Inflammation in the Peripheral Nervous System after Injury
Abstract
:1. Background
2. Inflammatory Response after Nerve Injury
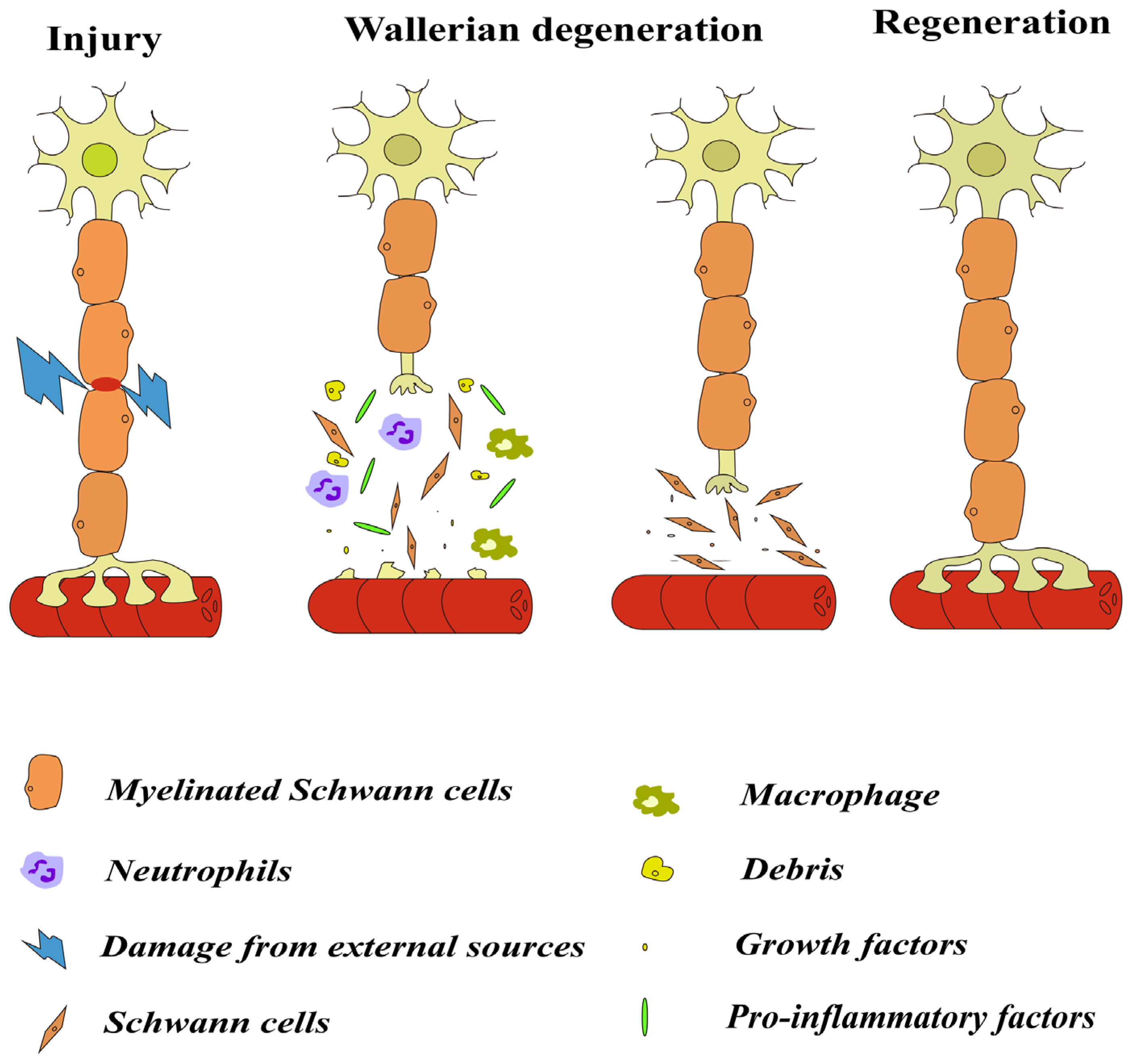
2.1. Wallerian Degeneration
2.2. Cells Involved in Inflammation after Injury
2.2.1. Macrophages
2.2.2. Neutrophils
2.2.3. Lymphocytes
2.2.4. Schwann Cells
2.3. Factors Involved in Inflammation
2.3.1. Cytokines and Chemokines
2.3.2. Neurotrophic Factors
2.4. Inflammasomes
2.5. Complement System
3. Relationship between Inflammation and Nerve Regeneration and Applications
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761. [Google Scholar] [CrossRef] [PubMed]
- Li, N.Y.; Onor, G.I.; Lemme, N.J.; Gil, J.A. Epidemiology of Peripheral Nerve Injuries in Sports, Exercise, and Recreation in the United States, 2009–2018. Physician Sportsmed. 2021, 49, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Maeng, W.Y.; Tseng, W.L.; Li, S.; Koo, J.; Hsueh, Y.Y. Electroceuticals for peripheral nerve regeneration. Biofabrication 2022, 14, 042002. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef]
- Estera, L.A.; Walsh, S.P.; Headen, J.A.; Williamson, R.E.; Kalinski, A.L. Neuroinflammation: Breaking barriers and bridging gaps. Neurosci. Res. 2023, 197, 9–17. [Google Scholar] [CrossRef]
- Kalinski, A.L.; Yoon, C.; Huffman, L.D.; Duncker, P.C.; Kohen, R.; Passino, R.; Hafner, H.; Johnson, C.; Kawaguchi, R.; Carbajal, K.S.; et al. Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement. eLife 2020, 9, e60223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Rovella, V.; Candi, E.; Jia, W.; Bernassola, F.; Bove, P.; Piacentini, M.; Scimeca, M.; Sica, G.; et al. Aged mesenchymal stem cells and inflammation: From pathology to potential therapeutic strategies. Biol. Direct 2023, 18, 40. [Google Scholar] [CrossRef]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflamm. 2011, 8, 109. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, M.; Taveggia, C. The Complex Work of Proteases and Secretases in Wallerian Degeneration: Beyond Neuregulin-1. Front. Cell. Neurosci. 2019, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.G.; Sim, H.J.; Song, E.K.; Kwon, T.; Park, T.J. Extracellular matrixes and neuroinflammation. BMB Rep. 2020, 53, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Conforti, L.; Gilley, J.; Coleman, M.P. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 2014, 15, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Huffman, L.D.; Hafner, H.; Athaiya, M.; Finneran, M.C.; Kalinski, A.L.; Kohen, R.; Flynn, C.; Passino, R.; Johnson, C.N.; et al. The injured sciatic nerve atlas (iSNAT), insights into the cellular and molecular basis of neural tissue degeneration and regeneration. eLife 2022, 11, e80881. [Google Scholar] [CrossRef]
- Au, N.P.B.; Ma, C.H.E. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front. Immunol. 2022, 13, 860070. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef]
- Xu, J.; Wen, J.; Fu, L.; Liao, L.; Zou, Y.; Zhang, J.; Deng, J.; Zhang, H.; Liu, J.; Wang, X.; et al. Macrophage-specific RhoA knockout delays Wallerian degeneration after peripheral nerve injury in mice. J. Neuroinflamm. 2021, 18, 234. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Jiang, M.; Qian, J.; Gu, D.; Bai, H.; Cai, M.; Yao, D. Role of transforming growth factor-beta in peripheral nerve regeneration. Neural Regen. Res. 2024, 19, 380–386. [Google Scholar] [CrossRef]
- Niemi, J.P.; Lindborg, J.A.; Zigmond, R.E. Detection of Neutrophils in the Sciatic Nerve Following Peripheral Nerve Injury. Methods Mol. Biol. 2020, 2143, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Xu, X.; Lu, S.; Yang, D.; Xie, C.; Jia, M.; Zhang, W.; Jin, L.; Wang, X.; et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics 2021, 11, 4187–4206. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Dunphy, G.; Heras-Murillo, I.; Mastrangelo, A.; Sancho, D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol. Immunol. 2022, 19, 384–408. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.E.; Zygelyte, E.; Grenier, J.K.; Edwards, M.G.; Cheetham, J. Temporal changes in macrophage phenotype after peripheral nerve injury. J. Neuroinflamm. 2018, 15, 185. [Google Scholar] [CrossRef]
- Boissonnas, A.; Louboutin, F.; Laviron, M.; Loyher, P.L.; Reboussin, E.; Barthelemy, S.; Reaux-Le Goazigo, A.; Lobsiger, C.S.; Combadiere, B.; Melik Parsadaniantz, S.; et al. Imaging resident and recruited macrophage contribution to Wallerian degeneration. J. Exp. Med. 2020, 217, e20200471. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef]
- Lu, C.Y.; Santosa, K.B.; Jablonka-Shariff, A.; Vannucci, B.; Fuchs, A.; Turnbull, I.; Pan, D.; Wood, M.D.; Snyder-Warwick, A.K. Macrophage-Derived Vascular Endothelial Growth Factor-A Is Integral to Neuromuscular Junction Reinnervation after Nerve Injury. J. Neurosci. 2020, 40, 9602–9616. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.A.; Holmes, A.; Rosin, N.L.; Sinha, S.; Vohra, M.; Burma, N.E.; Trang, T.; Midha, R.; Biernaskie, J. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018, 24, 2561–2572.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, S.; Halawani, D.; Wang, Y.; Junqueira Alves, C.; Ramakrishnan, A.; Estill, M.; Shen, L.; Li, F.; He, X.; et al. Macrophages facilitate peripheral nerve regeneration by organizing regeneration tracks through Plexin-B2. Genes Dev. 2022, 36, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. Targeting invading macrophage-derived PGE2, IL-6 and calcitonin gene-related peptide in injured nerve to treat neuropathic pain. Expert. Opin. Ther. Targets 2006, 10, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Balog, B.M.; Sonti, A.; Zigmond, R.E. Neutrophil biology in injuries and diseases of the central and peripheral nervous systems. Prog. Neurobiol. 2023, 228, 102488. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Acevedo-Cintron, J.A.; Sayanagi, J.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. The CCL2/CCR2 axis is critical to recruiting macrophages into acellular nerve allograft bridging a nerve gap to promote angiogenesis and regeneration. Exp. Neurol. 2020, 331, 113363. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Mack, M.; Zigmond, R.E. Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration. J. Neurosci. 2017, 37, 10258–10277. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Shim, H.B.; Shim, R.; Lee, W.Y.; Labit, E.; Sinha, S.; Keenan, C.M.; Surewaard, B.G.J.; Noh, J.Y.; Sun, Y.; et al. A monocyte-leptin-angiogenesis pathway critical for repair post-infection. Nature 2022, 609, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, T.J.; Cohen, H.C.; Kao, W.J. Poly(ethylene glycol)-containing hydrogels modulate α-defensin release from polymorphonuclear leukocytes and monocyte recruitment. J. Biomed. Mater. Res. A 2015, 103, 3772–3780. [Google Scholar] [CrossRef]
- Yuan, Y.S.; Niu, S.P.; Yu, F.; Zhang, Y.J.; Han, N.; Lu, H.; Yin, X.F.; Xu, H.L.; Kou, Y.H. Intraoperative single administration of neutrophil peptide 1 accelerates the early functional recovery of peripheral nerves after crush injury. Neural Regen. Res. 2020, 15, 2108–2115. [Google Scholar] [CrossRef]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Xu, K.; Yu, L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004, 129, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Bombeiro, A.L.; Pereira, B.T.N.; Bonfanti, A.P.; Oliveira, A.L.R. Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res. Bull. 2020, 160, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Beahrs, T.; Tanzer, L.; Sanders, V.M.; Jones, K.J. Functional recovery and facial motoneuron survival are influenced by immunodeficiency in crush-axotomized mice. Exp. Neurol. 2010, 221, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Stierli, S.; Imperatore, V.; Lloyd, A.C. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia 2019, 67, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury. J. Neurosci. 2016, 36, 9135–9147. [Google Scholar] [CrossRef] [PubMed]
- Brosius Lutz, A.; Lucas, T.A.; Carson, G.A.; Caneda, C.; Zhou, L.; Barres, B.A.; Buckwalter, M.S.; Sloan, S.A. An RNA-sequencing transcriptome of the rodent Schwann cell response to peripheral nerve injury. J. Neuroinflamm. 2022, 19, 105. [Google Scholar] [CrossRef]
- Rovak, J.M.; Bishop, D.K.; Boxer, L.K.; Wood, S.C.; Mungara, A.K.; Cederna, P.S. Peripheral nerve transplantation: The role of chemical acellularization in eliminating allograft antigenicity. J. Reconstr. Microsurg. 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Bleackley, R.C. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Meyer zu Horste, G.; Hu, W.; Hartung, H.P.; Lehmann, H.C.; Kieseier, B.C. The immunocompetence of Schwann cells. Muscle Nerve 2008, 37, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Mersha, T.B. Publicly available cytokine data: Limitations and opportunities. J. Allergy Clin. Immunol. 2022, 150, 1053–1056. [Google Scholar] [CrossRef]
- Lai, J.; Wu, H.; Qin, A. Cytokines in Febrile Diseases. J. Interferon Cytokine Res. 2021, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Broderick, L.; Hoffman, H.M. IL-1 and autoinflammatory disease: Biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 2022, 18, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Chen, M.; Mu, G.; Peng, S.; Liu, X.; Ou, C. NLRP3 Inflammasome Mediates Neurodegeneration in Rats with Chronic Neuropathic Pain. Shock 2021, 56, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Barthwal, M.K. IL-1 β genesis: The art of regulating the regulator. Cell. Mol. Immunol. 2018, 15, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhao, M.; Zhang, C.; Sun, X. IL-1β in atherosclerotic vascular calcification: From bench to bedside. Int. J. Biol. Sci. 2021, 17, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.I.; Su, M.; Cantuti-Castelvetri, L.; Müller, S.A.; Schifferer, M.; Djannatian, M.; Alexopoulos, I.; van der Meer, F.; Winkler, A.; van Ham, T.J.; et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med. 2020, 217, e20191390. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Glassman, C.R.; Garcia, K.C. Emerging principles of cytokine pharmacology and therapeutics. Nat. Rev. Drug Discov. 2023, 22, 21–37. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.; Rosario Claudio, J.; Sim, J.; Inyang, K.E.; Dagenais, A.; Monahan, K.; Lee, B.; Ramakrishnan, H.; Parmar, V.; Geron, M.; et al. Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response. Brain Behav. Immun. 2024, 116, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jancalek, R.; Svizenska, I.; Klusakova, I.; Dubovy, P. Bilateral changes of IL-10 protein in lumbar and cervical dorsal root ganglia following proximal and distal chronic constriction injury of peripheral nerve. Neurosci. Lett. 2011, 501, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kothur, K.; Wienholt, L.; Brilot, F.; Dale, R.C. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine 2016, 77, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.B.; Xian, H.; Wu, W.B.; Ma, S.Y.; Liu, Y.K.; Liang, Y.T.; Guo, H.; Kang, J.J.; Liu, Y.Y.; Zhang, H.; et al. CCL2 facilitates spinal synaptic transmission and pain via interaction with presynaptic CCR2 in spinal nociceptor terminals. Mol. Brain 2020, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Wu, L.; Liu, Y.; Du, J.; Luo, Z.; Xu, J.; Guo, L.; Liu, Y. Epigenetic regulation of chemokine (CC-motif) ligand 2 in inflammatory diseases. Cell Prolif. 2023, 56, e13428. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Miller, R.E.; Block, J.A. Targeting neurotrophic factors: Novel approaches to musculoskeletal pain. Pharmacol. Ther. 2020, 211, 107553. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Xu, F.; Sun, H.; Fu, X.; Zhao, Y. Preparation and Evaluation of BDNF Composite Conduits for Regeneration of Sciatic Nerve Defect in Rats. J. Pharm. Sci. 2020, 109, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Li, W.; Liang, J.; Li, S.; Wang, L.; Xu, S.; Jiang, S.; Song, M.; Meng, H.; Zhai, D.; Tang, L.; et al. Research progress of targeting NLRP3 inflammasome in peripheral nerve injury and pain. Int. Immunopharmacol. 2022, 110, 109026. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Cowie, A.M.; Dittel, B.N.; Stucky, C.L. A Novel Sex-Dependent Target for the Treatment of Postoperative Pain: The NLRP3 Inflammasome. Front. Neurol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liang, J.; Xu, D.; Zhao, L.; Zhang, X.; Zhang, L.; Ren, S.; Liu, D.; Niu, X.; Zang, Y.J.; et al. NLRP3 inflammasome is involved in nerve recovery after sciatic nerve injury. Int. Immunopharmacol. 2020, 84, 106492. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Leonard, F.; Zimmer, J.; Sonawane, A.R.; Perez, K.; Turner, M.E.; Kuraoka, S.; Pham, T.; Li, F.; Aikawa, M.; Singh, S.; et al. NLRP3 Inflammasome Activation in Peripheral Arterial Disease. J. Am. Heart Assoc. 2023, 12, e026945. [Google Scholar] [CrossRef]
- Gavriilaki, E.; de Latour, R.P.; Risitano, A.M. Advancing therapeutic complement inhibition in hematologic diseases: PNH and beyond. Blood 2022, 139, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Tenner, A.J.; Mollnes, T.E. Therapeutic Targeting of the Complement System: From Rare Diseases to Pandemics. Pharmacol. Rev. 2021, 73, 792–827. [Google Scholar] [CrossRef] [PubMed]
- Bohlson, S.S.; Tenner, A.J. Complement in the Brain: Contributions to Neuroprotection, Neuronal Plasticity, and Neuroinflammation. Annu. Rev. Immunol. 2023, 41, 431–452. [Google Scholar] [CrossRef]
- Zhou, J.; Wade, S.D.; Graykowski, D.; Xiao, M.F.; Zhao, B.; Giannini, L.A.A.; Hanson, J.E.; van Swieten, J.C.; Sheng, M.; Worley, P.F.; et al. The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci. Transl. Med. 2023, 15, eadf0141. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, V.; Daha, M.R.; Baas, F. The complement system in the peripheral nerve: Friend or foe? Mol. Immunol. 2008, 45, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, V.; Wolterman, R.; de Kok, M.; Vigar, M.A.; Wagenaar-Bos, I.; King, R.H.; Morgan, B.P.; Baas, F. Soluble complement receptor 1 protects the peripheral nerve from early axon loss after injury. Am. J. Pathol. 2008, 172, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Creswell, R.; Dombrowski, Y. Innate and adaptive immune mechanisms regulating central nervous system remyelination. Curr. Opin. Pharmacol. 2022, 63, 102175. [Google Scholar] [CrossRef] [PubMed]
- Yildiran, H.; Macit, M.S.; Özata Uyar, G. New approach to peripheral nerve injury: Nutritional therapy. Nutr. Neurosci. 2020, 23, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Wee Yong, V. Inflammation in neurological disorders: A help or a hindrance? Neuroscientist 2010, 16, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Sasso, L.L.; de Souza, L.G.; Girasol, C.E.; Marcolino, A.M.; de Jesus Guirro, R.R.; Barbosa, R.I. Photobiomodulation in Sciatic Nerve Crush Injuries in Rodents: A Systematic Review of the Literature and Perspectives for Clinical Treatment. J. Lasers Med. Sci. 2020, 11, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Turrin, N.P.; Rivest, S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J. Neurosci. 2006, 26, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.P.; Liu, S.; Kubes, P.; Yong, V.W. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 2009, 29, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Rawji, K.S.; Keough, M.B.; Kappen, J.; Dowlatabadi, R.; Vogel, H.J.; Chopra, S.; Distéfano-Gagné, F.; Dufour, A.; Gosselin, D.; et al. Harnessing the Benefits of Neuroinflammation: Generation of Macrophages/Microglia with Prominent Remyelinating Properties. J. Neurosci. 2021, 41, 3366–3385. [Google Scholar] [CrossRef] [PubMed]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic Pavlovic, B.; Vucevic, D.; Pekovic, S.; Bozic Nedeljkovic, B. Vitamin B Complex Treatment Attenuates Local Inflammation after Peripheral Nerve Injury. Molecules 2019, 24, 4615. [Google Scholar] [CrossRef] [PubMed]
- Albay, C.; Akkalp, A.K. Alpha-Tocopherol and Cyanocobalamin Combination Accelerates Peripheral Nerve Healing: An Experimental Animal Study. Turk. Neurosurg. 2021, 31, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Scaricamazza, S.; Salvatori, I.; Amadio, S.; Nesci, V.; Torcinaro, A.; Giacovazzo, G.; Primiano, A.; Gloriani, M.; Candelise, N.; Pieroni, L.; et al. Repurposing of Trimetazidine for amyotrophic lateral sclerosis: A study in SOD1(G93A) mice. Br. J. Pharmacol. 2022, 179, 1732–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.D.; Xian, Y.F.; Zhang, F.; Huang, P.Y.; Tang, Y.; Yuan, Q.J.; Lin, Z.X. Berberine enhances survival and axonal regeneration of motoneurons following spinal root avulsion and re-implantation in rats. Free Radic. Biol. Med. 2019, 143, 454–470. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, H.; Bao, H.; Zhang, D.; Feng, L.; Xiao, Y.; Zhu, K.; Hou, Y.; Luo, S.; Zhang, Y.; et al. Cordycepin (3′-deoxyadenosine) promotes remyelination via suppression of neuroinflammation in a cuprizone-induced mouse model of demyelination. Int. Immunopharmacol. 2019, 75, 105777. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, X.; Yang, Y.L.; Wang, Y.H.; Du, G.H. Ramulus Cinnamomi extract attenuates neuroinflammatory responses via downregulating TLR4/MyD88 signaling pathway in BV2 cells. Neural Regen. Res. 2017, 12, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Navaei-Alipour, N.; Mastali, M.; Ferns, G.A.; Saberi-Karimian, M.; Ghayour-Mobarhan, M. The effects of honey on pro- and anti-inflammatory cytokines: A narrative review. Phytother. Res. 2021, 35, 3690–3701. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Liu, Y.; Zhen, L.; Zhao, T.; Luo, L.; Zhang, J.; Deng, T.; Wu, M.; Cheng, G.; Hu, J. The structures of two glucomannans from Bletilla formosana and their protective effect on inflammation via inhibiting NF-κB pathway. Carbohydr. Polym. 2022, 292, 119694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, S.; Yin, J.J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tu, M.; Gao, W.; Cai, X.; Song, F.; Chen, Z.; Zhang, Q.; Wang, J.; Jin, C.; Shi, J.; et al. Hollow Prussian Blue Nanozymes Drive Neuroprotection against Ischemic Stroke via Attenuating Oxidative Stress, Counteracting Inflammation, and Suppressing Cell Apoptosis. Nano Lett. 2019, 19, 2812–2823. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, O.; Hu, M.; Hu, S.; Su, Z.; Liao, J.; Wang, W.; Wang, S.; Liu, L.; Liu, B.; et al. Multifunctional nanoparticles of sinomenine hydrochloride for treat-to-target therapy of rheumatoid arthritis via modulation of proinflammatory cytokines. J. Control Release 2022, 348, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, W.; Hao, J.; Wu, J.; Cai, X.; Zheng, Y. Self-synergistic effect of Prussian blue nanoparticles for cancer therapy: Driving photothermal therapy and reducing hyperthermia-induced side effects. J. Nanobiotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef] [PubMed]
| Cells | Functions | References |
|---|---|---|
| Macrophages | maintain homeostasis, repair wounds, tissue regeneration, phagocytose fragments, secrete factors and pro-inflammatory pain mediators, involve in axonal regeneration, promote neuroglial cells migration and proliferation, and produce VEGF-A | [6,26,28,29,34,35] |
| Neutrophils | phagocytose debris, release cytokines, chemokines, and NP-1 to participate in immune response responses | [37,41,42] |
| Lymphocytes | Produce pro-inflammatory or anti-inflammatory cytokines and participate in the immune response after injury | [46,47] |
| Schwann cells | constitute the blood-nerve interface, regulate nerve injury, clear myelin sheath and recruit immune cells, downregulate myelination genes, activate a series of repair axon genes, process and present antigens | [48,51,54,55,56] |
| Factors | Sources | Effect | Mechanism | References | |
|---|---|---|---|---|---|
| Cytokines | IL-1β | Lymphocytes and macrophages | Pro-inflammatory | directly involved in the inflammatory response, activating NF-κB and promoting the expression of inflammatory factors | [57,58,59,63] |
| TNF-α | Lymphocytes and macrophages | Pro-inflammatory | promote the secretion of pro-inflammatory mediators activate the immune system | [59,67] | |
| IL-10 | Macrophages and B cell | Anti-inflammatory | regulate the regeneration of damaged tissues, inhibit the secretion of pro-inflammatory factors and the expression of MHC, and inhibit the activity of immune cells | [59,69,70,71] | |
| Chemokines | CCL2 | Lymphocytes and macrophages | Pro-inflammatory effect | cause neuropathic pain, recruit cells, exacerbate inflammation | [73,74] |
| Neurotrophic factors | NGF | Target tissue and Schwann cells | Promoting growth | reduce neuronal apoptosis and promote nerve repair and regeneration | [76] |
| BDNF | Target tissue and Schwann cells | Promoting growth | promote neural growth during development, regulate synaptic transmission and plasticity in adulthood | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, D.; Xia, Y.; Ding, Z.; Qian, J.; Gu, X.; Bai, H.; Jiang, M.; Yao, D. Inflammation in the Peripheral Nervous System after Injury. Biomedicines 2024, 12, 1256. https://doi.org/10.3390/biomedicines12061256
Gu D, Xia Y, Ding Z, Qian J, Gu X, Bai H, Jiang M, Yao D. Inflammation in the Peripheral Nervous System after Injury. Biomedicines. 2024; 12(6):1256. https://doi.org/10.3390/biomedicines12061256
Chicago/Turabian StyleGu, Dandan, Yiming Xia, Zihan Ding, Jiaxi Qian, Xi Gu, Huiyuan Bai, Maorong Jiang, and Dengbing Yao. 2024. "Inflammation in the Peripheral Nervous System after Injury" Biomedicines 12, no. 6: 1256. https://doi.org/10.3390/biomedicines12061256





