Evaluation of Salivary Biomarkers and Spirometry for Diagnosing COPD in Non-Smokers and Smokers of Polish Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Population Characterization
2.2. Saliva and Serum Blood Collection
2.3. ELISA
2.4. Statistical Analysis
3. Results
3.1. Biomarkers (C-Reactive Protein (CRP), Procalcitonin (PCT), Neutrophil Elastase (NE) and Alpha-1 Antitrypsin (AAT)) Presence in Saliva and Serum
3.2. Characterization of Spirometry and Molecular Parameters of Healthy Non-Smoking and Smoking Groups
3.3. Examining Spirometry Parameters and Biomarker Levels within the Groups of Non-Smokers and Smokers with COPD (Comprising Both Non-Smokers and Smokers)
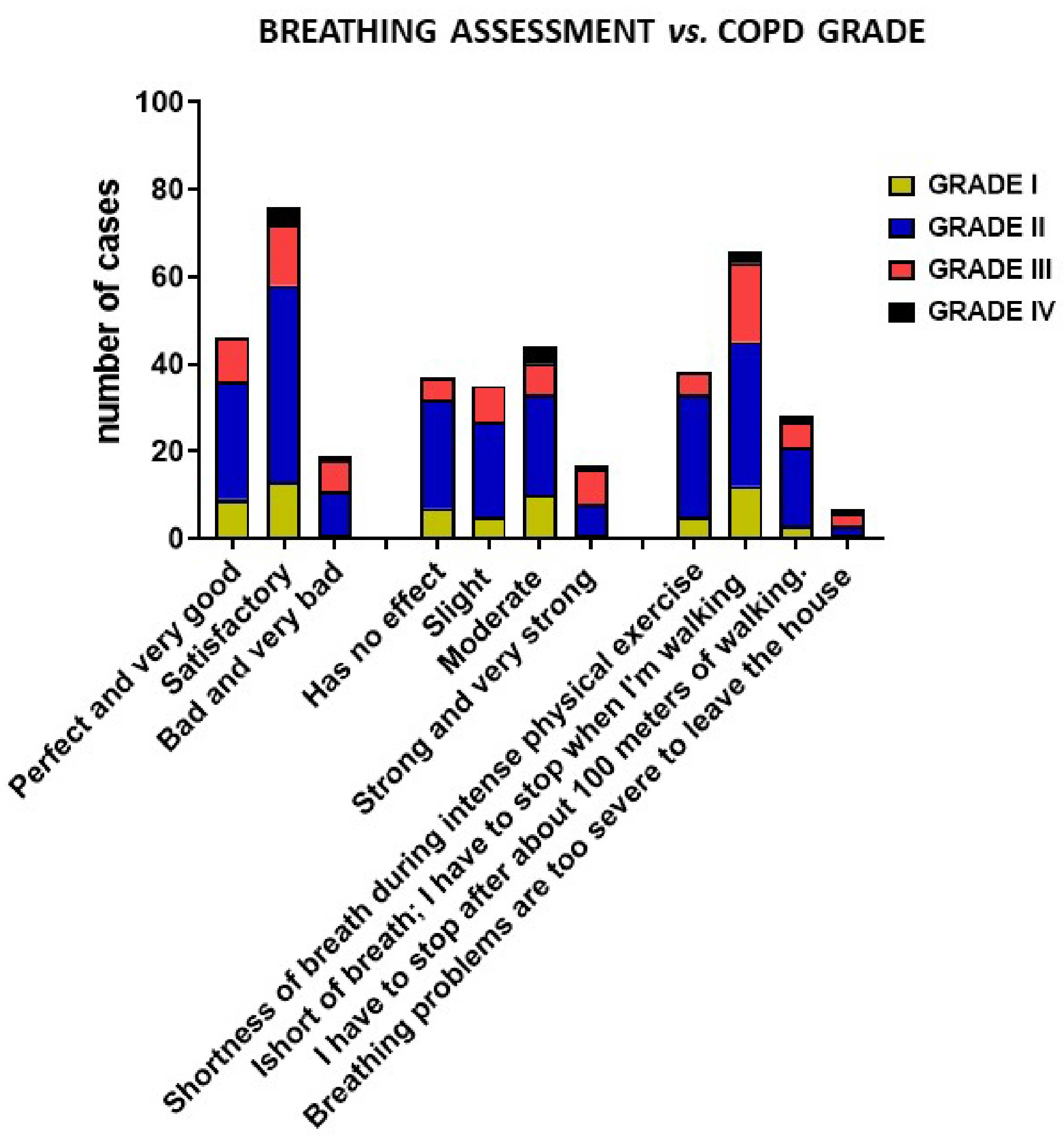
3.4. Breathing Assessment of COPD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| AAT | alpha-1 antitrypsin |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced expiratory volume |
| TNF | tumor necrosis factor |
| MMP | matrix metalloproteinase |
| MP | mass spectrometry |
| 2-DE | two-dimensional gel electrophoresis |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| FFE | free-flow electrophoresis |
| CRP | C-reactive protein |
| PCT | procalcitonin |
| NE | neutrophil elastase |
| GOLD | global initiative for chronic obstructive lung disease |
| CRF | case report form |
| BMI | body mass index |
| WHO | The World Health Organization |
References
- Bhatt, S.P.; Kim, Y.; Harrington, K.F.; Hokanson, J.E.; Lutz, S.M.; Cho, M.H.; DeMeo, D.L.; Wells, J.M.; Make, B.J.; Rennard, S.I.; et al. Smoking Duration Alone Provides Stronger Risk Estimates of Chronic Obstructive Pulmonary Disease than Pack-Years. Thorax 2018, 73, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, C.; Jamiyansuren, J.; Tumurbaatar, A.; Bayarmaa, A.; Enebish, T.; Dashtseren, I.; Jav, S. The Cumulative Effect of Gene–Gene Interactions between GSTM1, CHRNA3, CHRNA5 and SOD3 Gene Polymorphisms Combined with Smoking on COPD Risk. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Brøgger, J.; Steen, V.M.; Eiken, H.G.; Gulsvik, A.; Bakke, P. Genetic Association between COPD and Polymorphisms in TNF, ADRB2 and EPHX1. Eur. Respir. J. 2006, 27, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Chrebelska, K.H.; Mukherjee, D.; Maryanchik, S.V.; Rudzinska-Radecka, M. Biological and Genetic Mechanisms of COPD, Its Diagnosis, Treatment, and Relationship with Lung Cancer. Biomedicines 2023, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.L.; Fishbane, N.; Patel, J.; MacIsaac, J.L.; McEwen, L.M.; Fisher, A.J.; Brandsma, C.-A.; Nair, P.; Kobor, M.S.; Hackett, T.-L.; et al. Altered DNA Methylation Is Associated with Aberrant Gene Expression in Parenchymal but Not Airway Fibroblasts Isolated from Individuals with COPD. Clin. Epigenet. 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.-A.; et al. Burden of Chronic Obstructive Pulmonary Disease and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Nardini, S.; Annesi-Maesano, I.; Simoni, M.; Ponte, A.D.; Sanguinetti, C.M.; De Benedetto, F. Accuracy of Diagnosis of COPD and Factors Associated with Misdiagnosis in Primary Care Setting. E-DIAL (Early DIAgnosis of Obstructive Lung Disease) Study Group. Respir. Med. 2018, 143, 61–66. [Google Scholar] [CrossRef]
- Poland. Available online: https://international-respiratory-coalition.org/countries/poland/ (accessed on 6 March 2024).
- Blanco, I.; Diego, I.; Bueno, P.; Fernández, E.; Casas-Maldonado, F.; Esquinas, C.; Soriano, J.B.; Miravitlles, M. Geographical Distribution of COPD Prevalence in Europe, Estimated by an Inverse Distance Weighting Interpolation Technique. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 57–67. [Google Scholar] [CrossRef]
- Nizankowska-Mogilnicka, E.; Mejza, F.; Buist, A.S.; Vollmer, W.M.; Skucha, W.; Harat, R.; Pajak, A.; Gasowski, J.; Frey, J.; Nastalek, P.; et al. Prevalence of COPD and Tobacco Smoking in Malopolska Region—Results from the BOLD Study in Poland. Pol. Arch. Med. Wewn. 2007, 117, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.P.; Walters, J.A.E.; Walters, E.H. Diagnosis and Early Detection of COPD Using Spirometry. J. Thorac. Dis. 2014, 6, 1557–1569. [Google Scholar] [CrossRef]
- Barnes, T.A.; Fromer, L. Spirometry Use: Detection of Chronic Obstructive Pulmonary Disease in the Primary Care Setting. Clin. Interv. Aging 2011, 6, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Belzer, R.B.; Lewis, R.J. The Practical Significance of Measurement Error in Pulmonary Function Testing Conducted in Research Settings. Risk Anal. 2019, 39, 2316–2328. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the Diagnosis of Diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.; Lamster, I.B. The Diagnostic Applications of Saliva—A Review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Messana, I.; Cabras, T.; Inzitari, R.; Lupi, A.; Zuppi, C.; Olmi, C.; Fadda, M.B.; Cordaro, M.; Giardina, B.; Castagnola, M. Characterization of the Human Salivary Basic Proline-Rich Protein Complex by a Proteomic Approach. J. Proteome Res. 2004, 3, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xie, Y.; Ramachandran, P.; Ogorzalek Loo, R.R.; Li, Y.; Loo, J.A.; Wong, D.T. Large-Scale Identification of Proteins in Human Salivary Proteome by Liquid Chromatography/Mass Spectrometry and Two-Dimensional Gel Electrophoresis-Mass Spectrometry. Proteomics 2005, 5, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Rhodus, N.L.; Griffin, R.J.; Carlis, J.V.; Griffin, T.J. A Catalogue of Human Saliva Proteins Identified by Free Flow Electrophoresis-Based Peptide Separation and Tandem Mass Spectrometry. Mol. Cell. Proteom. 2005, 4, 1826–1830. [Google Scholar] [CrossRef]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative Human Salivary and Plasma Proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Aksu, F.; Çapan, N.; Aksu, K.; Ofluoğlu, R.; Canbakan, S.; Yavuz, B.; Akin, K.O. C-Reactive Protein Levels Are Raised in Stable Chronic Obstructive Pulmonary Disease Patients Independent of Smoking Behavior and Biomass Exposure. J. Thorac. Dis. 2013, 5, 414. [Google Scholar] [CrossRef]
- Pandey, S.; Garg, R.; Kant, S.; Verma, A.; Gaur, P. Serum Procalcitonin Levels in Chronic Obstructive Pulmonary Disease Patients in North Indian Population. Ann. Afr. Med. 2019, 18, 103–107. [Google Scholar] [CrossRef]
- Demkow, U.; van Overveld, F. Role of Elastases in the Pathogenesis of Chronic Obstructive Pulmonary Disease: Implications for Treatment. Eur. J. Med. Res. 2010, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.D.; Woods, A.; Hilleman, D.E.; Malesker, M.A. Alpha-1 Antitrypsin Replacement in Patients with COPD. Pharm. Ther. 2019, 44, 412–415. [Google Scholar]
- HSP|Home. Available online: https://www.salivaryproteome.org/ (accessed on 6 March 2024).
- Lau, W.W.; Hardt, M.; Zhang, Y.H.; Freire, M.; Ruhl, S. The Human Salivary Proteome Wiki: A Community-Driven Research Platform. J. Dent. Res. 2021, 100, 1510–1519. [Google Scholar] [CrossRef]
- Boncler, M.; Wu, Y.; Watala, C. The Multiple Faces of C-Reactive Protein—Physiological and Pathophysiological Implications in Cardiovascular Disease. Molecules 2019, 24, 2062. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Galeone, C.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. C-Reactive Protein Level Predicts Mortality in COPD: A Systematic Review and Meta-Analysis. Eur. Respir. Rev. 2017, 26, 160070. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Man, S.; Senthilselvan, A.; Sin, D. Association between Chronic Obstructive Pulmonary Disease and Systemic Inflammation: A Systematic Review and a Meta-Analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Maruna, P.; Nedelníková, K.; Gürlich, R. Physiology and Genetics of Procalcitonin. Physiol. Res. 2000, 49 (Suppl. S1), S57–S61. [Google Scholar]
- Gong, C.; Yang, Y.; Chen, M.; Xie, Z. Effect of Procalcitonin on the Prognosis of Patients with COPD. Biomed. Rep. 2020, 12, 313–318. [Google Scholar] [CrossRef]
- Voynow, J.A.; Shinbashi, M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Saputra, P.B.T.; Purwati, D.D.; Ulhaq, A.U.D.; Yolanda, S.; Djatioetomo, Y.C.E.D.; Rosyid, A.N.; Bakhtiar, A. Neutrophil Elastase in the Pathogenesis of Chronic Obstructive Pulmonary Disease: A Review. Curr. Respir. Med. Rev. 2023, 19, 29–35. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Murray, G.; Gogoi, D.; Yusuf, A.; McCarthy, C.; Wormald, M.R.; Casey, M.; Gabillard-Lefort, C.; McElvaney, N.G.; Reeves, E.P. A Review of Alpha-1 Antitrypsin Binding Partners for Immune Regulation and Potential Therapeutic Application. Int. J. Mol. Sci. 2022, 23, 2441. [Google Scholar] [CrossRef] [PubMed]
- Sample Size Calculator. Available online: https://clincalc.com/stats/samplesize.aspx (accessed on 15 May 2024).
- Salivary Proteome on LinkedIn: HSP. Available online: https://www.linkedin.com/feed/update/urn:li:activity:7166890619993333760 (accessed on 16 May 2024).
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (COPD) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Krenke, R.; Kuziemski, K.; Czajkowska-Malinowska, M.; Celejewska-Wójcik, N.; Kuźnar-Kamińska, B.; Farnik, M.; Bokiej, J.; Miszczuk, M.; Damps-Konstańska, I.; et al. Distribution and Characteristics of COPD Phenotypes—Results from the Polish Sub-Cohort of the POPE Study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in Diagnostic Applications of Saliva in Human Organ Diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Shanahan, L.; Copeland, W.E.; Worthman, C.M.; Erkanli, A.; Angold, A.; Costello, E.J. Sex-Differentiated Changes in C-Reactive Protein from Ages 9 to 21: The Contributions of BMI and Physical/Sexual Maturation. Psychoneuroendocrinology 2013, 38, 2209–2217. [Google Scholar] [CrossRef]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Zielenkiewicz, M.; Paczek, L. Inflammatory Markers Change with Age, but Do Not Fall Beyond Reported Normal Ranges. Arch. Immunol. Ther. Exp. 2016, 64, 249–254. [Google Scholar] [CrossRef]
- Pignatti, P.; Ragnoli, B.; Radaeli, A.; Moscato, G.; Malerba, M. Age-Related Increase of Airway Neutrophils in Older Healthy Nonsmoking Subjects. Rejuvenation Res. 2011, 14, 365–370. [Google Scholar] [CrossRef]
- Meyer, K.C.; Rosenthal, N.S.; Soergel, P.; Peterson, K. Neutrophils and Low-Grade Inflammation in the Seemingly Normal Aging Human Lung. Mech. Ageing Dev. 1998, 104, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gökhan, P.; Sema, A. Evaluation of Alpha-1-Antitrypsin Levels in Blood Serum of Patients with Chronic Obstructive Pulmonary Disease. Acta Biomed. 2019, 90, 37–43. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Avihai, B.; Sundel, E.P.; Lee, E.; Greenberg, P.J.; Cook, B.P.; Altomare, N.J.; Ko, T.M.; Chaia, A.I.; Parikh, P.D.; Blaser, M.J. CRP Monitoring in Early Hospitalization: Implications for Predicting Outcomes in Patients with COVID-19. Pathogens 2023, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Hesamizadeh, K.; Maleki, F.; Farahmand, M.; Zahednasab, H.; Mirzaei, R.; Banoun, H.; Zamani, F.; Hajibaba, M.; Tabibzadeh, A.; et al. A Possible Pathogenic Correlation between Neutrophil Elastase (NE) Enzyme and Inflammation in the Pathogenesis of Coronavirus Disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 100, 108137. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin Levels in COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Belcher, J.; Thorpe, G.; Forsyth, N.R.; Spiteri, M.A. Measurement of C-Reactive Protein, Procalcitonin and Neutrophil Elastase in Saliva of COPD Patients and Healthy Controls: Correlation to Self-Reported Wellbeing Parameters. Respir. Res. 2015, 16, 62. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Saini, V.; Kaur, J. Evaluation and Correlation of Serum C-Reactive Protein and Procalcitonin Levels in Acute Exacerbation and Stable Phase of Chronic Obstructive Pulmonary Disease Patients: An Observational Study. J. Clin. Diagn. Res. 2021, 15, 24–28. [Google Scholar] [CrossRef]
- Nikolakopoulou, S.; Hillas, G.; Perrea, D.; Tentolouris, N.; Loukides, S.; Kostikas, K.; Simoes, D.; Georgakopoulou, E.; Vogiatzakis, E.; Veldekis, D.; et al. Serum Angiopoietin-2 and CRP Levels during COPD Exacerbations. COPD 2014, 11, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, S.J.; Mistry, V.; Brightling, C.E.; Moffitt, K.L.; Ribeiro, D.; Bafadhel, M. Neutrophil Elastase as a Biomarker for Bacterial Infection in COPD. Respir. Res. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Abhang, S. RupaliPawar, Subodhini A AbhangEvaluation of Serum Level of Neutrophil Elastase, Superoxide Dismutase and Nitric Oxide in COPD Patients and Its Correlation with Lung Function Test. Int. J. Biochem. Res. Rev. 2015, 5, 153–161. [Google Scholar]
- Stocker, M.; Fontana, M.; El Helou, S.; Wegscheider, K.; Berger, T.M. Use of Procalcitonin-Guided Decision-Making to Shorten Antibiotic Therapy in Suspected Neonatal Early-Onset Sepsis: Prospective Randomized Intervention Trial. Neonatology 2010, 97, 165–174. [Google Scholar] [CrossRef]
- Guz, G.; Colak, B.; Hizel, K.; Reis, K.A.; Erten, Y.; Bali, M.; Sindel, S. Procalcitonin and Conventional Markers of Inflammation in Peritoneal Dialysis Patients and Peritonitis. Perit. Dial. Int. 2006, 26, 240–248. [Google Scholar] [CrossRef]
- Issa, S.A.; Elmahdy, M.A.; ElShimi, O.S.; ElNaggar, M.E.; Behairy, M.M. Asessment of Serum Procalcitonin Level in Patients with Chronic Obstructive Pulmonary Disease. Benha J. Appl. Sci. 2021, 6, 319–324. [Google Scholar] [CrossRef]
- Kelly-Robinson, G.A.; Reihill, J.A.; Lundy, F.T.; McGarvey, L.P.; Lockhart, J.C.; Litherland, G.J.; Thornbury, K.D.; Martin, S.L. The Serpin Superfamily and Their Role in the Regulation and Dysfunction of Serine Protease Activity in COPD and Other Chronic Lung Diseases. Int. J. Mol. Sci. 2021, 22, 6351. [Google Scholar] [CrossRef] [PubMed]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J. Effects of Cigarette Smoke on the Lung and Systemic Immunity. J. Physiol. Pharmacol. 2009, 59 (Suppl. S6), 19–34. [Google Scholar]
- Joshi, B.K.; Singh, S.; Samanta, S.; Mohapatra, T. To Study the Effect of Cigarette Smoking on Anthropometric Markers, Serum Alpha 1 Antitrypsin and Cotinine Levels. Int. J. Adv. Integr. Med. Sci. 2021, 6, 25–31. [Google Scholar]
- Bestall, J.; Paul, E.; Garrod, R.; Garnham, R.; Jones, P.; Wedzicha, J. Usefulness of the Medical Research Council (MRC) Dyspnoea Scale as a Measure of Disability in Patients with Chronic Obstructive Pulmonary Disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Paladini, L.; Hodder, R.; Cecchini, I.; Bellia, V.; Incalzi, R.A. The MRC Dyspnoea Scale by Telephone Interview to Monitor Health Status in Elderly COPD Patients. Respir. Med. 2010, 104, 1027–1034. [Google Scholar] [CrossRef]
- Weiss, S.T.; DeMeo, D.L.; Postma, D.S. COPD: Problems in Diagnosis and Measurement. Eur. Respir. J. Suppl. 2003, 41, 4s–12s. [Google Scholar] [CrossRef]
- Andreeva, E.; Pokhaznikova, M.; Lebedev, A.; Moiseeva, I.; Kuznetsova, O.; Degryse, J.-M. Spirometry Is Not Enough to Diagnose COPD in Epidemiological Studies: A Follow-up Study. npj Prim. Care Resp. Med. 2017, 27, 62. [Google Scholar] [CrossRef]








| No. | Inclusion Criteria for Non-Smokers and Smokers: | Exclusion Criteria for Groups |
|---|---|---|
| 1 | aged > 18 years (male or female) | for healthy smoking and non-smoking cohorts: individuals diagnosed with COPD or other respiratory diseases |
| 2 | general good health condition | pregnancy or breastfeeding |
| 3 | (1) for the non-smoking group: the non-smoking individuals; (2) for smoker’s cohort: addicted to nicotine | the likelihood of non-cooperation or participation in another clinical trial, a history of addiction to nicotine (for the non-smoking group), drugs, or alcohol, an implanted bioelectric device, such as a pacemaker, hearing aid, orthodontic appliance, or metal wires in the mouth |
| No. | Inclusion Criteria for COPD Patients: | |
| 1 | aged > 18 years (male or female) | |
| 2 | diagnosis of COPD at least 12 months before sample collections |
| Clinical Characteristic | Healthy Non-Smokers n = 70 | Healthy Smokers n = 70 | COPD n = 140 |
|---|---|---|---|
| Gender | |||
| Female | 55 (79%) | 51 (73%) | 73 (52%) |
| Male | 15 (21%) | 19 (27%) | 67 (48%) |
| Age | |||
| 18–39 | 30 (43%) | 30 (43%) | 0 (0%) |
| 40–55 | 28 (40%) | 24 (34%) | 4 (3%) |
| >55 | 12 (17%) | 16 (33%) | 136 (97%) |
| BMI (kg/m2) | |||
| 18.5–24.9 | 45 (64%) | 30 (43%) | 41 (29%) |
| 25.0–29.9 | 18 (26%) | 27 (39%) | 54 (39%) |
| >30 | 7 (10%) | 13 (18%) | 45 (32%) |
| Regular Medication (Unrelated COPD) | |||
| With Medicines (medicine for blood pressure and thyroid medications) | 12 (17%) | 16 (23%) | 109 (79%) |
| Without Medicines | 58 (83%) | 54 (77%) | 31 (21%) |
| Comorbidities | |||
| Yes | N/A * | N/A | 82 (59%) |
| No | N/A | N/A | 58 (41%) |
| COVID-19 | |||
| 0 | 33 (47%) | 35 (50%) | 96 (69%) |
| 1 | 29 (41%) | 24 (34%) | 44 (31%) |
| ≥2 | 8 (11%) | 11 (16%) | 0 (0%) |
| Doses OF COVID-19 Vaccines | |||
| 0 | 8 (11%) | 4 (6%) | 12 (9%) |
| 1 | 0 (0%) | 4 (6%) | 0 (0%) |
| 2 | 14 (20%) | 16 (23%) | 31 (22%) |
| 3 | 43 (61%) | 32 (46%) | 58 (41%) |
| 4 | 5 (8%) | 14 (20%) | 39 (28%) |
| Allergy | |||
| Yes (allergy to pollen, cosmetic, insect bites, food) | 6 (9%) | 11 (16%) | 122 (87%) |
| No | 64 (91%) | 59 (84%) | 18 (13%) |
| Frequent Respiratory Infections | |||
| Yes | 5 (7%) | 9 (13%) | 13 (9%) |
| No | 65 (93%) | 61 (87%) | 127 (91%) |
| COPD Grade | |||
| I | N/A | N/A | 23 (16%) |
| II | N/A | N/A | 81 (58%) |
| III | N/A | N/A | 31 (22%) |
| IV | N/A | N/A | 5 (4%) |
| Years since the COPD Diagnosis | |||
| 1–5 | N/A | N/A | 75 (54%) |
| 6–10 | N/A | N/A | 36 (26%) |
| >10 | N/A | N/A | 29 (20%) |
| Bronchial Asthma | |||
| Yes | 0 | 0 | 38 (27%) |
| No | 0 | 0 | 102 (33%) |
| Prediction for Patient | |||
| Very good and good | N/A | N/A | 22 (16%) |
| Moderate good | N/A | N/A | 45 (32%) |
| Moderate bad | N/A | N/A | 45 (32%) |
| Bad and very bad | N/A | N/A | 28 (20%) |
| Tobacco Addiction | |||
| Non-smoking | 70 (100%) | N/A | 87 (62%) |
| Standard cigarettes | N/A | 45 (64%) | 53 (38%) |
| Electronic cigarettes | N/A | 25 (36%) | 0 (0%) |
| Years of Smoking | |||
| 0 | N/A | 0 (0%) | 87 (62%) |
| 1–19 | N/A | 40 (57%) | 1 (1%) |
| ≥20–29 | N/A | 21 (30%) | 5 (3%) |
| ≥30 | N/A | 9 (13%) | 47 (34%) |
| Numbers of Cigarettes/Day | |||
| 0 | N/A | 0 (0%) | 87 (62%) |
| 1–9 | 0 | 47 (67%) | 7 (5%) |
| ≥10 | 0 | 23 (33%) | 46 (33%) |
| Exposure to Dust | |||
| No | 0 (0%) | 0 (0%) | 0 (0%) |
| Yes | 70 (100%) | 70 (100%) | 140 (100%) |
| Pain in Chest | |||
| No | 70 (100%) | 66 (94%) | 136 (97%) |
| Yes | 0 (0%) | 4 (6%) | 4 (3%) |
| Cough without Infection | |||
| No | 70 (100%) | 70 (100%) | 19 (14%) |
| Yes | 0 (0%) | 0 (0%) | 121 (86%) |
| Wheezing | |||
| No | 70 (100%) | 69 (99%) | 80 (57%) |
| Yes | 0 (0%) | 1 (1%) | 60 (43%) |
| Short of Breath during Physical Activity | |||
| No | 70 (100%) | 66 (94%) | 18 (13%) |
| Yes | 0 (0%) | 4 (6%) | 122 (87%) |
| Difficulty Inhaling | |||
| No | 70 (100%) | 66 (94%) | 95 (68%) |
| Yes | 0 (0%) | 4 (6%) | 45 (32%) |
| Sputum | |||
| No | 70 (100%) | 1 (1%) | 26 (19%) |
| Yes | 0 (0%) | 69 (99%) | 114 (81%) |
| Depression | |||
| No | 70 (100%) | 70 (100%) | 2 (1%) |
| Yes | 0 (0%) | 0 (0%) | 138 (99%) |
| Cyanosis | |||
| No | 70 (100%) | 70 (100%) | 140 (100%) |
| Yes | 0 (0%) | 0 (0%) | 0 (0%) |
| Breathing Assessment (Answer Category) | How Do You Rate Your Breathing? | |||||
|---|---|---|---|---|---|---|
| Number of Cases | FEV1/FVC Value | CRP | NE | PCT | AAT | |
| (1) Perfect | 46 | 77.8 ± 10 | 8.4 ± 11.9 | 170.9 ± 153 | 2.8 ± 1.4 | 83.6 ± 30 |
| (2) Satisfactory | 76 | 74.0 ± 15 | 6.5 ± 4.5 | 174.5 ± 125 | 2.6 ± 1.8 | 99.6 ± 57 |
| (3) Bad and very bad | 18 | 71.6 ± 13 | 8.5 ± 4.7 | 120 ± 84 | 2.0 ± 1.4 | 90.5 ± 31 |
| Significant differences | - | - | - | (1) vs. (3) * p = 0.03 | ||
| How Does Your Breathing Affect Everyday Activities, e.g., Walking, Cleaning, Cooking? | ||||||
| (1) Has no effect | 39 | 79.6 ± 9 | 8.1 ± 6.7 | 180 ± 115 | 3.1 ± 1.7 | 80.3 ± 23 |
| (2) Slight | 36 | 76.6 ± 9 | 6.6 ± 4.4 | 157.9 ± 137 | 2.7 ± 1.5 | 100.9 ± 67 |
| (3) Moderate | 48 | 73.7 ± 12 | 7.3 ± 10 | 187.8 ± 151 | 2.6 ± 1.7 | 98.6 ± 45 |
| (4) Strong and very strong | 17 | 64 ± 22 | 4.5 ± 4.3 | 92.6 ± 53 | 1.6 ± 1.2 | 90.3 ± 40 |
| Significant differences | (1) vs. (3) * p = 0.01 (1) vs. (6) *** p = 0.0006 (2) vs. (4) ** p = 0.007 (3) vs. (4) * p= 0.03 | (1) vs. (4) * p = 0.04 | - (1) vs. (4) ** p = 0.004 (3) vs. (4) * p = 0.01 | - (1) vs. (4) ** p = 0.001 (2) vs. (3) * p = 0.01 (3) vs. (4) * p = 0.01 | - (1) vs. (3) * p = 0.02 | |
| How Do You Rate the Degree of Apnea Associated with Your Activity? | ||||||
| (1) Shortness of breath during intense physical exercise or running | 38 | 77.7 ± 10 | 5.8 ± 3.9 | 169.3 ± 153 | 2.7 ± 1.9 | 86.5 ± 24 |
| (2) I get short of breath faster than people my age when walking. I have to stop when I’m walking at my own pace on a level. | 67 | 75.3 ± 12 | 8.7 ± 10 | 169.2 ± 128 | 2.8 ± 1.5 | 92.4 ± 47 |
| (3) I have to stop after about 100 m of walking. | 28 | 75.4 ± 11 | 5.7 ± 4.5 | 169.5 ± 122 | 2.3 ± 1.4 | 108 ± 70 |
| (4) Breathing problems are too severe to leave the house | 7 | 54.6 ± 28 | 2.8 ± 2.4 | 112 ± 66 | 1.5 ± 1.2 | 76 ± 30 |
| Significant differences | (1) vs. (4) *** p = 0.0003 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzinska-Radecka, M.; Bańcerowski, B.; Marczyński, R.; Mukherjee, D.; Sikora, T.; Morawska, K.; Mielczarek, A.; Moździerski, M.; Hajduk, B.; Kotowicz, B. Evaluation of Salivary Biomarkers and Spirometry for Diagnosing COPD in Non-Smokers and Smokers of Polish Origin. Biomedicines 2024, 12, 1206. https://doi.org/10.3390/biomedicines12061206
Rudzinska-Radecka M, Bańcerowski B, Marczyński R, Mukherjee D, Sikora T, Morawska K, Mielczarek A, Moździerski M, Hajduk B, Kotowicz B. Evaluation of Salivary Biomarkers and Spirometry for Diagnosing COPD in Non-Smokers and Smokers of Polish Origin. Biomedicines. 2024; 12(6):1206. https://doi.org/10.3390/biomedicines12061206
Chicago/Turabian StyleRudzinska-Radecka, Magdalena, Bartłomiej Bańcerowski, Robert Marczyński, Debjita Mukherjee, Tomasz Sikora, Karolina Morawska, Agnieszka Mielczarek, Marcin Moździerski, Bogdan Hajduk, and Beata Kotowicz. 2024. "Evaluation of Salivary Biomarkers and Spirometry for Diagnosing COPD in Non-Smokers and Smokers of Polish Origin" Biomedicines 12, no. 6: 1206. https://doi.org/10.3390/biomedicines12061206







