First Application of a Mixed Porcine–Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure
Abstract
:1. Introduction
2. Materials and Methods
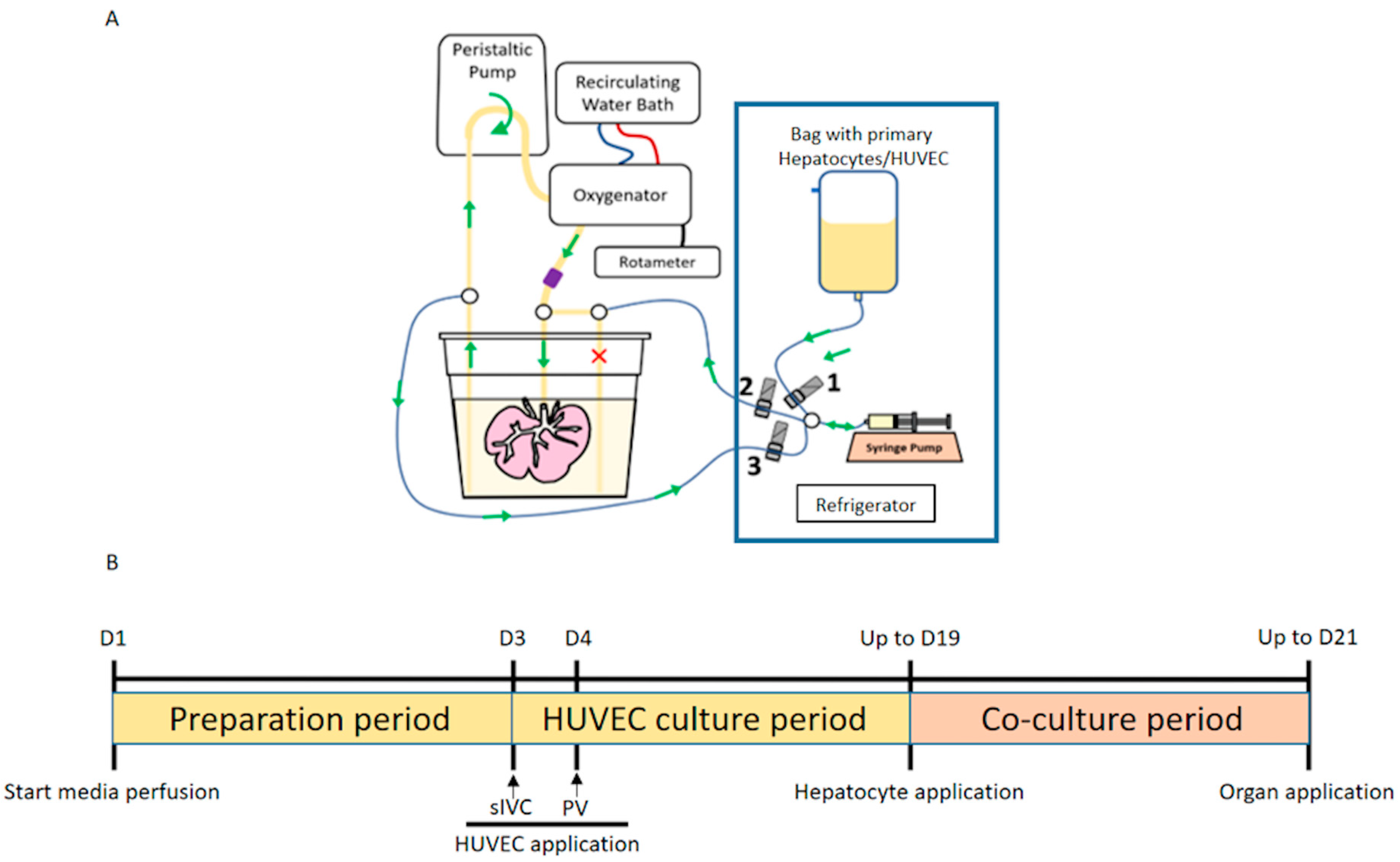
2.1. Production of Bioengineered Livers (BELs)
2.1.1. Porcine Organ Procurement and Whole Liver Decellularization
2.1.2. HUVEC Cell Culture
2.1.3. Isolation of Primary Porcine Hepatocytes (PPHCs)
2.1.4. Isolation of Primary Human Hepatocytes (PHHCs)
2.1.5. Production of Vascularized BEL Scaffolds
2.1.6. Production of MPH-BEL and FH-BEL Grafts
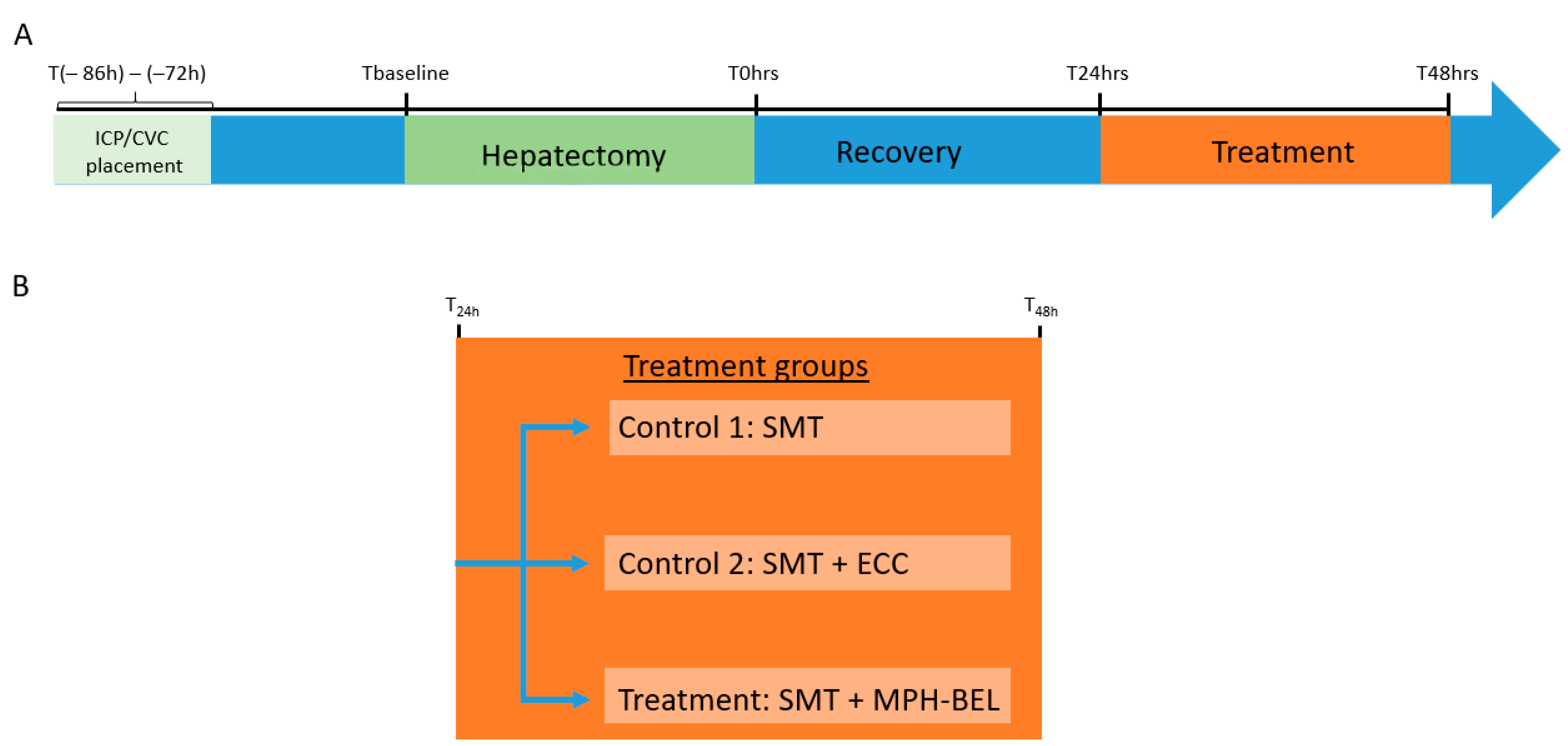
2.2. Porcine Model of Post-Resection Liver Failure (PRLF)
2.2.1. Anesthesia and Surgical Procedures
2.2.2. Placement of Intracranial Pressure Probe and Double Lumen Central Venous Catheter
2.2.3. The 85% Hepatectomy
2.2.4. Standard Medical Therapy (SMT) after 85% Hepatectomy
2.2.5. Extracorporeal Therapy
2.2.6. Blood Donation
2.2.7. Study Endpoints
- Survival to 48 h after hepatectomy
- Blood loss of more than 500 mL during liver resection
- Signs of hepatic encephalopathy stage III or IV
- Elevated ICP (>20 mmHg) for over 1 h
2.2.8. Animals Were Euthanized by Sodium Pentobarbital Overdose after Reaching One of These Endpoints
Histology
Immunofluorescence
Interleukin-6
2.3. In Vitro Perfusion of MPH-BEL and FH-BEL Grafts
2.3.1. Analysis of Metabolites
2.3.2. Ammonia Clearance by BELs
2.3.3. Statistical Analysis
3. Results
3.1. Animal Characteristics and Operative Variables
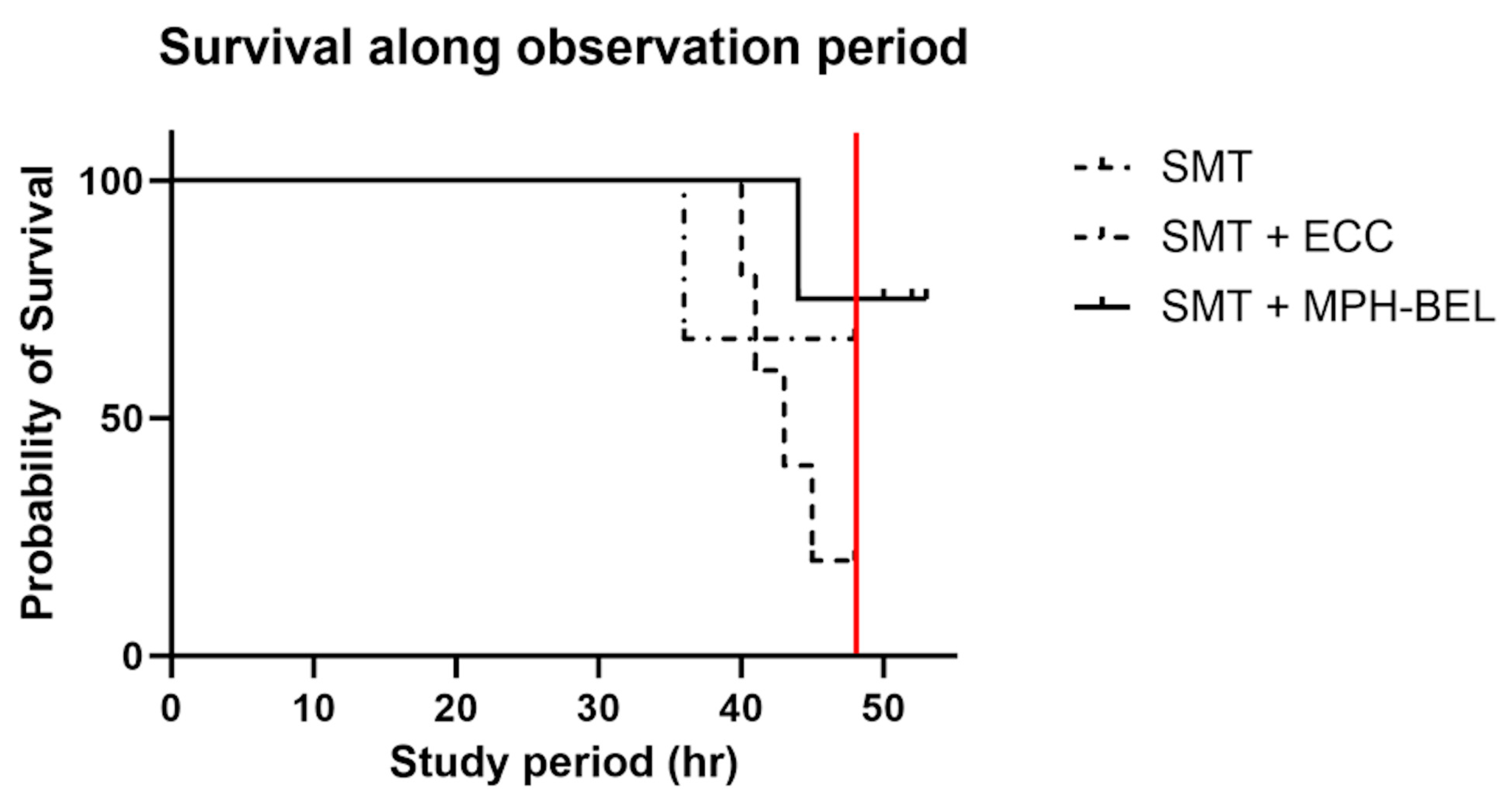
3.2. Survival
3.3. Evaluation of the MPH-BEL Functionality in the PRLF Model
3.4. Detoxification Function and Correlating ICP with Ammonia Levels of the MPH-BEL in the PRLF Model
3.5. Synthetic Function of the MPH-BEL in the PRLF Model
3.6. Hematological Markers during MPH-BEL Treatment
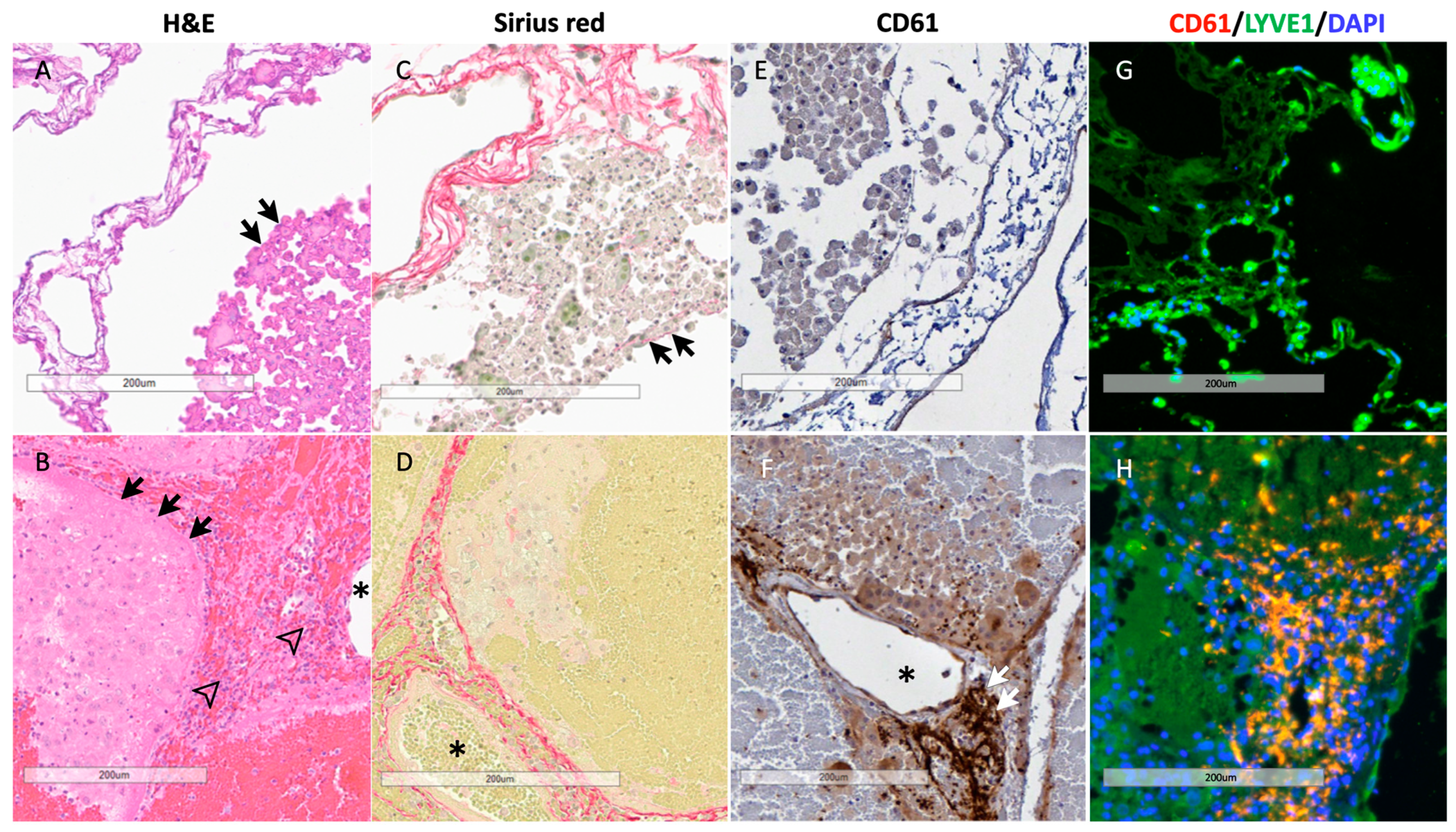
3.7. Histology of BEL Grafts
3.8. In Vitro Comparison of MPH- BEL and FH-BEL Grafts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine amino transferase |
| ALF | acute liver failure |
| AST | aspartate amino transferase |
| BD | common bile duct |
| BEL | bioengineered liver |
| CBC | central blood count |
| CVC | central venous catheter |
| ECC | extracorporeal circuit |
| FH-BEL | fully human bioengineered liver |
| HUVECs | human umbilical vein endothelial cells |
| IACUC | Institutional Animal Care and Use Committee |
| ICP | intracranial pressure |
| iIVC | infrahepatic Inferior Vena Cava |
| INR | international normalized ratio |
| MAP | mean arterial pressure |
| MPH-BEL | mixed porcine–human bioengineered liver |
| PHHCs | primary human hepatocytes |
| PPHCs | primary porcine hepatocytes |
| PRLF | post-resection liver failure |
| PV | portal vein |
| sIVC | suprahepatic Inferior Vena Cava |
| SMT | standard medical therapy |
| SPAD | single-pass albumin dialysis |
References
- Warrillow, S.; Bellomo, R. Intensive Care Management of Severe Acute Liver Failure. In Annual Update in Intensive Care and Emergency Medicine; Vincent, J.L., Ed.; Springer: Cham, Switzerland, 2015; pp. 415–430. [Google Scholar]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Tandon, R.; Froghi, S. Artificial liver support systems. J. Gastro Hepatol. 2021, 36, 1164–1179. [Google Scholar] [CrossRef]
- Kjaergard, L.L.; Liu, J.; Als-Nielsen, B.; Gluud, C. Artificial and Bioartificial Support Systems for Acute and Acute-on-Chronic Liver Failure: A Systematic Review. JAMA 2003, 289, 217. [Google Scholar] [CrossRef]
- Saliba, F.; Camus, C.; Durand, F.; Mathurin, P.; Letierce, A.; Delafosse, B.; Barange, K.; Perrigault, P.F.; Belnard, M.; Ichaï, P.; et al. Albumin Dialysis with a Noncell Artificial Liver Support Device in Patients with Acute Liver Failure: A Randomized, Controlled Trial. Ann. Intern. Med. 2013, 159, 522. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Subramanian, R.M.; Olson, J.C.; Speiser, J.L.; Durkalski-Mauldin, V.L.; Abraldes, J.G.; Bigam, D.L.; Flynn, M.M.; Rapaka, B.; Shropshire, B.M. Use of the Mo-lecular Adsorbent Recirculating System in Acute Liver Failure: Results of a Multicenter Propensity Score-Matched Study*. Critical Care Med. 2022, 50, 286–295. [Google Scholar] [CrossRef]
- Brophy, C.M.; Nyberg, S.L. Is there a future for the bioartificial liver? Current Opin. Organ Transplant. 2006, 11, 219–225. [Google Scholar] [CrossRef]
- Kelly, J.H.; Darlington, G.J. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. Vitr. Cell. Dev. Biol. 1989, 25, 217–222. [Google Scholar] [CrossRef]
- Mavri-Damelin, D.; Damelin, L.H.; Eaton, S.; Rees, M.; Selden, C.; Hodgson, H.J. Cells for bioartificial liver devices: The human hepatoma-derived cell line C3A produces urea but does not detoxify ammonia. Biotechnol. Bioeng. 2008, 99, 644–651. [Google Scholar] [CrossRef]
- Mavri-Damelin, D.; Eaton, S.; Damelin, L.H.; Rees, M.; Hodgson, H.J.; Selden, C. Ornithine transcarbamylase and arginase I deficiency are responsible for diminished urea cycle function in the human hepatoblastoma cell line HepG2. Int. J. Biochem. Cell Biol. 2007, 39, 555–564. [Google Scholar] [CrossRef]
- Shaheen, M.F.; Joo, D.J.; Ross, J.J.; Anderson, B.D.; Chen, H.S.; Huebert, R.C.; Li, Y.; Amiot, B.; Young, A.; Zlochiver, V. Sustained perfusion of revascularized bioengineered livers heterotopically transplanted into immunosuppressed pigs. Nat. Biomed. Eng. 2019, 4, 437–445. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nelson, E.D.; Joo, D.; Amiot, B.P.; Katane, A.A.; Mendenhall, A.; Steiner, B.G.; Stumbras, A.R.; Nelson, V.L.; Palumbo, R.N. Functional characterization of a bio-engineered liver after heterotopic implantation in pigs. Commun. Biol. 2021, 4, 1157. [Google Scholar]
- Chen, H.S.; Joo, D.J.; Shaheen, M.; Li, Y.; Wang, Y.; Yang, J.; Nicolas, C.T.; Predmore, K.; Amiot, B.; Michalak, G.; et al. Randomized Trial of Spheroid Reservoir Bioartificial Liver in Porcine Model of Posthepatectomy Liver Failure. Hepatology 2019, 69, 329–342. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lee, W.M.; Stravitz, T.R.; Larson, A.M. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology 2012, 55, 965–967. [Google Scholar] [CrossRef]
- Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Yaron, I.; Bernardi, M.; Simpson, K.J.; et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef]
- Li, K.; Tharwat, M.; Larson, E.L.; Felgendreff, P.; Hosseiniasl, S.M.; Abu Rmilah, A.; Safwat, K.; Ross, J.J.; Nyberg, S.L. Re-Endothelialization of Decellularized Liver Scaffolds: A Step for Bioengineered Liver Transplantation. Front. Bioeng. Biotechnol. 2022, 10, 833163. [Google Scholar] [CrossRef]
- Adwan, H.; Fuller, B.; Seldon, C.; Davidson, B.; Seifalian, A. Modifying three-dimensional scaffolds from novel nanocomposite materials using dissolvable porogen particles for use in liver tissue engineering. J. Biomater. Appl. 2013, 28, 250–261. [Google Scholar] [CrossRef]
- Ye, S.; Boeter, J.W.; Penning, L.C.; Spee, B.; Schneeberger, K. Hydrogels for Liver Tissue Engineering. Bioengineering 2019, 6, 59. [Google Scholar] [CrossRef]
- Clemmesen, J.O.; Larsen, F.S.; Kondrup, J.; Hansen, B.A.; Ott, P. Cerebral herniation in patients with acute E liver failure is correlated with arterial ammonia concentration. Hepatology 1999, 29, 648–653. [Google Scholar] [CrossRef]
- Scott, T.R. Pathophysiology of cerebral oedema in acute liver failure. World J. Gastroenterol. 2013, 19, 9240–9255. [Google Scholar] [CrossRef]
- Wendon, J.; Lee, W. Encephalopathy and Cerebral Edema in the Setting of Acute Liver Failure: Pathogenesis and Management. Neurocrit. Care 2008, 9, 97–102. [Google Scholar] [CrossRef]
- Glorioso, J.M.; Mao, S.A.; Rodysill, B.; Mounajjed, T.; Kremers, W.K.; Elgilani, F.; Hickey, R.D.; Haugaa, H.; Rose, C.F.; Amiot, B.; et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015, 63, 388–398. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6, 245sr2. [Google Scholar] [CrossRef]
- Afzal, Z.; Huguet, E.L. Bioengineering liver tissue by repopulation of decellularised scaffolds. World J. Hepatol. 2023, 15, 151–179. [Google Scholar] [CrossRef]
- Debnath, T.; Mallarpu, C.S.; Chelluri, L.K. Development of Bioengineered Organ Using Biological Acellular Rat Liver Scaffold and Hepatocytes. Organogenesis 2020, 16, 61–72. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016, 38, 82–93. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, Y.; Guo, Y.; Wang, L.; Ling, C.; Guo, Q.; Wang, Y.; Zhu, S.; Fan, X.; Zhu, M.; et al. Decellularization and Recellularization of Rat Livers with Hepatocytes and Endothelial Progenitor Cells. Artif. Organs 2015, 40, E25–E38. [Google Scholar] [CrossRef]
- Carrier, F.M.M.; Deshêtres, A.; Guerra, S.M.F.; Rioux-Massé, B.; Zaouter, C.M.; Lee, N.M.S.; Amzallag, M.; Joosten, A.; Massicotte, L.; Chassé, M. Preoperative Fibrinogen Level and Bleeding in Liver Transplantation for End-stage Liver Disease: A Cohort Study. Transplantation 2023, 107, 693–702. [Google Scholar] [CrossRef]
- Evans, D.W.; Moran, E.C.; Baptista, P.M.; Soker, S.; Sparks, J.L. Scale-dependent mechanical properties of native and decellularized liver tissue. Biomech. Model. Mechanobiol. 2012, 12, 569–580. [Google Scholar] [CrossRef]





| SMT | SMT + ECC | SMT + MPH-BEL | p-Value | |
|---|---|---|---|---|
| Animal weight (kg ± SD) | 31.4 ± 5.3 | 34.8 ± 2.3 | 32.8 ± 4.9 | 0.326 |
| Pre-hepatectomy volume (mL ± SD) | 941 ± 124 | 1003 ± 130 | 1020 ± 241 | 0.760 |
| Post-hepatectomy volume (mL ± SD) | 109 ± 32 | 133 ± 32 | 126 ± 38 | 0.718 |
| Percent resection (%) | 88.0 | 86.8 | 87.5 | 0.985 |
| Blood loss during resection (mL ± SD) | 254 ± 102 | 153.3 ± 50 | 193.8 ± 71 | 0.290 |
| Intraoperative fluid (mL ± SD) | 1242 ± 448 | 843 ± 266 | 681 ± 224 | 0.191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felgendreff, P.; Hosseiniasl, S.M.; Minshew, A.; Amiot, B.P.; Wilken, S.; Ahmadzada, B.; Huebert, R.C.; Sakrikar, N.J.; Engles, N.G.; Halsten, P.; et al. First Application of a Mixed Porcine–Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure. Biomedicines 2024, 12, 1272. https://doi.org/10.3390/biomedicines12061272
Felgendreff P, Hosseiniasl SM, Minshew A, Amiot BP, Wilken S, Ahmadzada B, Huebert RC, Sakrikar NJ, Engles NG, Halsten P, et al. First Application of a Mixed Porcine–Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure. Biomedicines. 2024; 12(6):1272. https://doi.org/10.3390/biomedicines12061272
Chicago/Turabian StyleFelgendreff, Philipp, Seyed Mohammad Hosseiniasl, Anna Minshew, Bruce P. Amiot, Silvana Wilken, Boyukkhanim Ahmadzada, Robert C. Huebert, Nidhi Jalan Sakrikar, Noah G. Engles, Peggy Halsten, and et al. 2024. "First Application of a Mixed Porcine–Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure" Biomedicines 12, no. 6: 1272. https://doi.org/10.3390/biomedicines12061272







