Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Serum Soluble CD14 (sCD14), CD163 (sCD163) and Endotoxin
2.3. Serum K18M30 and M65 Levels
2.4. Serum Cytokines, Chemokines, and Hormones
2.5. Statistical Analysis
3. Results
3.1. The Characteristics of Study Participants
3.2. Gut Microbial Translocation and Immune Activation Markers in Patients with T2DM
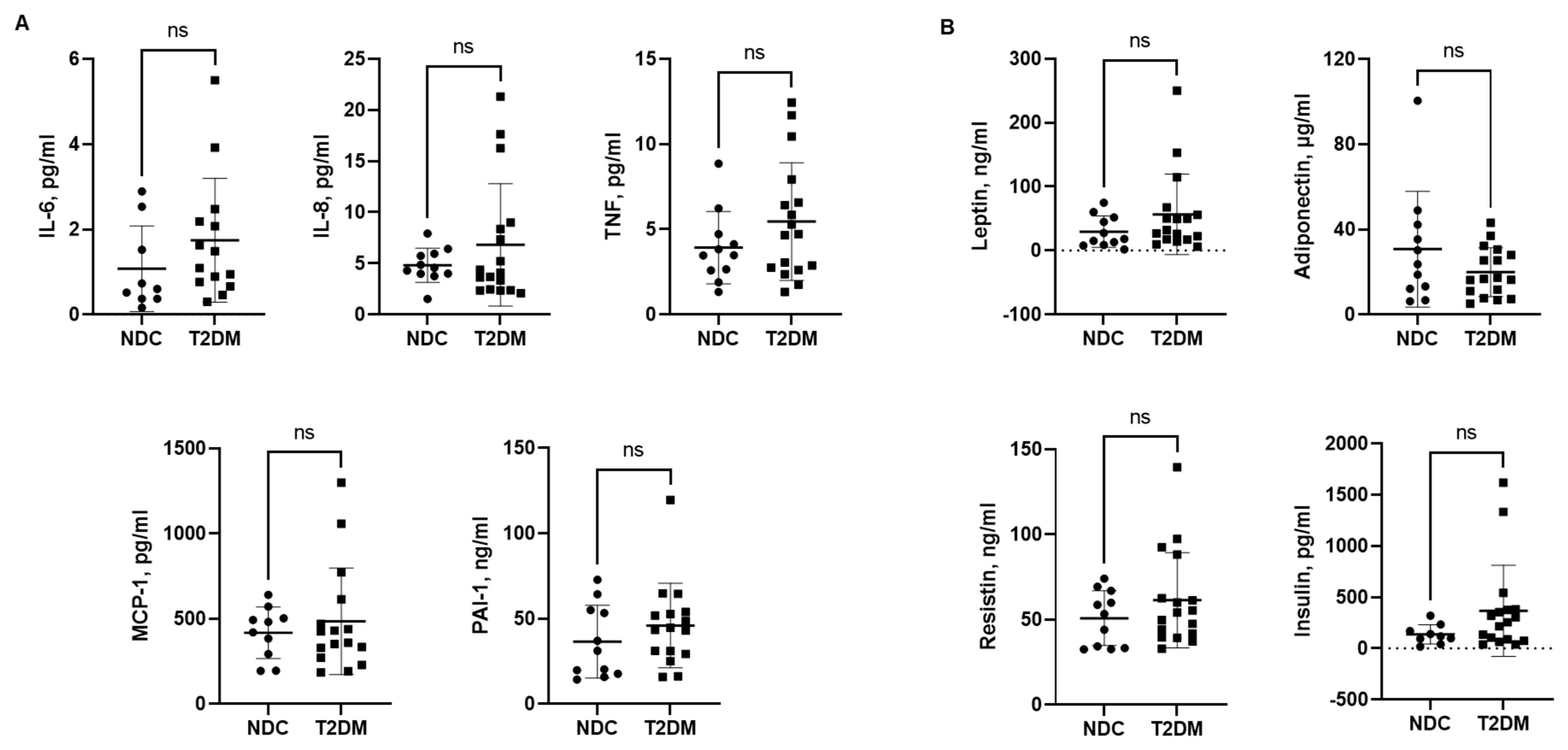
3.3. Serum Levels of Inflammatory Cytokines, Chemokines and Hormones
3.4. Liver Injury Markers—M30 and M65
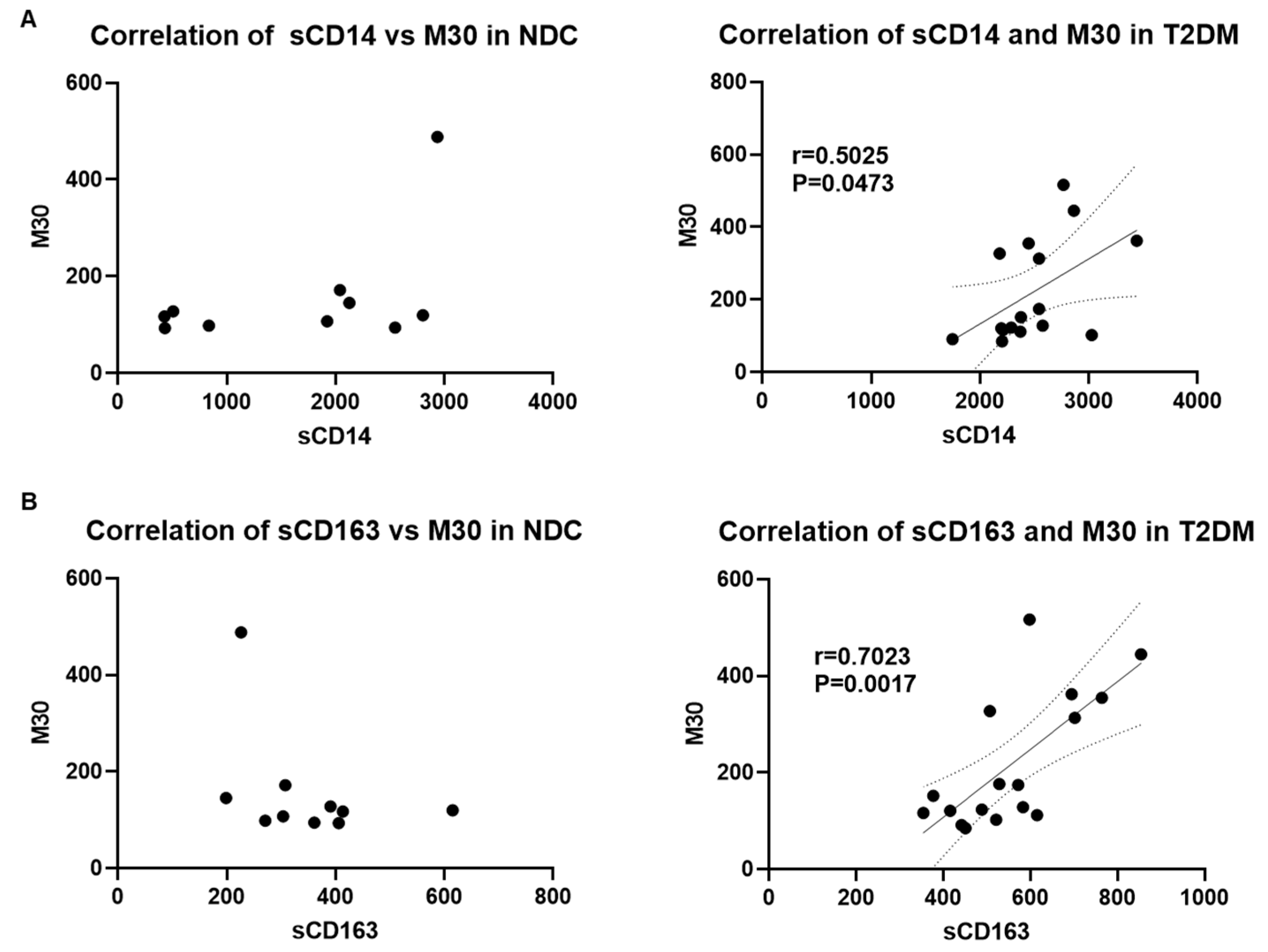
3.5. Correlation of Serum Markers of Microbial Translocation, Macrophage Activation, and Liver Injury in Patients with T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.J.; Nathan, D.M.; Arslanian, S.A.; Groop, L.; Rizza, R.A.; Rotter, J.I. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: What we know and what we need to know. J. Clin. Endocrinol. Metab. 2010, 95, 1566–1574. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef]
- Abeysekera, K.W.M.; Valenti, L.; Younossi, Z.; Dillon, J.F.; Allen, A.M.; Nourredin, M.; Rinella, M.E.; Tacke, F.; Francque, S.; Gines, P.; et al. Implementation of a liver health check in people with type 2 diabetes. Lancet Gastroenterol. Hepatol. 2024, 9, 83–91. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef]
- Petit, J.M.; Hamza, S.; Rollot, F.; Sigonney, V.; Crevisy, E.; Duvillard, L.; Raab, J.J.; Bronowicki, J.P.; Bernard-Chabert, B.; Di Martino, V.; et al. Impact of liver disease severity and etiology on the occurrence of diabetes mellitus in patients with liver cirrhosis. Acta Diabetol. 2014, 51, 455–460. [Google Scholar] [CrossRef]
- Hickman, I.J.; Macdonald, G.A. Impact of diabetes on the severity of liver disease. Am. J. Med. 2007, 120, 829–834. [Google Scholar] [CrossRef]
- Moscatiello, S.; Manini, R.; Marchesini, G. Diabetes and liver disease: An ominous association. Nutr. Metab. Cardiovasc. Dis. NMCD 2007, 17, 63–70. [Google Scholar] [CrossRef]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef]
- Leite, N.C.; Salles, G.F.; Araujo, A.L.; Villela-Nogueira, C.A.; Cardoso, C.R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009, 29, 113–119. [Google Scholar] [CrossRef]
- Miyasato, M.; Murase-Mishiba, Y.; Bessho, M.; Miyawaki, M.; Imbe, H.; Tsutsumi, C.; Tanimoto, K.; Imagawa, A.; Terasaki, J.; Hanafusa, T. The cytokeratin-18 fragment level as a biomarker of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Clin. Chim. Acta 2014, 433, 184–189. [Google Scholar] [CrossRef]
- Smith, B.W.; Adams, L.A. Nonalcoholic fatty liver disease and diabetes mellitus: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2011, 7, 456–465. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Pichiri, I.; Sorgato, C.; Zenari, L.; Bonora, E. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J. Hepatol. 2010, 53, 713–718. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Rodella, S.; Tessari, R.; Zenari, L.; Lippi, G.; Arcaro, G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007, 30, 2119–2121. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar] [CrossRef]
- Adams, L.A.; Harmsen, S.; St Sauver, J.L.; Charatcharoenwitthaya, P.; Enders, F.B.; Therneau, T.; Angulo, P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: A community-based cohort study. Am. J. Gastroenterol. 2010, 105, 1567–1573. [Google Scholar] [CrossRef]
- Lu, H.; Zeng, L.; Liang, B.; Shu, X.; Xie, D. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch. Med. Res. 2009, 40, 571–575. [Google Scholar] [CrossRef]
- Burcelin, R.; Serino, M.; Chabo, C.; Blasco-Baque, V.; Amar, J. Gut microbiota and diabetes: From pathogenesis to therapeutic perspective. Acta Diabetol. 2011, 48, 257–273. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ley, R.E. Obesity and the human microbiome. Curr. Opin. Gastroenterol. 2010, 26, 5–11. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef]
- Wu, X.; Ma, C.; Han, L.; Nawaz, M.; Gao, F.; Zhang, X.; Yu, P.; Zhao, C.; Li, L.; Zhou, A.; et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr. Microbiol. 2010, 61, 69–78. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Pickup, J.C. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. TEM 2008, 19, 10–16. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Rivero, A.; Mora, C.; Muros, M.; Garcia, J.; Herrera, H.; Navarro-Gonzalez, J.F. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci. 2009, 116, 479–492. [Google Scholar] [CrossRef]
- Kolb, H.; Mandrup-Poulsen, T. An immune origin of type 2 diabetes? Diabetologia 2005, 48, 1038–1050. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Tracy, R.P.; Haffner, S.M.; Insulin Resistance Atherosclerosis, S. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2002, 51, 1131–1137. [Google Scholar] [CrossRef]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef]
- Yang, M.; Gan, H.; Shen, Q.; Tang, W.; Du, X.; Chen, D. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation 2012, 35, 388–396. [Google Scholar] [CrossRef]
- Fernandez-Botran, R.; Joshi-Barve, S.; Ghare, S.; Barve, S.; Young, M.; Plankey, M.; Bordon, J. Systemic cytokine and interferon responsiveness Patterns in HIV and HCV mono and co-infections. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 885–893. [Google Scholar] [CrossRef]
- Landmann, R.; Knopf, H.P.; Link, S.; Sansano, S.; Schumann, R.; Zimmerli, W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect. Immun. 1996, 64, 1762–1769. [Google Scholar] [CrossRef]
- Kazankov, K.; Bojsen-Moller, K.N.; Moller, H.J.; Madsbad, S.; Gronbaek, H. Macrophage activation marker sCD163 is associated with liver injury and hepatic insulin resistance in obese patients before and after Roux-en-Y gastric bypass. Physiol. Rep. 2022, 10, e15157. [Google Scholar] [CrossRef]
- Sherman, K.E.; Meeds, H.L.; Rouster, S.D.; Abdel-Hameed, E.A.; Hernandez, J.; Tamargo, J.; Chen, J.; Ehman, R.L.; Baum, M. Soluble CD163 Identifies Those at Risk for Increased Hepatic Inflammation & Fibrosis. Open Forum Infect. Dis. 2021, 8, ofab203. [Google Scholar] [CrossRef]
- Glavind, E.; Gotthardt, D.N.; Pfeiffenberger, J.; Sandahl, T.D.; Bashlekova, T.; Willemoe, G.L.; Hasselby, J.P.; Weiss, K.H.; Moller, H.J.; Vilstrup, H.; et al. The macrophage activation marker soluble CD163 is elevated and associated with liver disease phenotype in patients with Wilson’s disease. Orphanet J. Rare Dis. 2020, 15, 173. [Google Scholar] [CrossRef]
- Siggaard, C.B.; Kazankov, K.; Rodgaard-Hansen, S.; Moller, H.J.; Donnelly, M.C.; Simpson, K.J.; Gronbaek, H. Macrophage markers soluble CD163 and soluble mannose receptor are associated with liver injury in patients with paracetamol overdose. Scand. J. Gastroenterol. 2019, 54, 623–632. [Google Scholar] [CrossRef]
- Anand, G.; Zarrinpar, A.; Loomba, R. Targeting Dysbiosis for the Treatment of Liver Disease. Semin. Liver Dis. 2016, 36, 37–47. [Google Scholar] [CrossRef]
- Quigley, E.M.; Monsour, H.P. The Gut Microbiota and Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2015, 35, 262–269. [Google Scholar] [CrossRef]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Mai, Y.; Meng, L.; Deng, G.; Qin, Y. The Role of Type 2 Diabetes Mellitus-Related Risk Factors and Drugs in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 159–171. [Google Scholar] [CrossRef]
- Albeshry, A.M.; Abdulrahman Alasmari, M.; Alshahrani, J.A.; Alshahrani, A.M.; Saad Almusma, A.; Alfaya, M.A.; Alfaifi, A.J.; Alshahrani, M.A.; Alharbi, H.K.D.; Ali Etwdi, A.S.; et al. Prevalence of Non-alcoholic Fatty Liver Disease (NAFLD) among Diabetic Mellitus Patients in Saudi Arabia: Systematic Review and Meta-Analysis. Cureus 2023, 15, e51092. [Google Scholar] [CrossRef]
- Colosimo, S.; Mitra, S.K.; Chaudhury, T.; Marchesini, G. Insulin resistance and metabolic flexibility as drivers of liver and cardiac disease in T2DM. Diabetes Res. Clin. Pract. 2023, 206, 111016. [Google Scholar] [CrossRef]
- Boeriu, A.; Dobru, D.; Fofiu, C. Non-Invasive Diagnostic of NAFLD in Type 2 Diabetes Mellitus and Risk Stratification: Strengths and Limitations. Life 2023, 13, 2262. [Google Scholar] [CrossRef]
- Qadri, S.; Yki-Jarvinen, H. Surveillance of the liver in type 2 diabetes: Important but unfeasible? Diabetologia 2024, 67, 961–973. [Google Scholar] [CrossRef]
- He, L.; Xuan, W.; Liu, D.; Zhong, J.; Luo, H.; Cui, H.; Zhang, X.; Chen, W. The role of adiponectin in the association between abdominal obesity and type 2 diabetes: A mediation analysis among 232,438 Chinese participants. Front. Endocrinol. 2024, 15, 1327716. [Google Scholar] [CrossRef]
- Vilarino-Garcia, T.; Polonio-Gonzalez, M.L.; Perez-Perez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Bansal, S.K.; Bansal, M.B. Review article: Pathogenesis of MASLD and MASH—Role of insulin resistance and lipotoxicity. Aliment. Pharmacol. Ther. 2024; early view. [Google Scholar] [CrossRef]
- Fruhbeck, G.; Gomez-Ambrosi, J.; Ramirez, B.; Becerril, S.; Rodriguez, A.; Mentxaka, A.; Valenti, V.; Moncada, R.; Reina, G.; Baixauli, J.; et al. Decreased expression of the NLRP6 inflammasome is associated with increased intestinal permeability and inflammation in obesity with type 2 diabetes. Cell. Mol. Life Sci. 2024, 81, 77. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, Q.; Xu, W.; Zhang, J. Gut microbiome in diabetic retinopathy: A systematic review and meta-analysis. Microb. Pathog. 2024, 189, 106590. [Google Scholar] [CrossRef]
- Wang, J.; Teng, M.; Feng, R.; Su, X.; Xu, K.; Wang, J.; Wang, G.; Zhang, Y.; Xu, P. Large-scale causal analysis of gut microbiota and six common complications of diabetes: A mendelian randomization study. Diabetol. Metab. Syndr. 2024, 16, 66. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Shi, M.; Zong, X.; Hur, J.; Birmann, B.M.; Martinez-Maza, O.; Epeldegui, M.; Chan, A.T.; Giovannucci, E.L.; Cao, Y. Circulating markers of microbial translocation and host response to bacteria with risk of colorectal cancer: A prospective, nested case-control study in men. EBioMedicine 2023, 91, 104566. [Google Scholar] [CrossRef]
- Awoyemi, A.; Troseid, M.; Arnesen, H.; Solheim, S.; Seljeflot, I. Markers of metabolic endotoxemia as related to metabolic syndrome in an elderly male population at high cardiovascular risk: A cross-sectional study. Diabetol. Metab. Syndr. 2018, 10, 59. [Google Scholar] [CrossRef]
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. 2010, 7, 15. [Google Scholar] [CrossRef]
- Nolan, J.P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology 2010, 52, 1829–1835. [Google Scholar] [CrossRef]
- Hiraoka, A.; Horiike, N.; Akbar, S.M.; Michitaka, K.; Matsuyama, T.; Onji, M. Expression of CD163 in the liver of patients with viral hepatitis. Pathol. Res. Pract. 2005, 201, 379–384. [Google Scholar] [CrossRef]
- Sandahl, T.D.; Gronbaek, H.; Moller, H.J.; Stoy, S.; Thomsen, K.L.; Dige, A.K.; Agnholt, J.; Hamilton-Dutoit, S.; Thiel, S.; Vilstrup, H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: A prospective cohort study. Am. J. Gastroenterol. 2014, 109, 1749–1756. [Google Scholar] [CrossRef]
- Rutherford, A.; King, L.Y.; Hynan, L.S.; Vedvyas, C.; Lin, W.; Lee, W.M.; Chung, R.T.; Group, A.L.F.S. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology 2012, 143, 1237–1243. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Jochum, C.; Kocabayoglu, P.; Sowa, J.P.; Kassalik, M.; Gieseler, R.K.; Saner, F.; Paul, A.; Trautwein, C.; Gerken, G.; et al. Cytokeratin 18-based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J. Hepatol. 2010, 53, 639–647. [Google Scholar] [CrossRef]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Apoptosis as a mechanism for liver disease progression. Semin. Liver Dis. 2010, 30, 402–410. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Loomba, R. The Role of Noninvasive Tests for Differentiating NASH From NAFL and Diagnosing Advanced Fibrosis Among Patients With NAFLD. J. Clin. Gastroenterol. 2020, 54, 107–113. [Google Scholar] [CrossRef]
- Maccioni, L.; Horsmans, Y.; Leclercq, I.; Schnabl, B.; Starkel, P. Serum keratin 18-M65 levels detect progressive forms of alcohol-associated liver disease in early noncirrhotic stages. Alcohol. Clin. Exp. Res. 2023, 47, 1079–1087. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, H.C.; Hwu, D.W.; Chang, D.M.; Lin, K.C.; Lee, Y.J. Elevated serum cytokeratin-18 concentration in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Ann. Clin. Biochem. 2019, 56, 141–147. [Google Scholar] [CrossRef]
- Morling, J.R.; Fallowfield, J.A.; Williamson, R.M.; Nee, L.D.; Jackson, A.P.; Glancy, S.; Reynolds, R.M.; Hayes, P.C.; Guha, I.N.; Strachan, M.W.; et al. Non-invasive hepatic biomarkers (ELF and CK18) in people with type 2 diabetes: The Edinburgh type 2 diabetes study. Liver Int. 2014, 34, 1267–1277. [Google Scholar] [CrossRef]




| Variables | T2DM (n = 17) | NDC (n = 11) | p-Value |
|---|---|---|---|
| Age, yrs. | 61.1 ± 10.4 | 39.9 ± 12.6 | ≤0.001 |
| Sex | 8 females, 9 males | 9 females, 2 males | NA |
| BMI | 36.1 ± 7.1 | 23.9 ± 3.1 | ≤0.001 |
| HgbA1c | 9.1 ± 1.9 | NA | NA |
| ALT | 19.1 ± 8.03 | 15.3 ± 15.33 | =0.031 |
| AST | 20.4 ± 6.67 | 17.6 ± 4.38 | =NS |
| Total Protein | 7.4 ± 0.45 | 7.8 ± 0.46 | =0.054 |
| Albumin | 4.9 ± 0.26 | 5 ± 0.32 | NS |
| Alkaline Phosphatase | 88.8 ± 23.37 | 62.1 ± 12.33 | =0.0029 |
| Glucose (mg/dL) | 187.4 ± 57.2 | 85.6 ± 3.9(5) | =0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobejishvili, L.; Vatsalya, V.; Avila, D.V.; Feygin, Y.B.; McClain, C.J.; Mokshagundam, S.; Barve, S. Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes. Biomedicines 2024, 12, 1227. https://doi.org/10.3390/biomedicines12061227
Gobejishvili L, Vatsalya V, Avila DV, Feygin YB, McClain CJ, Mokshagundam S, Barve S. Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes. Biomedicines. 2024; 12(6):1227. https://doi.org/10.3390/biomedicines12061227
Chicago/Turabian StyleGobejishvili, Leila, Vatsalya Vatsalya, Diana V. Avila, Yana B. Feygin, Craig J. McClain, Sriprakash Mokshagundam, and Shirish Barve. 2024. "Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes" Biomedicines 12, no. 6: 1227. https://doi.org/10.3390/biomedicines12061227
APA StyleGobejishvili, L., Vatsalya, V., Avila, D. V., Feygin, Y. B., McClain, C. J., Mokshagundam, S., & Barve, S. (2024). Association of Circulating Markers of Microbial Translocation and Hepatic Inflammation with Liver Injury in Patients with Type 2 Diabetes. Biomedicines, 12(6), 1227. https://doi.org/10.3390/biomedicines12061227






