The Impact of COVID-19 on the Guillain–Barré Syndrome Incidence
Abstract
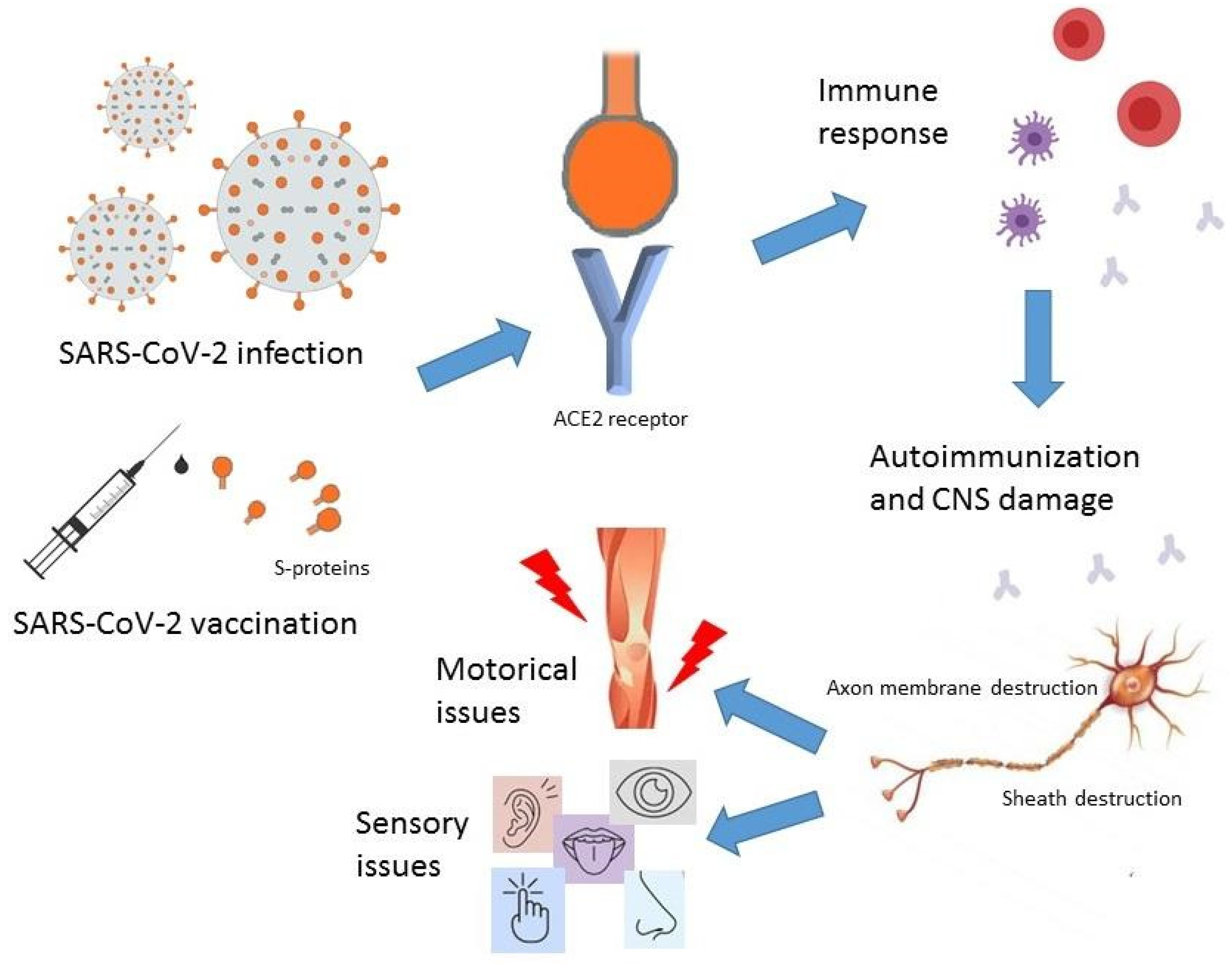
:1. Introduction
2. Neurological Symptoms of SARS-CoV-2 Infection
3. Pathomechanism of Guillain–Barré Syndrome Associated with SARS-CoV-2
4. COVID-19 and Guillain–Barre Association
5. COVID-19 Vaccine-Associated GBS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Novel Coronavirus (2019-nCoV). Situation Report–22. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=6f80d1b9_4 (accessed on 27 February 2024).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wang, Y.; Grunewald, M.; Perlman, S. Coronaviruses: An updated overview of their replication and pathogenesis. In Methods in Molecular Biology, 2nd ed.; Maier, H., Bickerton, E., Eds.; Humana Press: New York, NY, USA, 2020; Volume 2203, pp. 1–29. [Google Scholar] [CrossRef]
- Ricchio, M.; Tassone, B.; Pelle, M.C.; Mazzitelli, M.; Serapide, F.; Fusco, P.; Lionello, R.; Cancelliere, A.; Procopio, G.; Lio, E.; et al. Characteristics, Management, and Outcomes of Elderly Patients with Diabetes in a COVID-19 Unit: Lessons Learned from a Pilot Study. Medicina 2021, 57, 341. [Google Scholar] [CrossRef]
- Tsai, P.H.; Lai, W.Y.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Chen, H.K.; Chen, Y.M.; Lai, Y.C.; Kuo, L.C.; Chen, S.D.; et al. Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Çalıca Utku, A.; Budak, G.; Karabay, O.; Güçlü, E.; Okan, H.D.; Vatan, A. Main symptoms in patients presenting in the COVID-19 period. Scott. Med. J. 2020, 65, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- He, Y.; Bai, X.; Zhu, T.; Huang, J.; Zhang, H. What can the neurological manifestations of COVID-19 tell us: A meta-analysis. J. Transl. Med. 2021, 19, 1–35. [Google Scholar] [CrossRef]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology 2020, 296, E119–E120. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Sedaghat, Z.; Karimi, N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. 2020, 76, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Kaeley, N.; Kabi, A.; Pillai, A.; Shankar, T.; Ameena, M.S.S. Post-COVID-19 Guillain-Barré Syndrome: A Case Report With Literature Review. Cureus 2022, 14, e21246. [Google Scholar] [CrossRef] [PubMed]
- Keh, R.Y.S.; Scanlon, S.; Datta-Nemdharry, P.; Donegan, K.; Cavanagh, S.; Foster, M.; Skelland, D.; Palmer, J.; Machado, P.M.; Kaddie, S.; et al. COVID-19 vaccination and Guillain-Barré syndrome: Analyses using the National Immunoglobulin Database. Brain 2023, 146, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Hilts, A.; Schreiber, A.; Singh, A. A Clinical Case of COVID-19 Vaccine-Associated Guillain-Barré Syndrome. Am. J. Case Rep. 2022, 23, e936896. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.; Klein, C.J. Guillain-Barré Syndrome. Mayo Clin. Proc. 2017, 92, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, I.; Sanz-Gallego, I.; Rodríguez de Rivera, F.J.; Arpa, J. Guillain-Barré syndrome: Natural history and prognostic factors: A retrospective review of 106 cases. BMC Neurol. 2013, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Usuki, S.; Ariga, T. Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Infect. Immun. 2006, 74, 6517–6527. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Kohl, K.S.; Gidudu, J.; Amato, A.; Bakshi, N.; Baxter, R.; Burwen, D.R.; Cornblath, D.R.; Cleerbout, J.; Edwards, K.M.; et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011, 29, 599. [Google Scholar] [CrossRef]
- Shaheen, N.; Ramadan, A.; Nashwan, A.J.; Shaheen, A.; Ahmad, S.; Motawea, K.R.; Mohamed, S.; Mohamed, R.S.; Swed, S.; Aiash, H. Guillain-Barré syndrome following COVID-19 vaccination: An updated systematic review of cases. Clin. Case Rep. 2023, 11, e7456. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ranga, A.; Prakash, N.B.; Khanna, M. Rehabilitation outcomes in patients with post-COVID-19 vaccine-associated Guillain-Barre syndrome. J. Neurosci. Rural. Pract. 2022, 13, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Wakerley, B.R.; Uncini, A.; Yuki, N. Guillain-Barré and Miller Fisher syndromes--new diagnostic classification. Nat. Rev. Neurol. 2014, 10, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Desai, P.; Voloshko, A.; Jayasekara, D. COVID-19-Associated Miller Fisher Syndrome with Long Latency Period: A Case Report. Cureus 2022, 14, e24638. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Heo, J.H.; Kim, H.O.; Song, S.H.; Park, S.S.; Park, T.H.; Ahn, J.Y.; Kim, M.K.; Choi, J.P. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J. Clin. Neurol. 2017, 13, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Katsuno, M. Emerging infectious diseases, vaccines and Guillain-Barré syndrome. Clin. Exp. Neuroimmunol. 2021, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sauteur, P.M.M.; Huizinga, R.; Tio-Gillen, A.P.; Roodbol, J.; Hoogenboezem, T.; Jacobs, E.; van Rijn, M.; van der Eijk, A.A.; Vink, C.; de Wit, M.Y.; et al. Mycoplasma pneumoniae triggering the Guillain-Barré syndrome: A case-control study. Ann. Neurol. 2016, 80, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, H.; Subahi, A.; Shirah, B. Neurological Complications of Middle East Respiratory Syndrome Coronavirus: A Report of Two Cases and Review of the Literature. Case Rep. Neurol. Med. 2016, 2016, 3502683. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chu, X.; Xu, Y.; Ma, F. Vaccines and the risk of Guillain-Barré syndrome. Eur. J. Epidemiol. 2020, 35, 363–370. [Google Scholar] [CrossRef]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreira, M.L.B.; Cornblath, D.R.; van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Jasti, A.K.; Selmi, C.; Sarmiento-Monroy, J.C.; Vega, D.A.; Anaya, J.M.; Gershwin, M.E. Guillain-Barré syndrome: Causes, immunopathogenic mechanisms and treatment. Expert Rev. Clin. Immunol. 2016, 12, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- García-Azorín, D.; Martínez-Pías, E.; Trigo, J.; Hernández-Pérez, I.; Valle-Peñacoba, G.; Talavera, B.; Simón-Campo, P.; de Lera, M.; Chavarría-Miranda, A.; López-Sanz, C.; et al. Neurological Comorbidity Is a Predictor of Death in COVID-19 Disease: A Cohort Study on 576 Patients. Front. Neurol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Homma, Y.; Watanabe, M.; Inoue, K.; Moritaka, T. Coronavirus Disease-19 Pneumonia with Facial Nerve Palsy and Olfactory Disturbance. Intern. Med. 2020, 59, 1773–1775. [Google Scholar] [CrossRef]
- Sriwijitalai, W.; Wiwanitkit, V. Hearing loss and COVID-19: A note. Am. J. Otolaryngol. 2020, 41, 102473. [Google Scholar] [CrossRef]
- Ameer, M.Z.; Haiy, A.U.; Bajwa, M.H.; Abeer, H.; Mustafa, B.; Ameer, F.; Amjad, Z.; Rehman, A.U. Association of Parsonage-Turner syndrome with COVID-19 infection and vaccination: A systematic review. J. Int. Med. Res. 2023, 51, 3000605231187939. [Google Scholar] [CrossRef]
- Portela-Sánchez, S.; Sánchez-Soblechero, A.; Otalora, P.J.M.; López, R.; Alonso, G.V.; Palacios-Mendoza, M.A.; Caramé, C.C.; Pascasio, L.A.; Serrano, M.M.; Massot-Tarrús, A.; et al. Neurological complications of COVID-19 in hospitalized patients: The registry of a neurology department in the first wave of the pandemic. Eur. J. Neurol. 2021, 28, 3339–3347. [Google Scholar] [CrossRef]
- Restivo, D.A.; Centonze, D.; Alesina, A.; Marchese-Ragona, R. Myasthenia Gravis Associated With SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 1027–1028. [Google Scholar] [CrossRef]
- Herrero-Montes, M.; Fernández-De-Las-Peñas, C.; Ferrer-Pargada, D.; Tello-Mena, S.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Palacios-Ceña, D.; Parás-Bravo, P. Prevalence of Neuropathic Component in Post-COVID Pain Symptoms in Previously Hospitalized COVID-19 Survivors. Int. J. Clin. Pract. 2022, 2022, 3532917. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef]
- Ang, C.W.; Jacobs, B.C.; Laman, J.D. The Guillain-Barré syndrome: A true case of molecular mimicry. Trends Immunol. 2004, 25, 61–66. [Google Scholar] [CrossRef]
- Susuki, K.; Odaka, M.; Mori, M.; Hirata, K.; Yuki, N. Acute motor axonal neuropathy after Mycoplasma infection: Evidence of molecular mimicry. Neurology 2004, 62, 949–956. [Google Scholar] [CrossRef]
- Yuki, N.; Hartung, H.P. Guillain-Barré syndrome. N. Engl. J. Med. 2012, 366, 2294–2304. [Google Scholar] [CrossRef]
- Caress, J.B.; Castoro, R.J.; Simmons, Z.; Scelsa, S.N.; Lewis, R.A.; Ahlawat, A.; Narayanaswami, P. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve 2020, 62, 485–491. [Google Scholar] [CrossRef]
- Pimentel, V.; Luchsinger, V.W.; Carvalho, G.L.; Alcará, A.M.; Esper, N.B.; Marinowic, D.; Zanirati, G.; da Costa, J.C. Guillain–Barré syndrome associated with COVID-19: A systematic review. Brain Behav. Immun. 2023, 28, 100578. [Google Scholar] [CrossRef]
- Tawakul, A.A.; Al-Doboke, A.W.; Altayyar, S.A.; Alsulami, S.A.; Alfahmi, A.M.; Nooh, R.T. Guillain-Barré syndrome in the COVID-19 pandemic. Neurol. Int. 2021, 14, 34–48. [Google Scholar] [CrossRef]
- Bentley, S.A.; Ahmad, S.; Kobeissy, F.H.; Toklu, H.Z. Concomitant Guillain-Barré Syndrome and COVID-19: A Meta-Analysis of Cases. Medicina 2022, 58, 1835. [Google Scholar] [CrossRef]
- Racke, M.K.; Niles, J.K.; Lorenz, R.A.; Kaufman, H.W. Changes in ganglioside antibody positivity rates during the COVID-19 pandemic. J. Neuroimmunol. 2022, 367, 577877. [Google Scholar] [CrossRef]
- Ubogu, E.E. Biology of the Human Blood-Nerve Barrier in Health and Disease. Exp. Neurol. 2020, 328, 113272. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Zhu, J.; Cui, L.; Zhang, H.L. Biomarkers of Guillain-Barré Syndrome: Some Recent Progress, More Still to Be Explored. Mediat. Inflamm. 2015, 2015, 564098. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Duan, H.; Wang, Y.; Zhu, H. Biological analysis of the potential pathogenic mechanisms of Infectious COVID-19 and Guillain-Barré syndrome. Front. Immunol. 2023, 14, 1290578. [Google Scholar] [CrossRef]
- Yusari, I.G.A.A.A.; Sudira, P.G.; Samatra, D.P.G.P. Clinical characteristics of Guillain-Barre syndrome in COVID-19: A systematic review and meta-analysis of observational studies. Egypt. J. Neurol. Psychiatr. Neurosurg. 2023, 59, 40. [Google Scholar] [CrossRef]
- Hasan, I.; Saif-Ur-Rahman, K.M.; Hayat, S.; Papri, N.; Jahan, I.; Azam, R.; Ara, G.; Islam, Z. Guillain-Barré syndrome associated with SARS-CoV-2 infection: A systematic review and individual participant data meta-analysis. J. Peripher. Nerv. Syst. 2020, 25, 335–343. [Google Scholar] [CrossRef]
- Curtis, M.; Bhumbra, S.; Felker, M.V.; Jordan, B.L.; Kim, J.; Weber, M.; Friedman, M.L. Guillain-Barré Syndrome in a Child With COVID-19 Infection. Pediatrics 2021, 147, e2020015115. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Khalifa, M.; Zakaria, F.; Ragab, Y.; Saad, A.; Bamaga, A.; Emad, Y.; Rasker, J.J. Guillain-Barre syndrome associated with SARS-CoV-2 detection and a COVID-19 infection in a child. J. Pediatr. Infect. Dis. Soc. 2020, 9, 510–513. [Google Scholar] [CrossRef]
- Akçay, N.; Oğur, M.; Menentoglu, M.E.; Sofuoğlu, A.İ.; Işik, İ.; Şevketoğlu, E. Recurrent GBS With COVID-19 in a Child: A Case Report. Pediatr. Infect. Dis. J. 2023, 42, e129–e130. [Google Scholar] [CrossRef]
- Kanou, S.; Wardeh, L.; Govindarajan, S.; Macnay, K. Guillain-Barre syndrome (GBS) associated with COVID-19 infection that resolved without treatment in a child. BMJ Case Rep. 2022, 15, e245455. [Google Scholar] [CrossRef]
- Paybast, S.; Gorji, R.; Mavandadi, S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: A case report and review of the literature. Neurologist 2020, 25, 101–103. [Google Scholar] [CrossRef]
- Filosto, M.; Cotti Piccinelli, S.; Gazzina, S.; Foresti, C.; Frigeni, B.; Servalli, M.C.; Sessa, M.; Cosentino, G.; Marchioni, E.; Ravaglia, S.; et al. Guillain-Barré syndrome and COVID-19: An observational multicentre study from two Italian hotspot regions. J. Neurol. Neurosurg. Psychiatry 2021, 92, 751–756. [Google Scholar] [CrossRef]
- Fragiel, M.; Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberechts, E.J.; Jacob, J.; et al. Incidence, clinical, risk factors and outcomes of Guillain-Barré in COVID-19. Ann. Neurol. 2020, 89, 598–603. [Google Scholar] [CrossRef]
- Aquino Ferraz, L.D.; Marques, N.P.; Silveira, D.M.M.; de Magalhães, M.J.S.; Oliveira, E.A.; Martelli Júnior, H. Assessment of Guillain-Barre Syndrome Cases in Brazil in the COVID-19 Era. Neurologist 2022, 27, 155–156. [Google Scholar] [CrossRef]
- Keddie, S.; Pakpoor, J.; Mousele, C.; Pipis, M.; Machado, P.M.; Foster, M.; Record, C.J.; Keh, R.Y.S.; Fehmi, J.; Paterson, R.W.; et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain 2021, 144, 682–693. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, B.; Dalemo, E.; Elíasdóttir, Ó.; Ólafsson, E.; Axelsson, M. Decreased Incidence of Guillain-Barré Syndrome during the COVID-19 Pandemic: A Retrospective Population-Based Study. Neuroepidemiology 2023, 57, 1–6. [Google Scholar] [CrossRef]
- Choi, S.A.; Hwang, J.; Lim, B.C.; Chae, S.A. Incidence of Guillain–Barré syndrome in South Korea during the early COVID-19 pandemic. Front. Neurol. 2023, 14, 1125455. [Google Scholar] [CrossRef]
- Lee, H.; Heo, N.; Kwon, D.; Ha, J. Deciphering changes in the incidence of the Guillain-Barré syndrome during the COVID-19 pandemic: A nationwide time-series correlation study. BMJ Neurol. Open 2022, 4, e000378. [Google Scholar] [CrossRef]
- Vítor, J.; Dionísio, J.M.; Campos, C.; Santos, M.O.; Cruz, S.; Castelo, J.; Carvalho, M.; Vale, J.; Santos, M.; Castro, I.; et al. The Incidence of Guillain-Barré Syndrome during COVID-19 Pandemic: A Portuguese Multicentric Retrospective Study. Sinapse 2023, 23, 182–187. [Google Scholar] [CrossRef]
- Besharati, A.; Saberi, A.; Shirkouhi, S.G.; Ashraf, A.; Hatamian, H.; Kenarsari, H.E.; Andalib, S. Guillain-Barré syndrome during the COVID-19 pandemic and pre-pandemic periods. Caspian, J. Neurol. Sci. 2022, 8, 33–38. [Google Scholar] [CrossRef]
- Garg, D.; Dhamija, R.K.; Choudhary, A.; Shree, R.; Kumar, S.; Samal, P.; Pathak, A.; Vijaya, P.; Sireesha, Y.; Nair, S.S.; et al. Impact of the COVID-19 Pandemic on the Frequency, Clinical Spectrum and Outcomes of Pediatric Guillain-Barré Syndrome in India: A Multicentric Ambispective Cohort Study. Ann. Indian Acad. Neurol. 2022, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Aladawi, M.; Elfil, M.; Abu-Esheh, B.; Abu Jazar, D.; Armouti, A.; Bayoumi, A.; Piccione, E. Guillain Barre Syndrome as a Complication of COVID-19: A Systematic Review. Can. J. Neurol. Sci. 2022, 49, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain-Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2021, 268, 1133–1170. [Google Scholar] [CrossRef]
- Uncini, A.; Vallat, J.M.; Jacobs, B.C. Guillain-Barré syndrome in SARS-CoV-2 infection: An instant systematic review of the first six months of pandemic. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1105–1110. [Google Scholar] [CrossRef]
- Toydemir, H.E.; Mercan, M.; Yayla, V.A. The Profile of Guillain-Barré Syndrome Before and During COVID-19 Pandemic: A 5-Year Experience. Noro Psikiyatr. Ars. 2023, 60, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Kataria, S.; Tandon, M.; Patel, J.; Patel, R.; Jowkar, A.; Daimee, M.; Bernitsas, E.; Jaiswal, P.; Lisak, R.P. Guillain Barré Syndrome and its variants as a manifestation of COVID-19: A systematic review of case reports and case series. J. Neurol. Sci. 2021, 420, 117263. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 27 February 2024).
- Fathizadeh, H.; Afshar, S.; Masoudi, M.R.; Gholizadeh, P.; Asgharzadeh, M.; Ganbarov, K.; Köse, Ş.; Yousefi, M.; Kafil, H.S. SARS-CoV-2 (COVID-19) vaccines structure, mechanisms and effectiveness: A review. Int. J. Biol. Macromol. 2021, 188, 740–750. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Huynh-Truong-Anh, D.; Nguyen-Quang, T.; Nguyen-Thi-Kim, T.; Tran-Ngoc-Anh, T.; Nguyen-Van-Hoai, N.; Do-Thi-Thu, M.; Nguyen-Van, T.; Tang-Thi-Thao, T.; Nguyen-Tuan, A.; et al. Guillain-Barré Syndrome due to COVID-19 Vero Cell Vaccination Associated with Concomitant COVID-19 Infection-induced ARDS and Treated Successfully by Therapeutic Plasma Exchange: A First Case Report from Vietnam. Pulm. Ther. 2023, 9, 271–280. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Göbel, C.H.; Heinze, A.; Karstedt, S.; Morscheck, M.; Tashiro, L.; Cirkel, A.; Hamid, Q.; Halwani, R.; Temsah, M.H.; Ziemann, M.; et al. Clinical characteristics of headache after vaccination against COVID-19 (coronavirus SARS-CoV-2) with the BNT162b2 mRNA vaccine: A multicentre observational cohort study. Brain Commun. 2021, 3, fcab169. [Google Scholar] [CrossRef]
- Undugodage, C.; Dissanayake, U.; Kumara, H.; Samarasekera, B.; Yapa, L.; Ganegma, R.; Wickramasinghe, W.K.; Samarakoon, S.; Wijesinghe, P.; Wijemanne, U.; et al. Reactogenicity to ChAdOx1 nCoV-19 vaccine in health care workers: A multicenter observational study in Sri Lanka. Ceylon Med. J. 2021, 66, 177–184. [Google Scholar] [CrossRef]
- Kaulen, L.D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H.M.; Nagel, S.; Wildemann, B.; et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: A case series. Eur. J. Neurol. 2022, 29, 555–563. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Colella, G.; Orlandi, M.; Cirillo, N. Bell’s palsy following COVID-19 vaccination. J. Neurol. 2021, 268, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Vastarella, M.; Picone, V.; Martora, F.; Fabbrocini, G. Herpes zoster after ChAdOx1 nCoV-19 vaccine: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e845–e846. [Google Scholar] [CrossRef]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef]
- Oo, W.M.; Giri, P.; de Souza, A. AstraZeneca COVID-19 vaccine and Guillain- Barré Syndrome in Tasmania: A causal link? J. Neuroimmunol. 2021, 360, 577719. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, O.B.; Tsalamandris, G.; Paladini, A.; Varrassi, G.; Zis, P. Guillain-Barré Syndrome Induced by Vaccination Against COVID-19: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e37578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Min, Y.G.; Shin, J.; Kwon, Y.N.; Bae, J.S.; Sung, J.J.; Hong, Y.H. Guillain-Barré syndrome and variants following COVID-19 vaccination: Report of 13 cases. Front. Neurol. 2022, 12, 820723. [Google Scholar] [CrossRef] [PubMed]
- Le Vu, S.; Bertrand, M.; Botton, J.; Jabagi, M.J.; Drouin, J.; Semenzato, L.; Weill, A.; Dray-Spira, R.; Zureik, M. Risk of Guillain-Barré Syndrome Following COVID-19 Vaccines: A Nationwide Self-Controlled Case Series Study. Neurology 2023, 101, e2094–e2102. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A.; Scorza, C.A. Post SARS-CoV-2 vaccination Guillain-Barre syndrome in 19 patients. Clinics 2021, 76, e3286. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. GBS (Guillain-Barré Syndrome) and Vaccines. Available online: https://www.cdc.gov/vaccinesafety/concerns/guillain-barre-syndrome.html (accessed on 27 February 2024).
- Aomar-Millán, I.F.; Martínez de Victoria-Carazo, J.; Peregrina-Rivas, J.A.; Villegas-Rodríguez, I. COVID-19, Guillain-Barré syndrome, and the vaccine. A dangerous combination. Rev. Clin. Esp. 2021, 221, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, M.; Germano, F.; Grisanti, S.; Castellano, C.; Tazza, F.; Mobilia, E.M.; Visigalli, D.; Novi, G.; Massa, F.; Rossi, S.; et al. Case Report: Post-COVID-19 Vaccine Recurrence of Guillain-Barré Syndrome Following an Antecedent Parainfectious COVID-19-Related GBS. Front. Immunol. 2022, 13, 894872. [Google Scholar] [CrossRef] [PubMed]
- Anjum, Z.; Iyer, C.; Naz, S.; Jaiswal, V.; Nepal, G.; Laguio-Vila, M.; Anandaram, S.; Thapaliya, S. Guillain-Barré syndrome after mRNA-1273 (Moderna) COVID-19 vaccination: A case report. Clin. Case Rep. 2022, 10, e05733. [Google Scholar] [CrossRef]
- Finsterer, J.; Matovu, D.; Scorza, F.A. SARS-CoV-2 vaccinations reduce the prevalence of post-COVID Guillain-Barre syndrome. Clinics 2022, 77, 100064. [Google Scholar] [CrossRef]
- Noseda, R.; Ripellino, P.; Ghidossi, S.; Bertoli, R.; Ceschi, A. Reporting of Acute Inflammatory Neuropathies with COVID-19 Vaccines: Subgroup Disproportionality Analyses in VigiBase. Vaccines 2021, 9, 1022. [Google Scholar] [CrossRef]
- Bishara, H.; Arbel, A.; Barnett-Griness, O.; Bloch, S.; Cohen, S.; Najjar-Debbiny, R.; Gronich, N.; Auriel, E.; Saliba, W. Association Between Guillain-Barré Syndrome and COVID-19 Infection and Vaccination: A Population-Based Nested Case-Control Study. Neurology 2023, 101, e2035–e2042. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Bisaccia, G.; Gallina, S.; Tomassini, V.; Uncini, A. Guillain-Barré syndrome and COVID-19 vaccination: A systematic review and meta-analysis. J.Neurol. 2024, 271, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
| Type of Vaccine | Count of GBS Cases | Associated Neurological Complications |
|---|---|---|
| Pfizer | 1 [70] 1 [93] 28 [95] 5 [96] 4 [98] 1 [100] 1 [101] Total: 41 | difficulty walking [94] shoulder pain, arm weakness and areflexia [94] numbness, swelling, pain on the left side of the face and neck [98] |
| AstraZeneca | 15 [24] 1 [70] 77 [95] 8 [96] 14 [98] 1 [100] Total: 116 | acute tingling and weakness in hands and feet, severe back and chest pain, altered taste, paresthesia in the tongue and perioral area, distal paresthesia, facial paresthesia, weakness, areflexic quadriplegia, facial diplegia and respiratory failure, paraplegia, ophthalmoplegia, bulbar symptoms, abducens palsy [93,94,97,98] |
| J&J | 9 [93] 1 [98] Total: 10 | back and leg pain, headache, double vision, facial diplegia, paraparesis of lower legs [98] |
| Moderna | 7 [93] 2 [102] | progressive, ascending bilateral lower extremity paresthesia and weakness [102] |
| Sputnik | 9 [93] | Data not available |
| Sinopharm | 1 [83] | paralysis of the lower and upper extremities [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopańko, M.; Zabłudowska, M.; Zajkowska, M.; Gudowska-Sawczuk, M.; Mucha, M.; Mroczko, B. The Impact of COVID-19 on the Guillain–Barré Syndrome Incidence. Biomedicines 2024, 12, 1248. https://doi.org/10.3390/biomedicines12061248
Kopańko M, Zabłudowska M, Zajkowska M, Gudowska-Sawczuk M, Mucha M, Mroczko B. The Impact of COVID-19 on the Guillain–Barré Syndrome Incidence. Biomedicines. 2024; 12(6):1248. https://doi.org/10.3390/biomedicines12061248
Chicago/Turabian StyleKopańko, Magdalena, Magdalena Zabłudowska, Monika Zajkowska, Monika Gudowska-Sawczuk, Mateusz Mucha, and Barbara Mroczko. 2024. "The Impact of COVID-19 on the Guillain–Barré Syndrome Incidence" Biomedicines 12, no. 6: 1248. https://doi.org/10.3390/biomedicines12061248
APA StyleKopańko, M., Zabłudowska, M., Zajkowska, M., Gudowska-Sawczuk, M., Mucha, M., & Mroczko, B. (2024). The Impact of COVID-19 on the Guillain–Barré Syndrome Incidence. Biomedicines, 12(6), 1248. https://doi.org/10.3390/biomedicines12061248










