Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress
Abstract
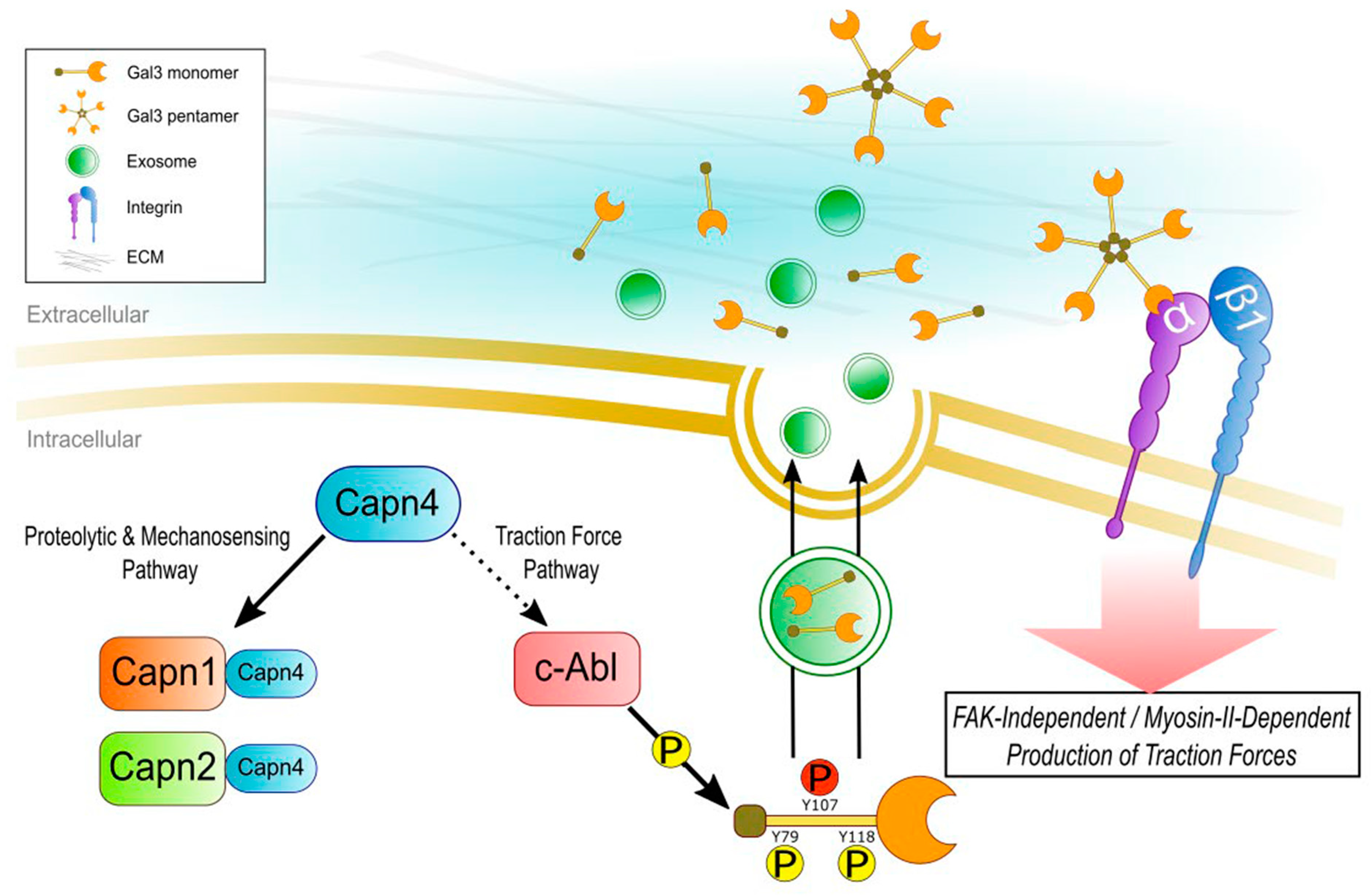
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Traction Force Microscopy and Analysis
2.3. Mechanosensing Experiments
2.4. Cell Adhesion Assay
2.5. Cell Migration Assay
2.6. Immunofluorescence
2.7. Microscopy
2.8. Polyacrylamide Gel Electrophoresis and Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Gal3 Is Essential for the Generation of Cellular Traction Force
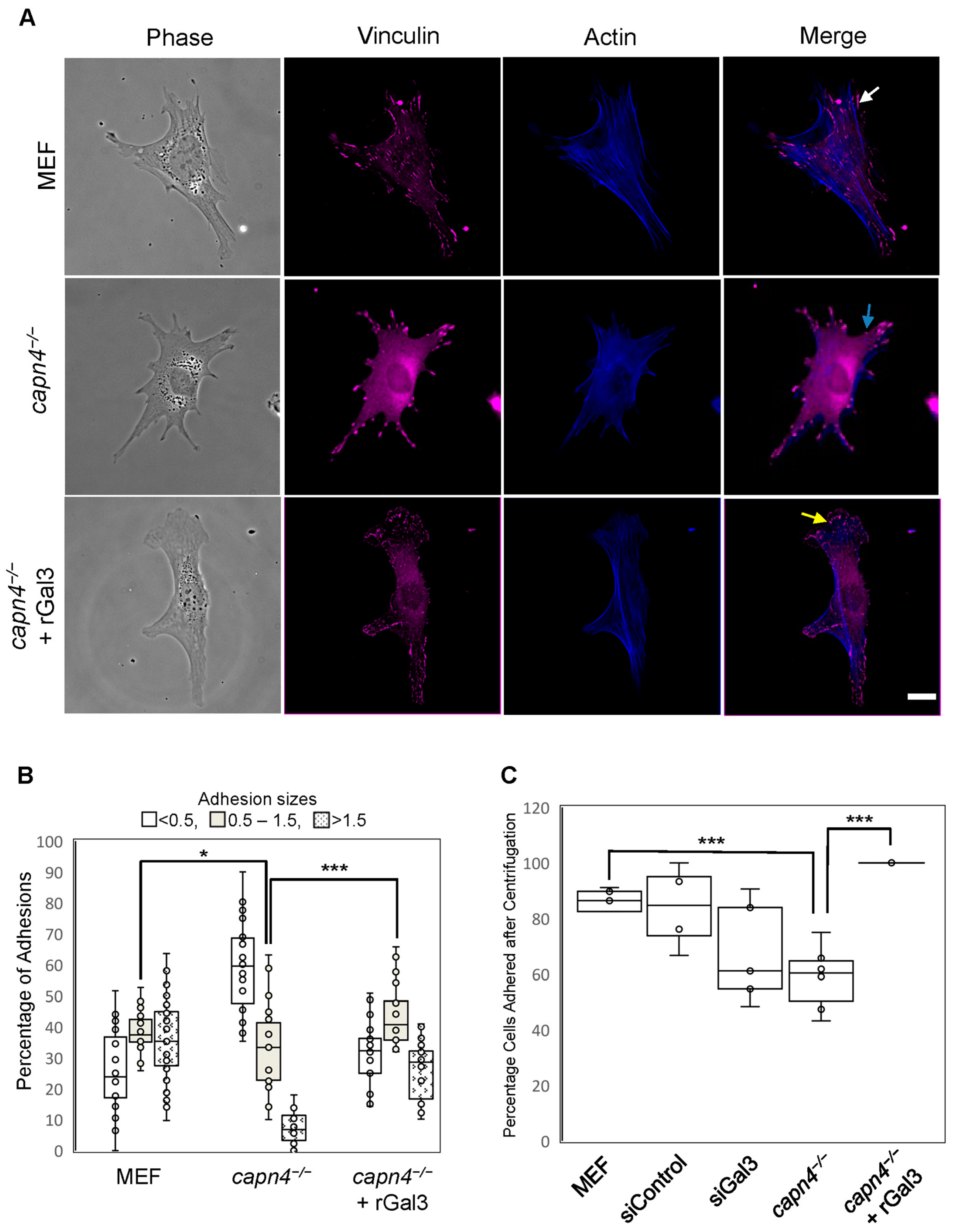
3.2. Extracellular Gal3 Affects the Numbers, Localization, Morphology, and Strength of Focal Adhesions
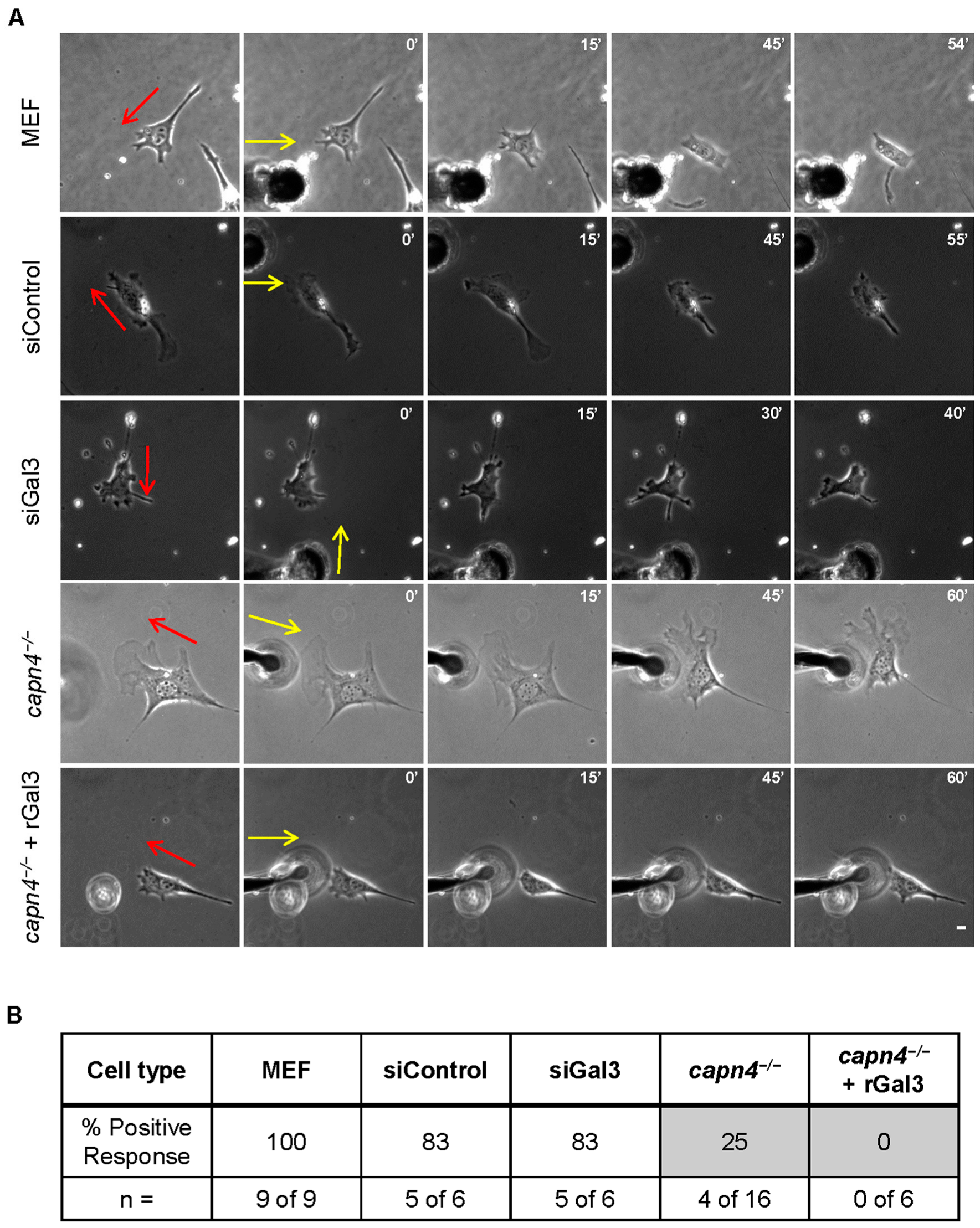
3.3. Extracellular Gal3 Impacts Linear Speed and Persistence of Migration
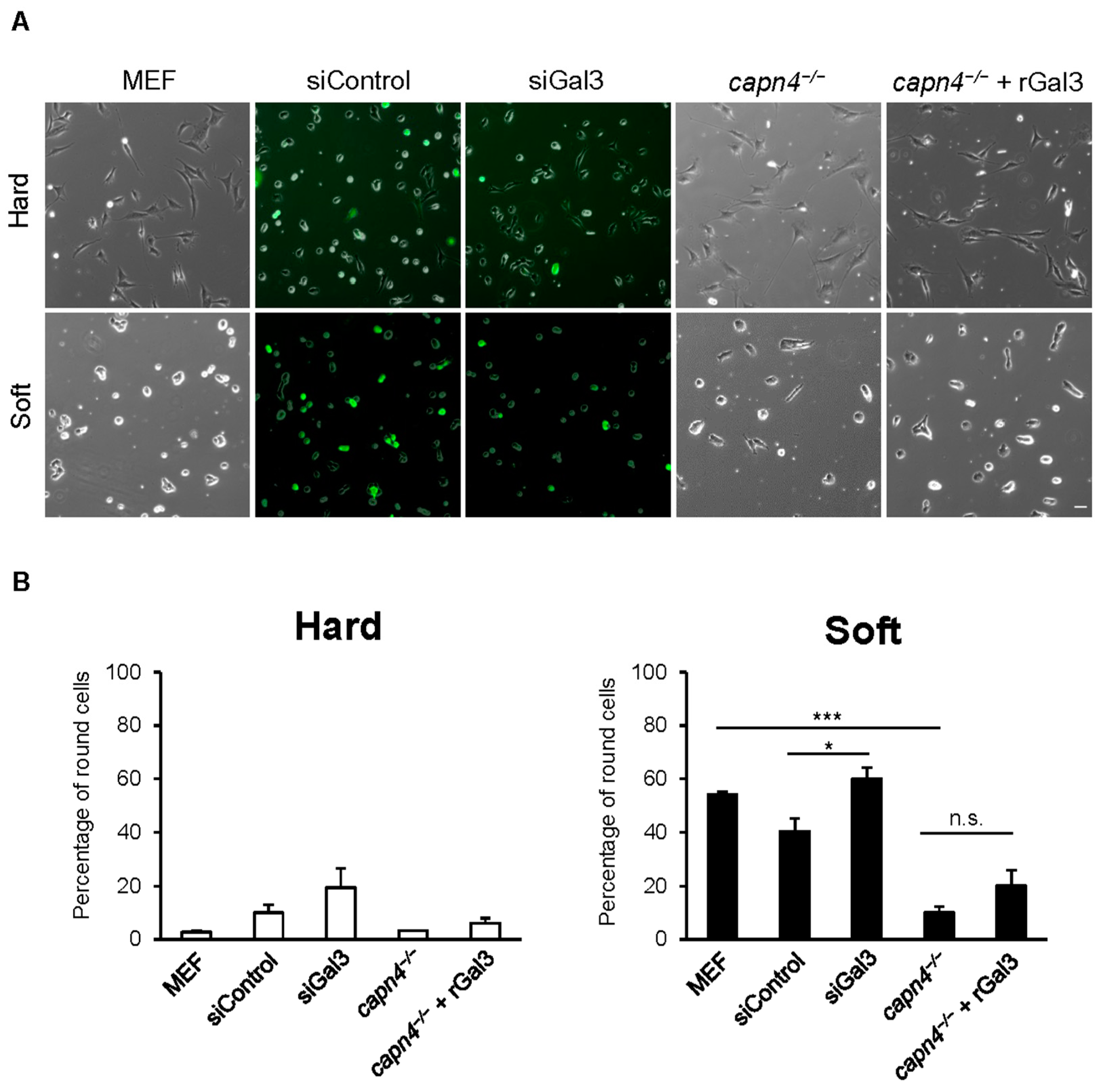
3.4. Extracellular Gal3 Does Not Rescue the Mechanosensing Defect of Capn4-Deficient Cells
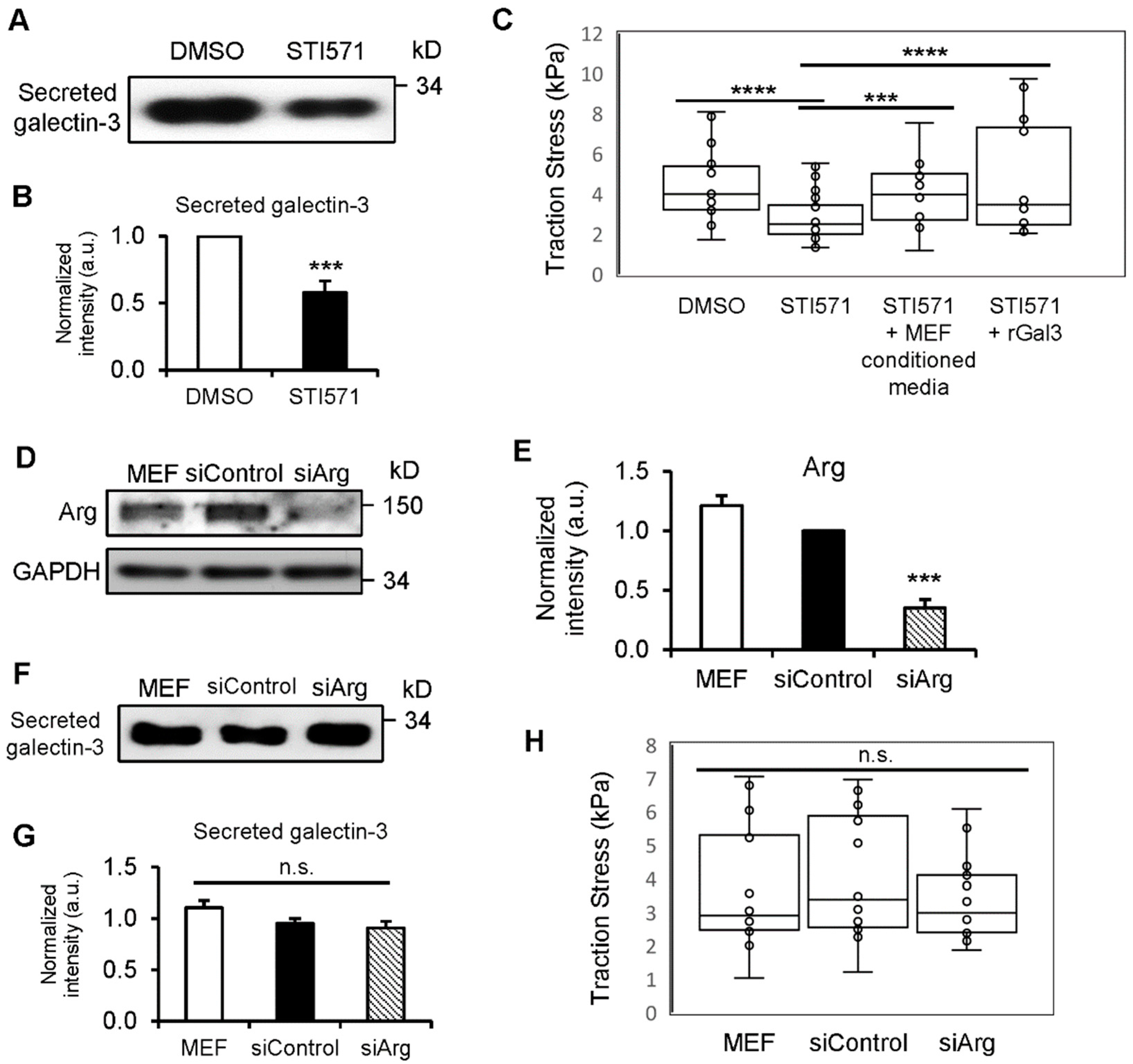
3.5. c-Abl Kinase Enhances Gal3 Secretion and Production of Traction Force
3.6. Y107 Phosphorylation of Gal3 Is Critical for Its Secretion and the Generation of Traction Forces
3.7. Active Integrin β1 May Participate in Extracellular Gal3-Mediated Traction Force Production, Possibly through a FAK-Independent Mechanism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huttenlocher, A.; Sandborg, R.R.; Horwitz, A.F. Adhesion in cell migration. Curr. Opin. Cell Biol. 1995, 7, 697–706. [Google Scholar] [PubMed]
- Li, S.; Guan, J.L.; Chien, S. Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 2005, 7, 105–150. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Actomyosin contractility and collective migration: May the force be with you. Curr. Opin. Cell Biol. 2017, 48, 87–96. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Zemel, A.; Safran, S.A. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010, 98, 143–175. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.A.; Atherton, P.; Ballestrem, C. Mechanotransduction at the cell-matrix interface. Semin. Cell Dev. Biol. 2017, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Fouchard, J.; Mitrossilis, D.; Asnacios, A. Acto-myosin based response to stiffness and rigidity sensing. Cell Adh. Migr. 2011, 5, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef]
- Weng, S.; Fu, J. Synergistic regulation of cell function by matrix rigidity and adhesive pattern. Biomaterials 2011, 32, 9584–9593. [Google Scholar] [CrossRef] [PubMed]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L. Traction forces and rigidity sensing of adherent cells. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 3339–3340. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.E.; Odde, D.J. Traction dynamics of filopodia on compliant substrates. Science 2008, 322, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Undyala, V.V.; Dembo, M.; Cembrola, K.; Perrin, B.J.; Huttenlocher, A.; Elce, J.S.; Greer, P.A.; Wang, Y.L.; Beningo, K.A. The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration. J. Cell Sci. 2008, 121, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Kang, C.M.; Beningo, K.A. Galectin-3 secretion and tyrosine phosphorylation is dependent on the calpain small subunit, Calpain 4. Biochem. Biophys. Res. Commun. 2011, 410, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Lipinska, A. Galectin-3 as a multifunctional protein. Cell Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar] [PubMed]
- Nakahara, S.; Raz, A. On the role of galectins in signal transduction. Methods Enzymol. 2006, 417, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Houzelstein, D.; Goncalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 2004, 21, 1177–1187. [Google Scholar] [CrossRef]
- Balan, V.; Nangia-Makker, P.; Jung, Y.S.; Wang, Y.; Raz, A. Galectin-3: A novel substrate for c-Abl kinase. Biochim. Biophys. Acta 2010, 1803, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Q.; Wang, J.; Liu, X.; Yang, Y.; Zhao, H.; Wang, Y.; Jin, Y.; Zeng, J.; Li, J.; et al. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ. 2010, 17, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta 2010, 1800, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta 2002, 1572, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Dange, M.C.; Agarwal, A.K.; Kalraiya, R.D. Extracellular galectin-3 induces MMP9 expression by activating p38 MAPK pathway via lysosome-associated membrane protein-1 (LAMP1). Mol. Cell Biochem. 2015, 404, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Akita, K.; Yashiro, M.; Sawada, T.; Hirakawa, K.; Murata, T.; Nakada, H. Binding of Galectin-3, a beta-Galactoside-binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy. J. Biol. Chem. 2015, 290, 26125–26140. [Google Scholar] [CrossRef]
- Colomb, F.; Wang, W.; Simpson, D.; Zafar, M.; Beynon, R.; Rhodes, J.M.; Yu, L.G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017, 292, 8381–8389. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2004, 19, 527–535. [Google Scholar] [CrossRef]
- Boscher, C.; Nabi, I.R. Galectin-3- and phospho-caveolin-1-dependent outside-in integrin signaling mediates the EGF motogenic response in mammary cancer cells. Mol. Biol. Cell 2013, 24, 2134–2145. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.; Butera, D.; Junqueira Mde, S.; Hsu, D.K.; da Silva, A.M.; Liu, F.T.; Santos, M.F.; Chammas, R. The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase. PLoS ONE 2011, 6, e29313. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.H.; Rode, J.; Howlader, M.A.; Eckermann, M.; Santos, J.T.; Hernandez Armada, D.; Zheng, R.; Zou, C.; Cairo, C.W. Galectin-3 alters the lateral mobility and clustering of beta1-integrin receptors. PLoS ONE 2017, 12, e0184378. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G.; Joshi, B.; Lajoie, P.; Strugnell, S.S.; Scudamore, T.; Kojic, L.D.; Nabi, I.R. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J. Cell Biol. 2008, 180, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.; Goetz, J.G.; Cheung, P.; Raz, A.; Dennis, J.W.; Nabi, I.R. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell Biol. 2006, 26, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.C.; Honjo, Y.; Nangia-Makker, P.; Hogan, V.; Mazurak, N.; Bresalier, R.S.; Raz, A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999, 59, 6239–6245. [Google Scholar] [PubMed]
- Lindstedt, R.; Apodaca, G.; Barondes, S.H.; Mostov, K.E.; Leffler, H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J. Biol. Chem. 1993, 268, 11750–11757. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Burdett, I.; Hughes, R.C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: A pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 1993, 207, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Q.; Ochieng, J. Rapid release of intracellular galectin-3 from breast carcinoma cells by fetuin. Cancer Res. 2001, 61, 1869–1873. [Google Scholar] [PubMed]
- Banfer, S.; Schneider, D.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef] [PubMed]
- Papusheva, E.; Heisenberg, C.P. Spatial organization of adhesion: Force-dependent regulation and function in tissue morphogenesis. EMBO J. 2010, 29, 2753–2768. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Henis, Y.I.; Geiger, B.; Bershadsky, A.D. The heel and toe of the cell’s foot: A multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton 2009, 66, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Dourdin, N.; Bhatt, A.K.; Dutt, P.; Greer, P.A.; Arthur, J.S.; Elce, J.S.; Huttenlocher, A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem. 2001, 276, 48382–48388. [Google Scholar] [CrossRef]
- Arthur, J.S.; Elce, J.S.; Hegadorn, C.; Williams, K.; Greer, P.A. Disruption of the murine calpain small subunit gene, Capn4: Calpain is essential for embryonic development but not for cell growth and division. Mol. Cell Biol. 2000, 20, 4474–4481. [Google Scholar] [CrossRef]
- Franco, S.; Perrin, B.; Huttenlocher, A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp. Cell Res. 2004, 299, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Beningo, K.A.; Lo, C.M.; Wang, Y.L. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 2002, 69, 325–339. [Google Scholar] [PubMed]
- Dembo, M.; Wang, Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999, 76, 2307–2316. [Google Scholar] [CrossRef]
- Marganski, W.A.; Dembo, M.; Wang, Y.L. Measurements of cell-generated deformations on flexible substrata using correlation-based optical flow. Methods Enzymol. 2003, 361, 197–211. [Google Scholar] [PubMed]
- Guo, W.H.; Frey, M.T.; Burnham, N.A.; Wang, Y.L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006, 90, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Petrie, R.J.; Doyle, A.D.; Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Munevar, S.; Wang, Y.L.; Dembo, M. Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol. Biol. Cell 2001, 12, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Freund, J.B.; Goetz, J.G.; Hill, K.L.; Vermot, J. Fluid flows and forces in development: Functions, features and biophysical principles. Development 2012, 139, 1229–1245. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Menon, S.; Beningo, K.A. Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS ONE 2011, 6, e17277. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J., Jr.; Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.A.; Ju, Y.E.; Marg, B.; Osterfield, M.; Janmey, P.A. Neurite branching on deformable substrates. Neuroreport 2002, 13, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, O.; Rix, U.; Superti-Furga, G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk. Lymphoma 2008, 49, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; Gauthier, N.C.; Del Rio, A.; Sheetz, M.P. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 2009, 106, 16245–16250. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.E.; Dumbauld, D.W.; Burns, K.L.; Hanks, S.K.; Garcia, A.J. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell 2009, 20, 2508–2519. [Google Scholar] [CrossRef]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006, 174, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Schober, M.; Raghavan, S.; Nikolova, M.; Polak, L.; Pasolli, H.A.; Beggs, H.E.; Reichardt, L.F.; Fuchs, E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007, 176, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Dembo, M.; Hanks, S.K.; Wang, Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA 2001, 98, 11295–11300. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Carey, S.P.; Califano, J.P.; Reinhart-King, C.A. Quantifying traction stresses in adherent cells. Methods Cell Biol. 2012, 110, 139–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lin, J.S. Cell traction force and measurement methods. Biomech. Model. Mechanobiol. 2007, 6, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Beningo, K.A.; Dembo, M.; Kaverina, I.; Small, J.V.; Wang, Y.L. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001, 153, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Arias-Salgado, E.G.; Lizano, S.; Sarkar, S.; Brugge, J.S.; Ginsberg, M.H.; Shattil, S.J. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 2003, 100, 13298–13302. [Google Scholar] [CrossRef]
- Cox, E.A.; Sastry, S.K.; Huttenlocher, A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol. Cell 2001, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Rape, A.; Guo, W.H.; Wang, Y.L. Microtubule depolymerization induces traction force increase through two distinct pathways. J. Cell Sci. 2011, 124, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, G.; Gal, A.; Kutsche, K. AlphaPIX associates with calpain 4, the small subunit of calpain, and has a dual role in integrin-mediated cell spreading. J. Biol. Chem. 2005, 280, 6879–6889. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Spencer, S.D.; Lasky, L.A. Tyrosine phosphorylation regulates the SH3-mediated binding of the Wiskott-Aldrich syndrome protein to PSTPIP, a cytoskeletal-associated protein. J. Biol. Chem. 1998, 273, 5765–5770. [Google Scholar] [CrossRef] [PubMed]
- Baum, W.; Kirkin, V.; Fernandez, S.B.; Pick, R.; Lettau, M.; Janssen, O.; Zornig, M. Binding of the intracellular Fas ligand (FasL) domain to the adaptor protein PSTPIP results in a cytoplasmic localization of FasL. J. Biol. Chem. 2005, 280, 40012–40024. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Spencer, S.; Cote, J.F.; Wu, Y.; Tremblay, M.L.; Lasky, L.A.; Goff, S.P. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol. Cell 2000, 6, 1413–1423. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, I.; Menon, S.; Indra, I.; Basith, R.; Beningo, K.A. Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress. Biomedicines 2024, 12, 1247. https://doi.org/10.3390/biomedicines12061247
Jang I, Menon S, Indra I, Basith R, Beningo KA. Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress. Biomedicines. 2024; 12(6):1247. https://doi.org/10.3390/biomedicines12061247
Chicago/Turabian StyleJang, Imjoo, Shalini Menon, Indrajyoti Indra, Rabiah Basith, and Karen A. Beningo. 2024. "Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress" Biomedicines 12, no. 6: 1247. https://doi.org/10.3390/biomedicines12061247
APA StyleJang, I., Menon, S., Indra, I., Basith, R., & Beningo, K. A. (2024). Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress. Biomedicines, 12(6), 1247. https://doi.org/10.3390/biomedicines12061247







