xie, M.; Sun, X.; Sandhow, L.; Artemov, A.V.; Ivashkin, E.; et al. A Radical Switch in Clonality Reveals a Stem Cell Niche in the Epiphyseal Growth Plate. Nature 2019, 567, 234–238. [Google Scholar] [CrossRef]
- Jee, W. Integrated Bone Tissue Physiology: Anatomy and Physiology. In Bone Mechanics Handbook; CRC Press: Boca Raton, FL, USA, 2001; pp. 1.1–1.68. [Google Scholar]
- Shen, F.; Huang, X.; He, G.; Shi, Y. The Emerging Studies on Mesenchymal Progenitors in the Long Bone. Cell Biosci. 2023, 13, 105. [Google Scholar] [CrossRef]
- Akiyama, H.; Kim, J.E.; Nakashima, K.; Balmes, G.; Iwai, N.; Deng, J.M.; Zhang, Z.; Martin, J.F.; Behringer, R.R.; Nakamura, T.; et al. Osteo-Chondroprogenitor Cells Are Derived from Sox9 Expressing Precursors. Proc. Natl. Acad. Sci. USA 2005, 102, 14665–14670. [Google Scholar] [CrossRef]
- Shah, H.N.; Jones, R.E.; Borrelli, M.R.; Robertson, K.; Salhotra, A.; Wan, D.C.; Longaker, M.T. Craniofacial and Long Bone Development in the Context of Distraction Osteogenesis. Plast. Reconstr. Surg. 2021, 147, 54e–65e. [Google Scholar] [CrossRef]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a Periosteal Stem Cell Mediating Intramembranous Bone Formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Perrin, S.; Colnot, C. Periosteal Skeletal Stem and Progenitor Cells in Bone Regeneration. Curr. Osteoporos. Rep. 2022, 20, 334–343. [Google Scholar] [CrossRef]
- Colnot, C.; Lu, C.; Hu, D.; Helms, J.A. Distinguishing the Contributions of the Perichondrium, Cartilage, and Vascular Endothelium to Skeletal Development. Dev. Biol. 2004, 269, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.S.; Wu, J.S.; Eisenberg, R.L. Periosteal Reaction. AJR Am. J. Roentgenol. 2009, 193, W259–W272. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulos, C.K.; Kanellopoulos, A.D.; Trovas, G.P.; Dontas, I.A.; Lyritis, G.P. The Biomechanical Capacity of the Periosteum in Intact Long Bones. Arch. Orthop. Trauma Surg. 2008, 128, 117–120. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef]
- Colnot, C.; Zhang, X.; Tate, M.L.K. Current Insights on the Regenerative Potential of the Periosteum: Molecular, Cellular, and Endogenous Engineering Approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Colnot, C. Skeletal Cell Fate Decisions within Periosteum and Bone Marrow during Bone Regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Thompson, Z.; Miclau, T.; Hu, D.; Helms, J.A. A Model for Intramembranous Ossification during Fracture Healing. J. Orthop. Res. 2002, 20, 1091–1098. [Google Scholar] [CrossRef]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered Fracture Repair in the Absence of Mmp9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef]
- Ortinau, L.C.; Wang, H.; Lei, K.; Deveza, L.; Jeong, Y.; Hara, Y.; Grafe, I.; Rosenfeld, S.B.; Lee, D.; Lee, B.; et al. Identification of Functionally Distinct Mx1+Alphasma+ Periosteal Skeletal Stem Cells. Cell Stem Cell 2019, 25, 784–796.e5. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Li, Z.; Tian, Y.; Li, Z.; Lu, A.; Hsu, C.Y.; Negri, S.; Tang, C.; Tower, R.J.; et al. Pdgfralpha Reporter Activity Identifies Periosteal Progenitor Cells Critical for Bone Formation and Fracture Repair. Bone Res. 2022, 10, 7. [Google Scholar] [CrossRef]
- Matthews, B.G.; Novak, S.; Sbrana, F.V.; Funnell, J.L.; Cao, Y.; Buckels, E.J.; Grcevic, D.; Kalajzic, I. Heterogeneity of Murine Periosteum Progenitors Involved in Fracture Healing. eLife 2021, 10, e58534. [Google Scholar] [CrossRef]
- Tournaire, G.; Stegen, S.; Giacomini, G.; Stockmans, I.; Moermans, K.; Carmeliet, G.; van Gastel, N. Nestin-Gfp Transgene Labels Skeletal Progenitors in the Periosteum. Bone 2020, 133, 115259. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, G.; Lee, W.C.; McKenzie, J.A.; Silva, M.J.; Long, F. Gli1 Identifies Osteogenic Progenitors for Bone Formation and Fracture Repair. Nat. Commun. 2017, 8, 2043. [Google Scholar] [CrossRef]
- Ransom, R.C.; Hunter, D.J.; Hyman, S.; Singh, G.; Ransom, S.C.; Shen, E.Z.; Perez, K.C.; Gillette, M.; Li, J.; Liu, B.; et al. Axin2-Expressing Cells Execute Regeneration after Skeletal Injury. Sci. Rep. 2016, 6, 36524. [Google Scholar] [CrossRef]
- Bok, S.; Yallowitz, A.R.; Sun, J.; McCormick, J.; Cung, M.; Hu, L.; Lalani, S.; Li, Z.; Sosa, B.R.; Baumgartner, T.; et al. A Multi-Stem Cell Basis for Craniosynostosis and Calvarial Mineralization. Nature 2023, 621, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dong, J.; Fan, X.; Yong, J.; Yang, M.; Liu, Y.; Zhang, X.; Lv, L.; Wen, L.; Qiao, J.; et al. Characterization of Mesenchymal Stem Cells in Human Fetal Bone Marrow by Single-Cell Transcriptomic and Functional Analysis. Signal Transduct. Target Ther. 2023, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, D.D.; Mabuchi, Y.; Morikawa, S.; Niibe, K.; Araki, D.; Suzuki, S.; Okano, H.; Matsuzaki, Y. Isolation of Mouse Mesenchymal Stem Cells on the Basis of Expression of Sca-1 and Pdgfr-Alpha. Nat. Protoc. 2012, 7, 2103–2111. [Google Scholar] [CrossRef]
- Lin, W.; Li, Q.; Zhang, D.; Zhang, X.; Qi, X.; Wang, Q.; Chen, Y.; Liu, C.; Li, H.; Zhang, S.; et al. Mapping the Immune Microenvironment for Mandibular Alveolar Bone Homeostasis at Single-Cell Resolution. Bone Res. 2021, 9, 17. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using Cellchat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Ishida, K.; Nagatake, T.; Saika, A.; Kawai, S.; Node, E.; Hosomi, K.; Kunisawa, J. Induction of Unique Macrophage Subset by Simultaneous Stimulation with Lps and Il-4. Front. Immunol. 2023, 14, 1111729. [Google Scholar] [CrossRef]
- Rios, F.J.; Touyz, R.M.; Montezano, A.C. Isolation and Differentiation of Human Macrophages. Methods Mol. Biol. 2017, 1527, 311–320. [Google Scholar] [PubMed]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum Derived Stem Cells for Regenerative Medicine Proposals: Boosting Current Knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum Contains Skeletal Stem Cells with High Bone Regenerative Potential Controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef]
- Matthews, B.G.; Grcevic, D.; Wang, L.; Hagiwara, Y.; Roguljic, H.; Joshi, P.; Shin, D.G.; Adams, D.J.; Kalajzic, I. Analysis of Alphasma-Labeled Progenitor Cell Commitment Identifies Notch Signaling as an Important Pathway in Fracture Healing. J. Bone Miner. Res. 2014, 29, 1283–1294. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Raggatt, L.J.; Millard, S.; Batoon, L.; Wu, A.C.-K.; Chang, M.K.; Hume, D.A.; Pettit, A.R. Resting and Injury-Induced Inflamed Periosteum Contain Multiple Macrophage Subsets That Are Located at Sites of Bone Growth and Regeneration. Immunol. Cell Biol. 2017, 95, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Wullschleger, M.E.; Alexander, K.A.; Wu, A.C.; Millard, S.M.; Kaur, S.; Maugham, M.L.; Gregory, L.S.; Steck, R.; Pettit, A.R. Fracture Healing Via Periosteal Callus Formation Requires Macrophages for Both Initiation and Progression of Early Endochondral Ossification. Am. J. Pathol. 2014, 184, 3192–3204. [Google Scholar] [CrossRef]
- Qiu, P.; Li, M.; Chen, K.; Fang, B.; Chen, P.; Tang, Z.; Lin, X.; Fan, S. Periosteal Matrix-Derived Hydrogel Promotes Bone Repair through an Early Immune Regulation Coupled with Enhanced Angio- and Osteogenesis. Biomaterials 2020, 227, 119552. [Google Scholar] [CrossRef]
- Chang, M.K.; Raggatt, L.J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal Tissue Macrophages Are Intercalated Throughout Human and Mouse Bone Lining Tissues and Regulate Osteoblast Function in Vitro and in Vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in Bone Fracture Healing: Their Essential Role in Endochondral Ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Deng, R.; Chai, Y.; Chen, H.; Hu, B.; Wang, X.; Zhu, S.; Cao, Y.; Ni, S.; Wan, M.; et al. Macrophage-Lineage Trap+ Cells Recruit Periosteum-Derived Cells for Periosteal Osteogenesis and Regeneration. J. Clin. Investig. 2019, 129, 2578–2594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bose, T.; Unger, R.E.; Jansen, J.A.; Kirkpatrick, C.J.; van den Beucken, J. Macrophage Type Modulates Osteogenic Differentiation of Adipose Tissue Mscs. Cell Tissue Res. 2017, 369, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, V.; Wong, M.M.; Redpath, A.N.; Ersek, A.; Baban, D.F.; Williams, L.M.; Cope, A.P.; Horwood, N.J. Monocytes Induce Stat3 Activation in Human Mesenchymal Stem Cells to Promote Osteoblast Formation. PLoS ONE 2012, 7, e39871. [Google Scholar] [CrossRef] [PubMed]
- Nikovics, K.; Durand, M.; Castellarin, C.; Burger, J.; Sicherre, E.; Collombet, J.M.; Oger, M.; Holy, X.; Favier, A.L. Macrophages Characterization in an Injured Bone Tissue. Biomedicines 2022, 10, 1385. [Google Scholar] [CrossRef]
- Jarde, T.; Chan, W.H.; Rossello, F.J.; Kahlon, T.K.; Theocharous, M.; Arackal, T.K.; Flores, T.; Giraud, M.; Richards, E.; Chan, E.; et al. Mesenchymal Niche-Derived Neuregulin-1 Drives Intestinal Stem Cell Proliferation and Regeneration of Damaged Epithelium. Cell Stem Cell 2020, 27, 646–662.e7. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Zhang, Y.; Liang, S.; Li, Y.; Qing, J.; Gu, L.; Xu, X.; Wang, Z.; Gao, X.; Liu, H.; et al. M2 Macrophages Guide Periosteal Stromal Cell Recruitment and Initiate Bone Injury Regeneration. Biomedicines 2024, 12, 1205. https://doi.org/10.3390/biomedicines12061205
Lu D, Zhang Y, Liang S, Li Y, Qing J, Gu L, Xu X, Wang Z, Gao X, Liu H, et al. M2 Macrophages Guide Periosteal Stromal Cell Recruitment and Initiate Bone Injury Regeneration. Biomedicines. 2024; 12(6):1205. https://doi.org/10.3390/biomedicines12061205
Chicago/Turabian StyleLu, Dazhuang, Yingfei Zhang, Shimin Liang, Yang Li, Jia Qing, Lanxin Gu, Xiuyun Xu, Zeying Wang, Xin Gao, Hao Liu, and et al. 2024. "M2 Macrophages Guide Periosteal Stromal Cell Recruitment and Initiate Bone Injury Regeneration" Biomedicines 12, no. 6: 1205. https://doi.org/10.3390/biomedicines12061205




