Effects of Lithium Ions on tPA-Induced Hemorrhagic Transformation under Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Photothrombosis Model
2.3. Limb-Placing Test
2.4. Assessing Asymmetry of Forelimb Use in a Test Cylinder
2.5. Evans Blue Extravasation
2.6. MRI Scan Evaluation of Brain Damage
2.7. Oxygen-Glucose Deprivation in Cell Culture
2.8. Cell Viability Assay
2.9. Drug Treatment
2.10. Western Blot Analysis
2.11. MMP Zymograghy
2.12. Analysis of Glycolysis and Respiration
2.13. Statistical Analysis
3. Results
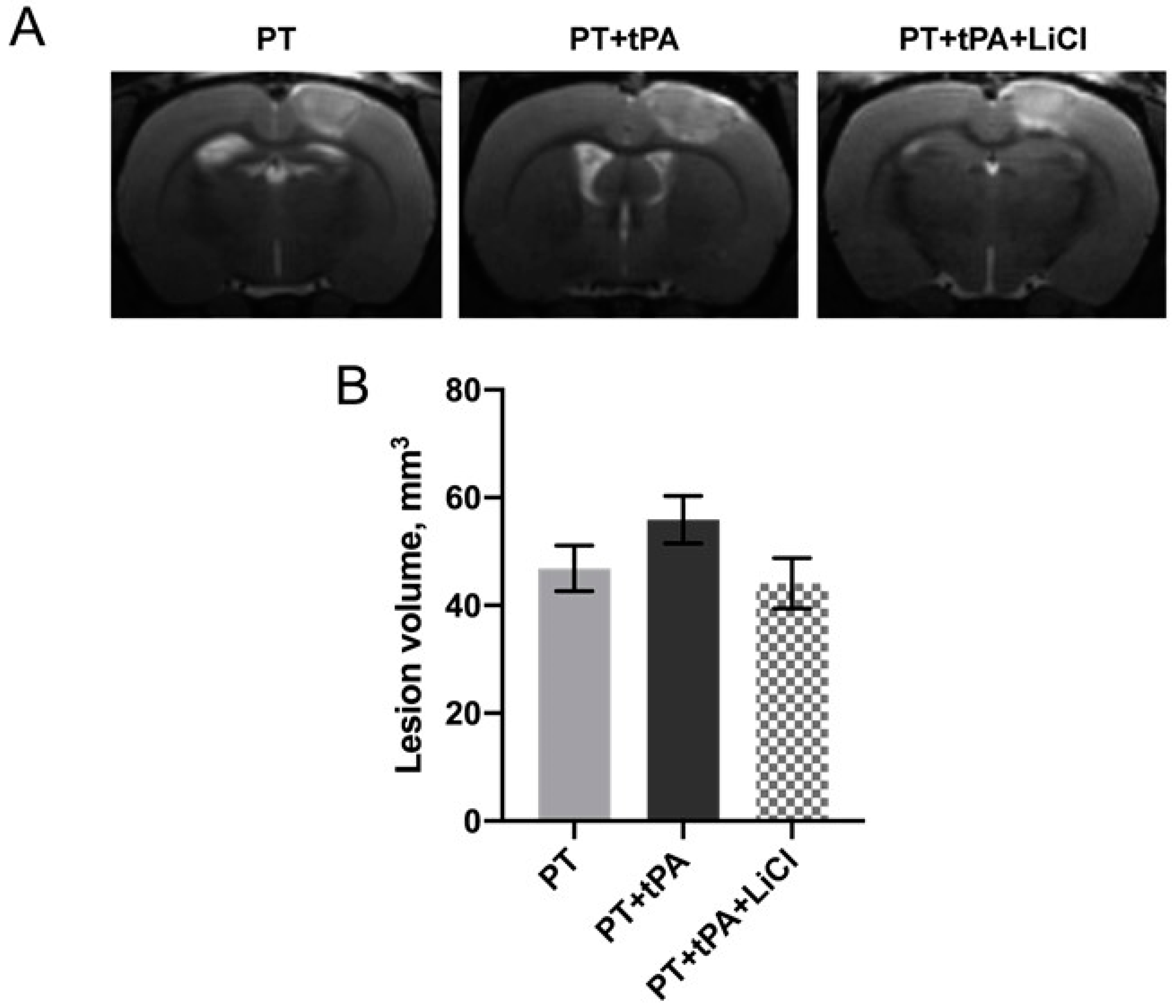
3.1. The Effect of Concurrent Thrombolytic Therapy with LiCl on Brain Damage
3.2. Evaluation of the Blood–Brain Barrier Integrity Using EBD
3.3. Effects of tPA on OGD-Induced Death of Endothelial Cells
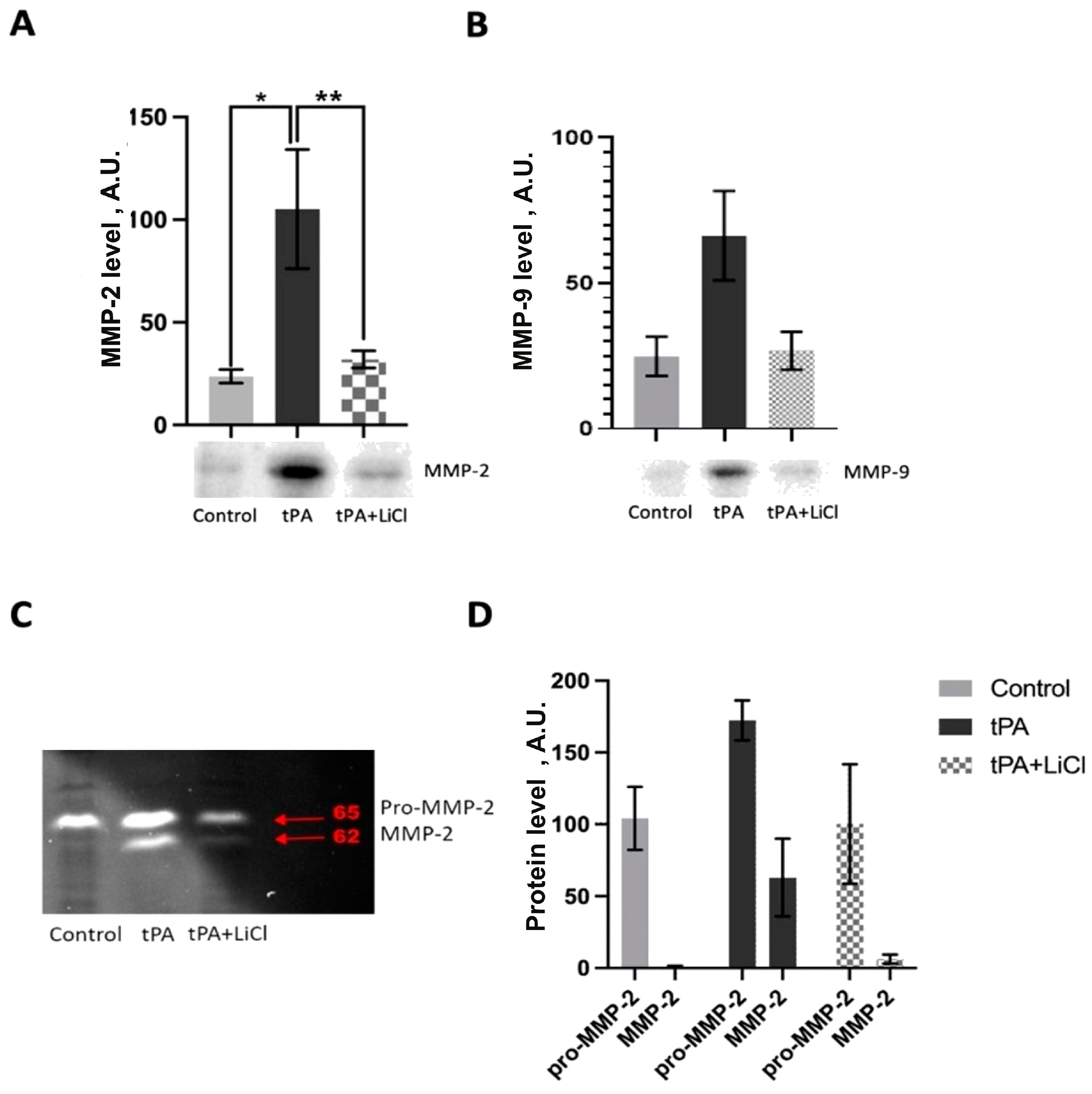
3.4. Effects of LiCl on tPA-Induced MMPs Increase
3.5. Energetic Metabolism Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Stroke Collaborators. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, J.; Jin, W.; Li, X.; Zhang, Y. Danhong Injection Combined With T-PA Improves Thrombolytic Therapy in Focal Embolic Stroke. Front. Pharmacol. 2018, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Lee, J.H.; Wali, B.; Stein, D.G.; Sayeed, I. Progesterone Attenuates Hemorrhagic Transformation after Delayed tPA Treatment in an Experimental Model of Stroke in Rats: Involvement of the VEGF-MMP Pathway. J. Cereb. Blood Flow Metab. 2014, 34, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Aguiar de Sousa, D.; von Martial, R.; Abilleira, S.; Gattringer, T.; Kobayashi, A.; Gallofré, M.; Fazekas, F.; Szikora, I.; Feigin, V.; Caso, V.; et al. Access to and Delivery of Acute Ischaemic Stroke Treatments: A Survey of National Scientific Societies and Stroke Experts in 44 European Countries. Eur. Stroke J. 2019, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.; Choi, S.; Mican, J.; Toul, M.; Ryu, W.-S.; Damborsky, J.; Mikulik, R.; Kim, D.-E. Development and Testing of Thrombolytics in Stroke. J. Stroke Cerebrovasc. Dis. 2021, 23, 12–36. [Google Scholar] [CrossRef]
- Babenko, V.A.; Fedulova, K.S.; Silachev, D.N.; Rahimi-Moghaddam, P.; Kalyuzhnaya, Y.N.; Demyanenko, S.V.; Plotnikov, E.Y. The Role of Matrix Metalloproteinases in Hemorrhagic Transformation in the Treatment of Stroke with Tissue Plasminogen Activator. J. Pers. Med. 2023, 13, 1175. [Google Scholar] [CrossRef]
- Sumii, T.; Lo, E.H. Involvement of Matrix Metalloproteinase in Thrombolysis-Associated Hemorrhagic Transformation after Embolic Focal Ischemia in Rats. Stroke 2002, 33, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gu, J.; Liu, Z.; Xu, C.; Qian, S.; Zhang, X.; Zhou, B.; Guan, Q.; Sun, Y.; Wang, Y.; et al. Inhibition of HIF-1α Reduced Blood Brain Barrier Damage by Regulating MMP-2 and VEGF During Acute Cerebral Ischemia. Front. Cell. Neurosci. 2018, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Lucero, J.; Abumiya, T.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Matrix Metalloproteinases Increase Very Early during Experimental Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 624–633. [Google Scholar] [CrossRef]
- Hamann, G.F.; Okada, Y.; del Zoppo, G.J. Hemorrhagic Transformation and Microvascular Integrity during Focal Cerebral Ischemia/reperfusion. J. Cereb. Blood Flow Metab. 1996, 16, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of Matrix Metalloproteinase-9 Gene Knock-out on the Proteolysis of Blood-Brain Barrier and White Matter Components after Cerebral Ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, E.; Ortega, L.; Hernández-Guillamon, M.; Penalba, A.; Fernández-Cadenas, I.; Rosell, A.; Montaner, J. Tissue Plasminogen Activator (t-PA) Promotes Neutrophil Degranulation and MMP-9 Release. J. Leukoc. Biol. 2008, 84, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cheng, Y.; Bi, G.; Zhu, Y.; Jun, W.; Ma, W.; Wu, H. Release of Matrix Metalloproteinases-2 and 9 by S-Nitrosylated Caveolin-1 Contributes to Degradation of Extracellular Matrix in tPA-Treated Hypoxic Endothelial Cells. PLoS ONE 2016, 11, e0149269. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A.; Skulachev, M.V.; Zorov, D.B. Lithium Salts—Simple but Magic. Biochemistry 2014, 79, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Singulani, M.P.; Ford, A.H.; Hackett, M.L.; Etherton-Beer, C.; Flicker, L.; Hankey, G.J.; De Paula, V.J.R.; Penteado, C.T.; Forlenza, O.V. Lithium and Stroke Recovery: A Systematic Review and Meta-Analysis of Stroke Models in Rodents and Human Data. Stroke 2022, 53, 2935–2944. [Google Scholar] [CrossRef]
- Mohammadianinejad, S.E.; Majdinasab, N.; Sajedi, S.A.; Abdollahi, F.; Moqaddam, M.M.; Sadr, F. The Effect of Lithium in Post-Stroke Motor Recovery: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Clin. Neuropharmacol. 2014, 37, 73–78. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Babenko, V.A.; Savchenko, E.S.; Zorova, L.D.; Pevzner, I.B.; Gulyaev, M.V.; Pirogov, Y.A.; Sukhikh, G.T.; Zorov, D.B. Protection of Neurovascular Unit Cells with Lithium Chloride and Sodium Valproate Prevents Brain Damage in Neonatal Ischemia/Hypoxia. Bull. Exp. Biol. Med. 2016, 160, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Andrejew, R.; Figueiro, F.; Bergamin, L.; Kapczinski, F.; Magalhães, P.V.d.S.; Battastini, A.M.O. Lithium-Induced Neuroprotective Activity in Neuronal and Microglial Cells: A Purinergic Perspective. Psychiatry Res. 2021, 295, 113562. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Zechmeister, B.; Bosche, B.; Lieschke, S.; Zheng, X.; Zhang, L.; Venkataramani, V.; Jin, F.; Hein, K.; Weber, M.S.; et al. Lithium Enhances Post-Stroke Blood-Brain Barrier Integrity, Activates the MAPK/ERK1/2 Pathway and Alters Immune Cell Migration in Mice. Neuropharmacology 2020, 181, 108357. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; van Meer, M.P.A.; Tejima, E.; Murata, Y.; Mandeville, J.B.; Dai, G.; Chuang, D.-M.; Rosen, B.R.; Lo, E.H. Functional MRI of Delayed Chronic Lithium Treatment in Rat Focal Cerebral Ischemia. Stroke 2008, 39, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of Reproducible Brain Infarction by Photochemically Initiated Thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Romanova, G.A.; Shakova, F.M.; Kovaleva, O.I.; Pivovarov, V.V.; Khlebnikova, N.N.; Karganov, M.Y. Relationship between Changes in Rat Behavior and Integral Biochemical Indexes Determined by Laser Correlation Spectroscopy after Photothrombosis of the Prefrontal Cortex. Bull. Exp. Biol. Med. 2004, 137, 135–138. [Google Scholar] [CrossRef] [PubMed]
- De Ryck, M.; Van Reempts, J.; Borgers, M.; Wauquier, A.; Janssen, P.A. Photochemical Stroke Model: Flunarizine Prevents Sensorimotor Deficits after Neocortical Infarcts in Rats. Stroke 1989, 20, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Jolkkonen, J.; Puurunen, K.; Rantakömi, S.; Härkönen, A.; Haapalinna, A.; Sivenius, J. Behavioral Effects of the alpha(2)-Adrenoceptor Antagonist, Atipamezole, after Focal Cerebral Ischemia in Rats. Eur. J. Pharmacol. 2000, 400, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS Plasticity and Assessment of Forelimb Sensorimotor Outcome in Unilateral Rat Models of Stroke, Cortical Ablation, Parkinsonism and Spinal Cord Injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Maeda, S.; Sasaki, K.; Halder, S.K.; Fujita, W.; Ueda, H. Neuroprotective DAMPs Member Prothymosin Alpha Has Addi-tional Beneficial Actions against Cerebral Ischemia-Induced Vascular Damages. J. Pharmacol. Sci. 2016, 132, 100–104. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent Cell Line Expressing Human Factor VIII-Related Antigen Established by Hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Ostrova, I.V.; Babkina, A.S.; Lyubomudrov, M.A.; Grechko, A.V.; Golubev, A.M. Photochemicallly Induced Thrombosis as a Model of Ischemic Stroke. Gen. Reanimatol. 2023, 19, 54–65. [Google Scholar] [CrossRef]
- Yepes, M.; Roussel, B.D.; Ali, C.; Vivien, D. Tissue-Type Plasminogen Activator in the Ischemic Brain: More than a Thrombolytic. Trends Neurosci. 2009, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Seners, P.; Turc, G.; Oppenheim, C.; Baron, J.-C. Incidence, Causes and Predictors of Neurological Deterioration Occurring within 24 H Following Acute Ischaemic Stroke: A Systematic Review with Pathophysiological Implications. J. Neurol. Neurosurg. Psychiatry 2015, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kenna, J.E.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Assessment of Recombinant Tissue Plasminogen Activator (rtPA) Toxicity in Cultured Neural Cells and Subsequent Treatment with Poly-Arginine Peptide R18D. Neurochem. Res. 2020, 45, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, M.; Zou, C.; Tian, Q.; Xu, Z. Tissue Plasminogen Activator Causes Brain Microvascular Endothelial Cell Injury After Oxygen Glucose Deprivation by Inhibiting Sonic Hedgehog Signaling. Neurochem. Res. 2019, 44, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Ortega-Aznar, A.; Alvarez-Sabín, J.; Fernández-Cadenas, I.; Ribó, M.; Molina, C.A.; Lo, E.H.; Montaner, J. Increased Brain Expression of Matrix Metalloproteinase-9 after Ischemic and Hemorrhagic Human Stroke. Stroke 2006, 37, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Florczak-Rzepka, M.; Grond-Ginsbach, C.; Montaner, J.; Steiner, T. Matrix Metalloproteinases in Human Spontaneous In-tracerebral Hemorrhage: An Update. Cerebrovasc. Dis. 2012, 34, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liao, Y.; Sun, Q.; Tang, H.; Wang, G.; Zhao, F.; Jin, Y. Upregulation of Matrix Metalloproteinase-9 in Primary Cul-tured Rat Astrocytes Induced by 2-Chloroethanol Via MAPK Signal Pathways. Front. Cell. Neurosci. 2017, 11, 218. [Google Scholar] [CrossRef]
- Yang, C.-Q.; Li, W.; Li, S.-Q.; Li, J.; Li, Y.-W.; Kong, S.-X.; Liu, R.-M.; Wang, S.-M.; Lv, W.-M. MCP-1 Stimulates MMP-9 Expres-sion via ERK 1/2 and p38 MAPK Signaling Pathways in Human Aortic Smooth Muscle Cells. Cell. Physiol. Biochem. 2014, 34, 266–276. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-B.; Gao, Q.; Tan, X.-X.; Huang, X.-W.; Ma, Y.-Z.; Fang, C.; Wang, S.-N.; Qiu, L.-H.; Cheng, Y.-X.; Guo, F.-Y.; et al. Lithium Alleviates Blood-Brain Barrier Breakdown after Cerebral Ischemia and Reperfusion by Upregulating Endothelial Wnt/β-Catenin Signaling in Mice. Neuropharmacology 2021, 186, 108474. [Google Scholar] [CrossRef]
- Wu, B.; Crampton, S.P.; Hughes, C.C.W. Wnt Signaling Induces Matrix Metalloproteinase Expression and Regulates T Cell Transmigration. Immunity 2007, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Park, J.-H.; Rhyu, S.Y.; Oh, S.-T.; Kang, W.-K.; Kim, H.-N. Wnt3a Expression Is Associated with MMP-9 Expression in Primary Tumor and Metastatic Site in Recurrent or Stage IV Colorectal Cancer. BMC Cancer 2014, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, W.; Xiong, Y.; Liu, H.; Li, N.; Du, Y.; Liu, Y. Intracellular Wnt/Beta-Catenin Signaling Underlying 17beta-Estradiol-Induced Matrix Metalloproteinase 9 Expression in Human Endometriosis. Biol. Reprod. 2016, 94, 70. [Google Scholar] [CrossRef] [PubMed]
- Edatt, L.; Haritha, K.; Sruthi, T.V.; Aswini, P.; Sameer Kumar, V.B. 2-Deoxy Glucose Regulate MMP-9 in a SIRT-1 Dependent and NFkB Independent Mechanism. Mol. Cell. Biochem. 2016, 423, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Sheu, J.-R.; Chung, C.-L.; Chen, C.-Y.; Lin, F.-L.; Hsu, M.-J.; Kuo, Y.-H.; Hsiao, G. Nuclear-Targeted Inhibition of NF-kappaB on MMP-9 Production by N-2-(4-Bromophenyl) Ethyl Caffeamide in Human Monocytic Cells. Chem. Biol. Interact. 2010, 184, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Shen, J.-X.; Wang, Y.; Liu, Y.; Shen, D.-Y.; Quan, S. Tankyrase Promotes Aerobic Glycolysis and Proliferation of Ovarian Cancer through Activation of Wnt/-Catenin Signaling. Biomed Res. Int. 2019, 2019, 2686340. [Google Scholar] [CrossRef]
- Kong, L.; Ma, Y.; Wang, Z.; Liu, N.; Ma, G.; Liu, C.; Shi, R.; Du, G. Inhibition of Hypoxia Inducible Factor 1 by YC-1 Attenuates Tissue Plasminogen Activator Induced Hemorrhagic Transformation by Suppressing HMGB1/TLR4/NF-κB Mediated Neutrophil Infiltration in Thromboembolic Stroke Rats. Int. Immunopharmacol. 2021, 94, 107507. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babenko, V.A.; Yakupova, E.I.; Pevzner, I.B.; Bocharnikov, A.D.; Zorova, L.D.; Fedulova, K.S.; Grebenchikov, O.A.; Kuzovlev, A.N.; Grechko, A.V.; Silachev, D.N.; et al. Effects of Lithium Ions on tPA-Induced Hemorrhagic Transformation under Stroke. Biomedicines 2024, 12, 1325. https://doi.org/10.3390/biomedicines12061325
Babenko VA, Yakupova EI, Pevzner IB, Bocharnikov AD, Zorova LD, Fedulova KS, Grebenchikov OA, Kuzovlev AN, Grechko AV, Silachev DN, et al. Effects of Lithium Ions on tPA-Induced Hemorrhagic Transformation under Stroke. Biomedicines. 2024; 12(6):1325. https://doi.org/10.3390/biomedicines12061325
Chicago/Turabian StyleBabenko, Valentina A., Elmira I. Yakupova, Irina B. Pevzner, Alexey D. Bocharnikov, Ljubava D. Zorova, Kseniya S. Fedulova, Oleg A. Grebenchikov, Artem N. Kuzovlev, Andrey V. Grechko, Denis N. Silachev, and et al. 2024. "Effects of Lithium Ions on tPA-Induced Hemorrhagic Transformation under Stroke" Biomedicines 12, no. 6: 1325. https://doi.org/10.3390/biomedicines12061325





