The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Setting an Animal Model of Liver Fibrosis
2.3. Determination of the Liver Profibrogenic Mediators (TGF-β1 and PDGF-BB)
2.4. Determination of Liver MMP-2, MMP-9, Dimer MMP-9 and TIMP-1 by Zymography
2.5. Histology Analysis of Liver Tissue
2.6. Statistical Analysis
3. Results
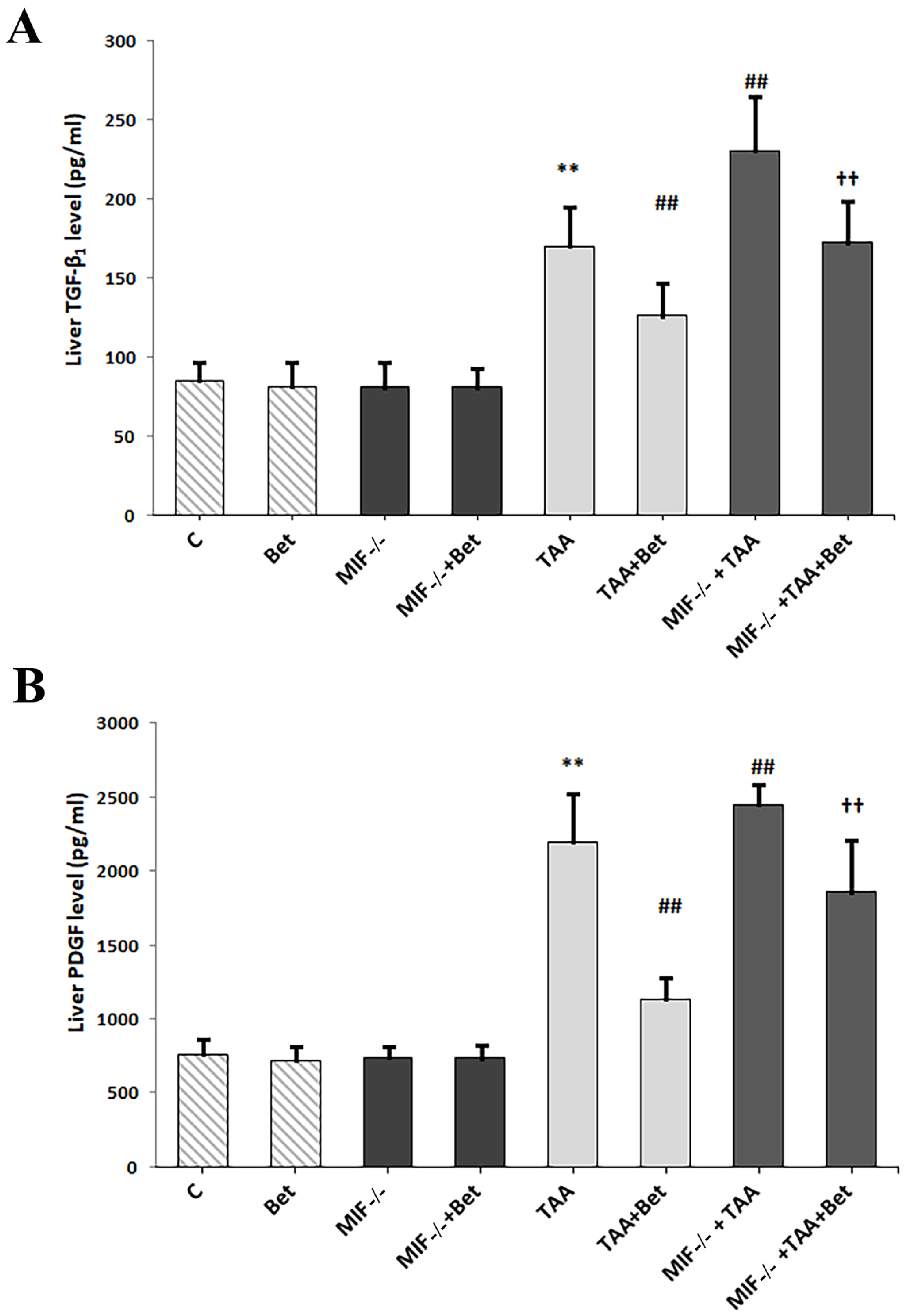
3.1. The Effects of MIF and Betaine on Profibrogenic Mediators (TGF-β1 and PDGF-BB) in TAA-Induced Liver Fibrosis
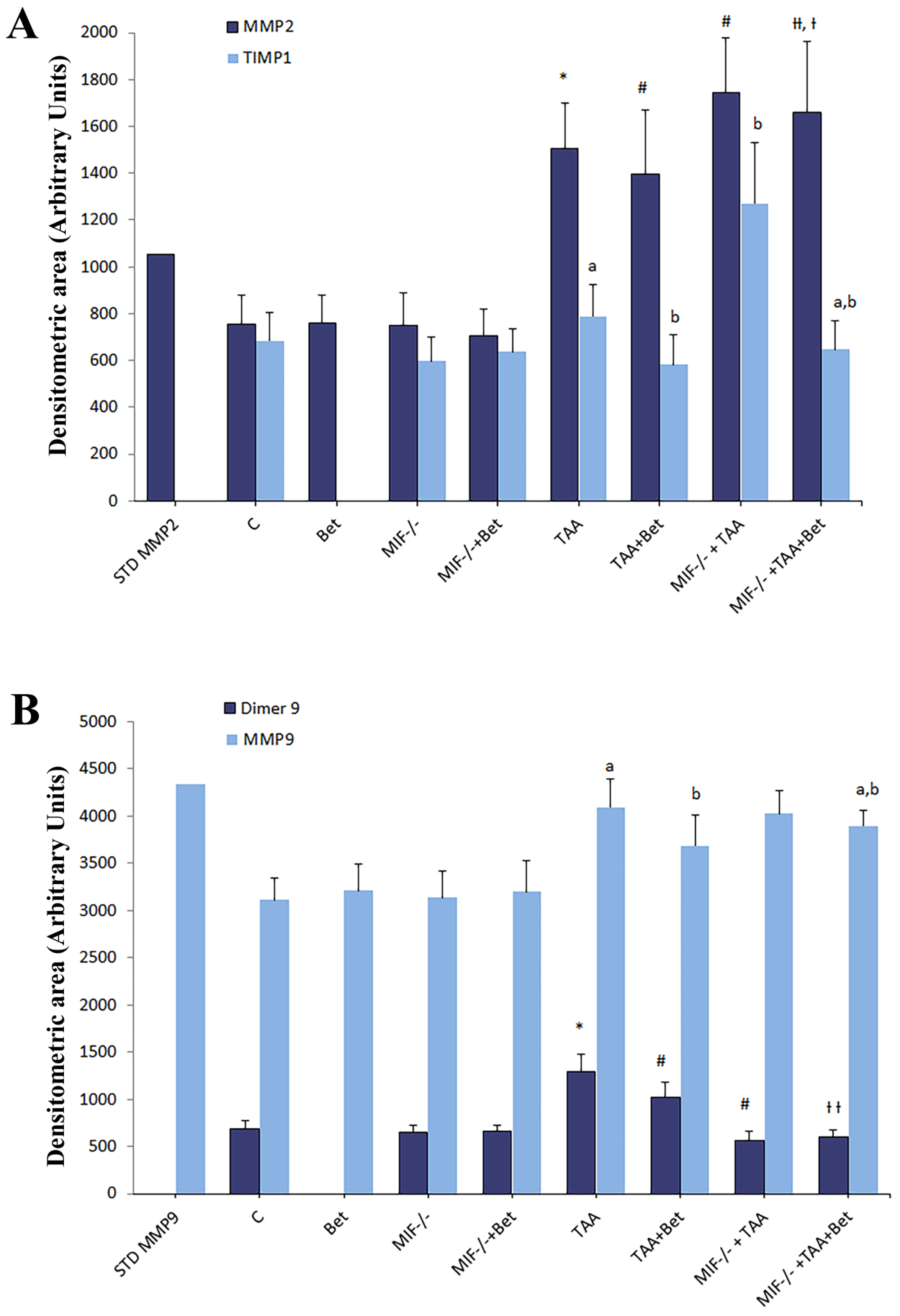
3.2. Effects of MIF and Betaine on MMP-2, MMP-9, Dimer MMP-9 and TIMP-1 Activity in TAA-Induced Liver Fibrosis
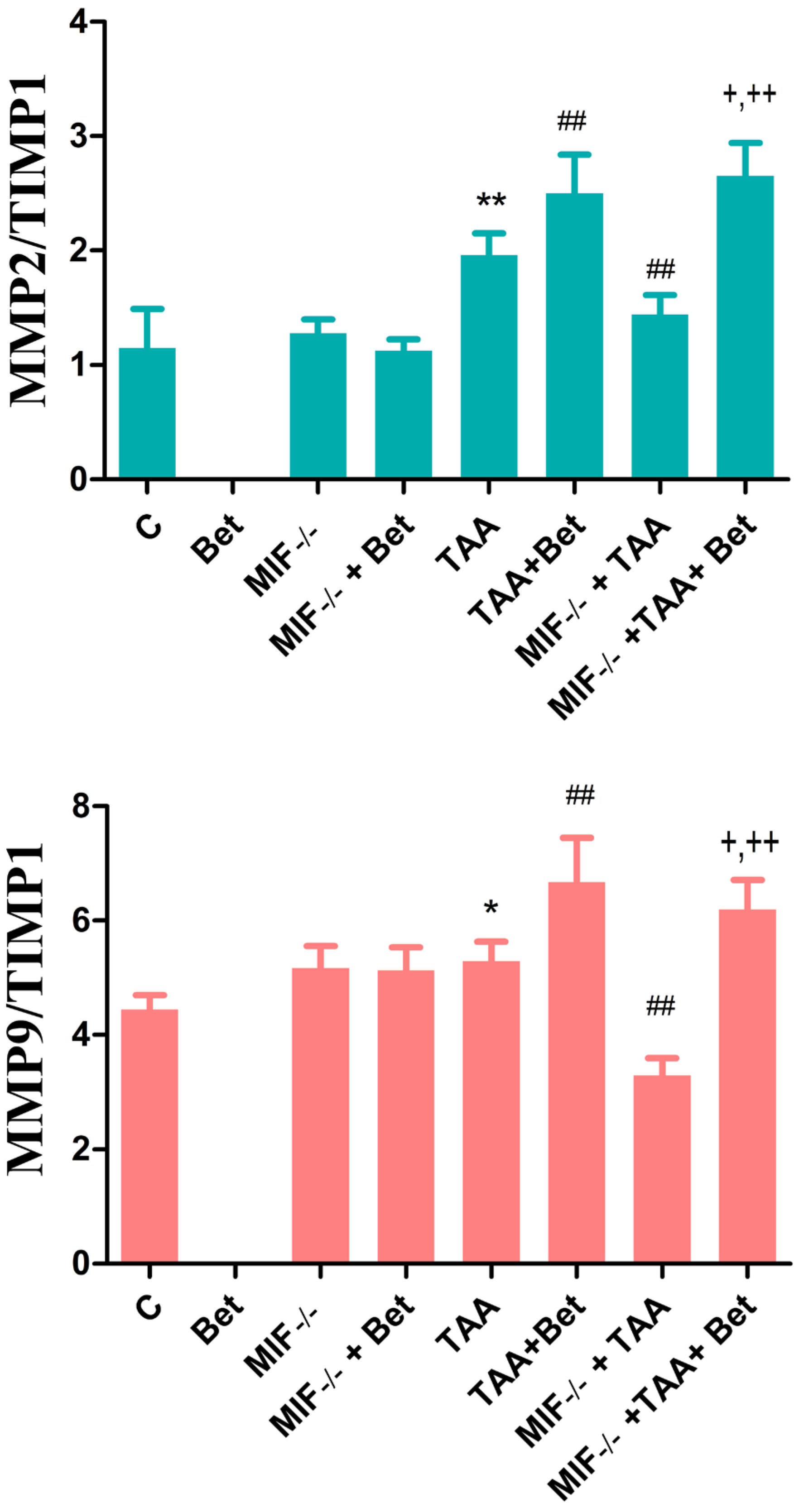
3.3. The Effects of MIF and Betaine on the Imbalance of MMP2/TIMP1 and MMP9/TIMP1 in the Livers of Mice with TAA-Induced Liver Fibrosis
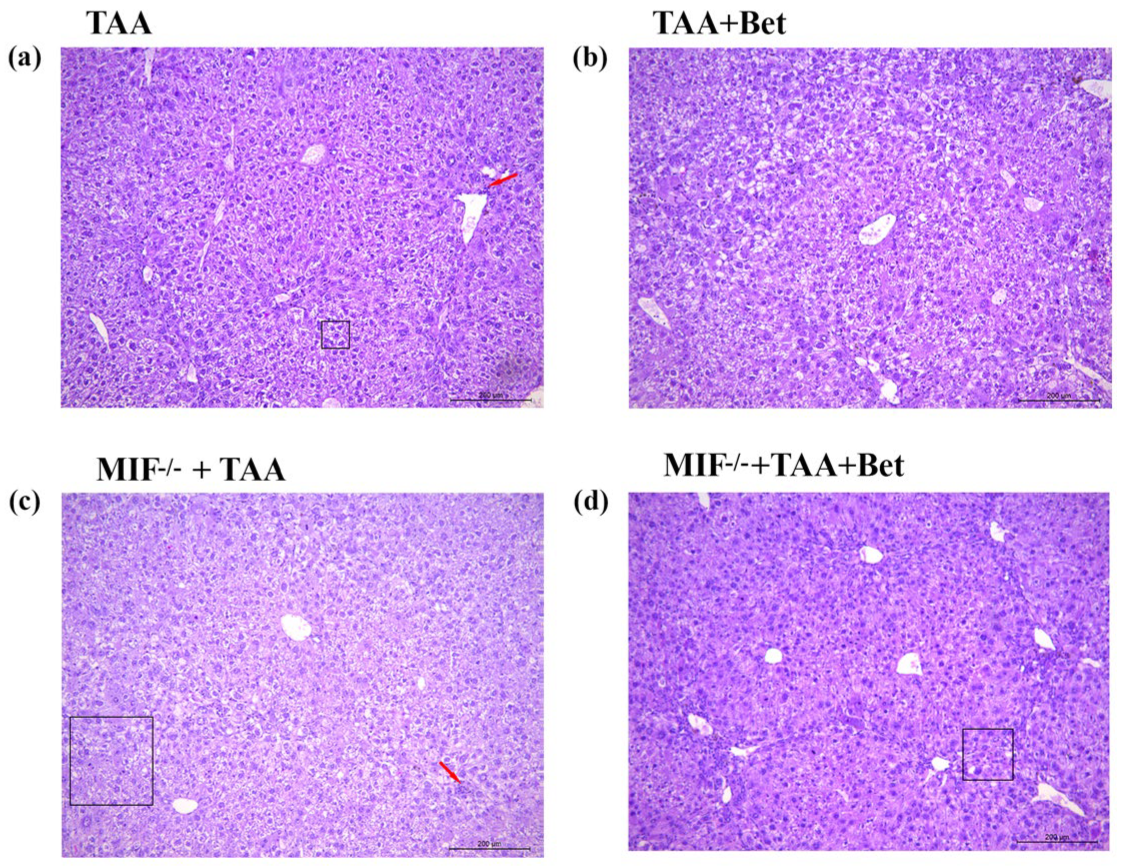
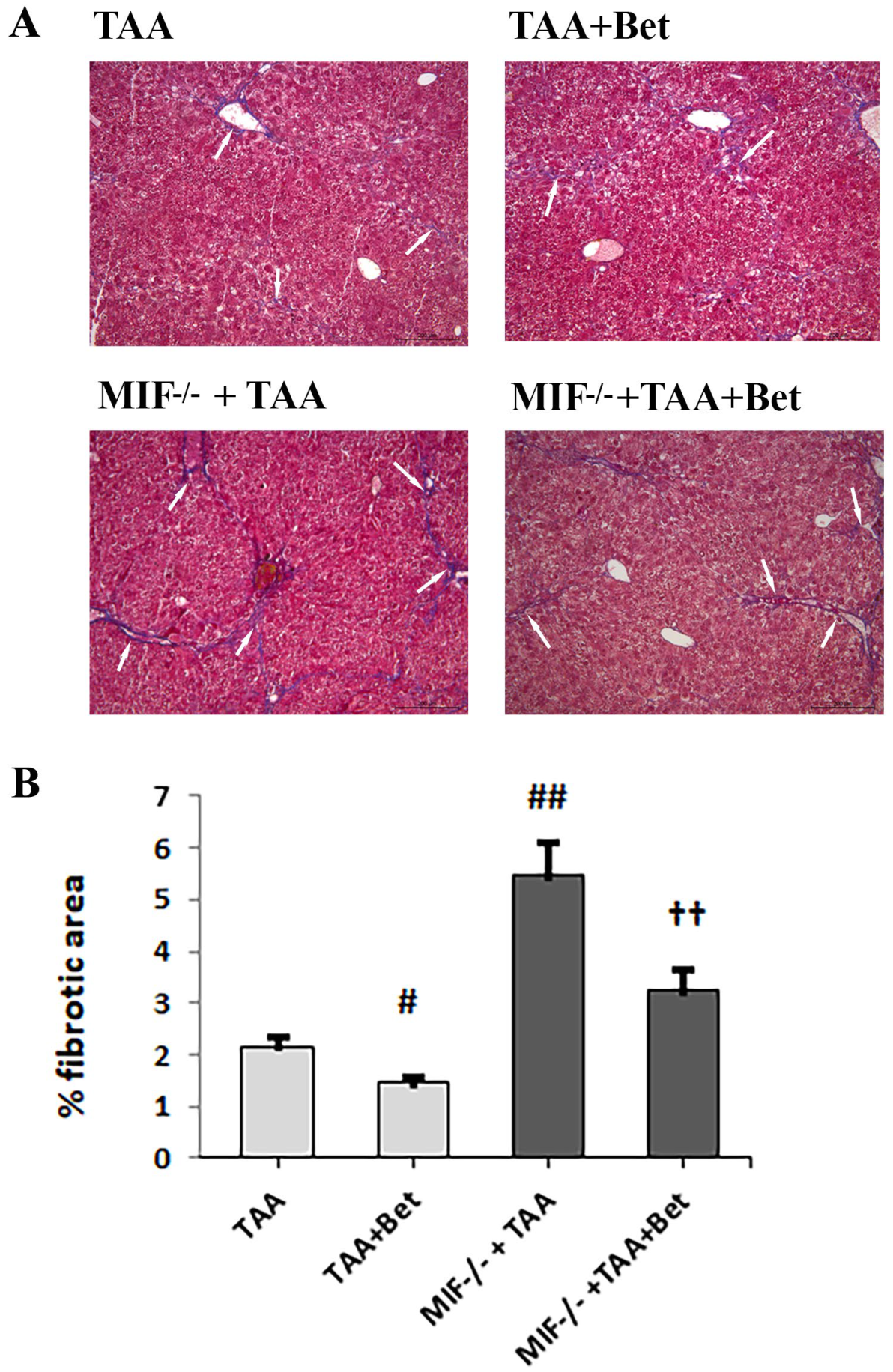
3.4. Effects of MIF and Betaine on Histology Findings of Liver Morphology in Mice with TAA-Induced Liver Fibrosis
3.5. The Effects of MIF and Betaine on TAA-Induced Liver Fibrosis in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, M.; Kisseleva, T. Reversibility of Liver Fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S60–S63. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. A review of liver fibrosis and cirrhosis regression. J. Pathol. Transl. Med. 2023, 57, 189–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Friedman, S.L. Hepatic Fibrosis: A Convergent Response to Liver Injury That Is Reversible. J. Hepatol. 2020, 73, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of Liver Fibrosis and Its Role in Liver Cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ignat, S.-R.; Dinescu, S.; Hermenean, A.; Costache, M. Cellular Interplay as a Consequence of Inflammatory Signals Leading to Liver Fibrosis Development. Cells 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Odena, G.; Poulsen, K.; Tiribelli, C.; Bellentani, S.; Barchetti, A.; Bru, P.S.; Rosso, N.; Bataller, R.; Nagy, L.E. Role of MIF in Hepatic Inflammatory Diseases and Fibrosis. In MIF Family Cytokines in Innate Immunity and Homeostasis. Progress in Inflammation Research; Bucala, R., Bernhagen, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 109–134. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Weiskirchen, R. The PDGF System and Its Antagonists in Liver Fibrosis. Cytokine Growth Factor Rev. 2016, 28, 53–61. [Google Scholar] [CrossRef]
- Liang, S.; Wang, F.; Zhai, D.; Meng, X.; Li, J.; Lv, X. Matrix Metalloproteinases Induce Extracellular Matrix Degradation through Various Pathways to Alleviate Hepatic Fibrosis. Biomed. Pharmacother. 2023, 161, 114472. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.J.; Huang, T.J.; Zhang, Q.Q.; Zhang, H.Y.; Guo, X.H.; Fan, H.Q.; Li, R.K.; Liu, L.X. Insulin-like growth factor binding protein related protein 1 knockdown attenuates hepatic fibrosis via the regulation of MMPs/TIMPs in mice. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sumaiya, K.; Langford, D.; Natarajaseenivasan, K.; Shanmughapriya, S. Macrophage Migration Inhibitory Factor (MIF): A Multifaceted Cytokine Regulated by Genetic and Physiological Strategies. Pharmacol. Ther. 2021, 233, 108024. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.-S.; Jiang, J.X.; Wu, J.; Halsted, C.; Friedman, S.L.; Zern, M.A.; Torok, N.J. Phagocytosis of Apoptotic Bodies by Hepatic Stellate Cells Induces NADPH Oxidase and Is Associated with Liver Fibrosisin Vivo. Hepatology 2006, 43, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Mizue, Y.; Ghani, S.; Leng, L.; McDonald, C.; Kong, P.; Baugh, J.; Lane, S.J.; Craft, J.; Nishihira, J.; Donnelly, S.C.; et al. Role for Macrophage Migration Inhibitory Factor in Asthma. Proc. Natl. Acad. Sci. USA 2005, 102, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Hoi, A.; Iskander, M.; Morand, E. Macrophage Migration Inhibitory Factor: A Therapeutic Target across Inflammatory Diseases. Inflamm. Allergy Drug Targets 2007, 6, 183–190. [Google Scholar] [CrossRef]
- Akbar, S.F.; Abe, M.; Murakami, H.; Tanimoto, K.; Kumagi, T.; Yamashita, Y.; Michitaka, K.; Horiike, N.; Onji, M. Macrophage Migration Inhibitory Factor in Hepatocellular Carcinoma and Liver Cirrhosis; Relevance to Pathogenesis. Cancer Lett. 2001, 171, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kozaci, L.D.; Sari, I.; Alacacioglu, A.; Akar, S.; Akkoc, N. Evaluation of Inflammation and Oxidative Stress in Ankylosing Spondylitis: A Role for Macrophage Migration Inhibitory Factor. Mod. Rheumatol. 2010, 20, 34–39. [Google Scholar] [CrossRef]
- Lu, H.; Bai, Y.; Wu, L.; Hong, W.; Liang, Y.; Chen, B.; Bai, Y. Inhibition of Macrophage Migration Inhibitory Factor Protects against Inflammation and Matrix Deposition in Kidney Tissues after Injury. Mediat. Inflamm. 2016, 2016, 2174682. [Google Scholar] [CrossRef]
- de Azevedo, R.A.; Shoshan, E.; Whang, S.; Markel, G.; Jaiswal, A.R.; Liu, A.; Curran, M.A.; Travassos, L.R.; Bar-Eli, M. MIF Inhibition as a Strategy for Overcoming Resistance to Immune Checkpoint Blockade Therapy in Melanoma. OncoImmunology 2020, 9, 1846915. [Google Scholar] [CrossRef]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.M.; Groothuis, G.M.M.; Olinga, P.; Boersema, M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.A.; McMullen, M.R.; Roychowdhury, S.; Pisano, S.G.; Liu, X.; Stavitsky, A.B.; Bucala, R.; Nagy, L.E. Macrophage Migration Inhibitory Factor Contributes to Ethanol-Induced Liver Injury by Mediating Cell Injury, Steatohepatitis and Steatosis. Hepatology 2013, 57, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Molecular Mechanisms That Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernández-Gea, V. The Endothelium as a Driver of Liver Fibrosis and Regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and Physiological Role of Reactive Oxygen Species—The Good, the Bad and the Ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Torok, N.J. Dysregulation of Redox Pathways in Liver Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G667–G674. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Hemmati-Dinarvand, M.; Saedi, S.; Valilo, M.; Kalantary-Charvadeh, A.; Alizadeh Sani, M.; Kargar, R.; Safari, H.; Samadi, N. Oxidative Stress and Parkinson’s Disease: Conflict of Oxidant-Antioxidant Systems. Neurosci. Lett. 2019, 709, 134296. [Google Scholar] [CrossRef]
- Maurya, P.K.; Dua, K. Role of Oxidative Stress in Pathophysiology of Diseases, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A. Oxidative Stress and Alcoholic Liver Disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef]
- McQuitty, C.E.; Williams, R.; Chokshi, S.; Urbani, L. Immunomodulatory Role of the Extracellular Matrix within the Liver Disease Microenvironment. Front. Immunol. 2020, 11, 574276. [Google Scholar] [CrossRef]
- Li, H.; You, H.; Fan, X.; Jia, J. Hepatic Macrophages in Liver Fibrosis: Pathogenesis and Potential Therapeutic Targets. BMJ Open Gastroenterol. 2016, 3, e000079. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, S.P.; Li, H.; Taniguchi, G.T. Zymography and Reverse Zymography for Detecting MMPs and TIMPs. Methods Mol. Biol. 2010, 622, 257–269. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, J.; Khalil, R.A. Zymography as a Research Tool in the Study of Matrix Metalloproteinase Inhibitors. Methods Mol. Biol. 2017, 1626, 79–102. [Google Scholar] [CrossRef]
- Heinrichs, D.; Knauel, M.; Offermanns, C.; Berres, M.-L.; Nellen, A.; Leng, L.; Schmitz, P.; Bucala, R.; Trautwein, C.; Weber, C.; et al. Macrophage Migration Inhibitory Factor (MIF) Exerts Antifibrotic Effects in Experimental Liver Fibrosis via CD74. Proc. Natl. Acad. Sci. USA 2011, 108, 17444–17449. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF Signal Transduction Initiated by Binding to CD74. J. Exp. Med. 2003, 197, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-Y.; Oh, M.-A.; Kim, W.H.; Sohn, H.-Y.; Park, S.I. AMP-Activated Protein Kinase Inhibits TGF-β-Induced Fibrogenic Responses of Hepatic Stellate Cells by Targeting Transcriptional Coactivator P300. J. Cell. Physiol. 2011, 227, 1081–1089. [Google Scholar] [CrossRef]
- Thủy, T.; Kawada, N. Antifibrotic Role of Macrophage Migration Inhibitory Factor: Discovery of an Unexpected Function. Hepatology 2012, 55, 1295–1297. [Google Scholar] [CrossRef]
- Qin, L.; Tan, J.; Lv, X.; Zhang, J. Vanillic Acid Alleviates Liver Fibrosis through Inhibiting Autophagy in Hepatic Stellate Cells via the MIF/CD74 Signaling Pathway. Biomed. Pharmacother. 2023, 168, 115673. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Sato, S.; Yamate, J.; Kurasaki, M.; Nishihira, J.; Hosokawa, T.; Fujita, H.; Saito, T. Immunohistochemical Study of Macrophage Migration Inhibitory Factor in Rat Liver Fibrosis Induced by Thioacetamide. Eur. J. Histochem. 2003, 47, 317–324. [Google Scholar] [CrossRef][Green Version]
- Wirtz, T.H.; Reuken, P.A.; Jansen, C.; Fischer, P.; Bergmann, I.; Backhaus, C.; Emontzpohl, C.; Reißing, J.; Brandt, E.F.; Koenen, M.T.; et al. Balance between Macrophage Migration Inhibitory Factor and SCD74 Predicts Outcome in Patients with Acute Decompensation of Cirrhosis. JHEP Rep. 2020, 3, 100221. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent Insights into the Role of Immune Cells in Alcoholic Liver Disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Marin, V.; Poulsen, K.; Odena, G.; McMullen, M.R.; Altamirano, J.; Sancho-Bru, P.; Tiribelli, C.; Caballeria, J.; Rosso, N.; Bataller, R.; et al. Hepatocyte-Derived Macrophage Migration Inhibitory Factor Mediates Alcohol-Induced Liver Injury in Mice and Patients. J. Hepatol. 2017, 67, 1018–1025. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Döring, Y.; Weber, C. Chemokines and Their Receptors in Atherosclerosis. J. Mol. Med. 2015, 93, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, L.; Tian, L.; Li, W.; Yang, L.; Li, L. Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury. Sci. Rep. 2016, 6, 27665. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic Macrophages in Liver Homeostasis and Diseases-Diversity, Plasticity and Therapeutic Opportunities. Cell. Mol. Immunol. 2020, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz, M.; Gunsar, F.; Nart, D.; Sahin, O.; Yilmaz, F.; Akay, S.; Ersoz, G.; Karasu, Z.; Ilter, T.; Batur, Y.; et al. Macrophage Migration Inhibitory Factor Expression and MIF Gene −173 G/c Polymorphism in Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 192–198. [Google Scholar] [CrossRef]
- Heinrichs, D.; Berres, M.-L.; Coeuru, M.; Knauel, M.; Nellen, A.; Fischer, P.; Philippeit, C.; Bucala, R.; Trautwein, C.; Wasmuth, H.E.; et al. Protective Role of Macrophage Migration Inhibitory Factor in Nonalcoholic Steatohepatitis. FASEB J. 2014, 28, 5136–5147. [Google Scholar] [CrossRef]
- Heinrichs, D.; Brandt, E.F.; Fischer, P.; Köhncke, J.; Wirtz, T.H.; Guldiken, N.; Djudjaj, S.; Boor, P.; Kroy, D.; Weiskirchen, W.; et al. Unexpected Pro-Fibrotic Effect of MIF in Non-Alcoholic Steatohepatitis Is Linked to a Shift in NKT Cell Populations. Cells 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving Complexity of MIF Signaling. Cell Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Geervliet, E.; Bansal, R. Matrix Metalloproteinases as Potential Biomarkers and Therapeutic Targets in Liver Diseases. Cells 2020, 9, 1212. [Google Scholar] [CrossRef]
- Takahara, T.; Furui, K.; Yata, Y.; Jin, B.; Zhang, L.P.; Nambu, S.; Sato, H.; Seiki, M.; Watanabe, A. Dual Expression of Matrix Metalloproteinase-2 and Membrane-Type 1-Matrix Metalloproteinase in Fibrotic Human Livers. Hepatology 1997, 26, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-B.; Cai, W.-M.; Weng, H.-L.; Hu, Z.-R.; Lu, J.; Zheng, M.; Liu, R.-H. Diagnostic Value of Platelet Derived Growth Factor-BB, Transforming Growth Factor-β1, Matrix Metalloproteinase-1, and Tissue Inhibitor of Matrix Metalloproteinase-1 in Serum and Peripheral Blood Mononuclear Cells for Hepatic Fibrosis. World J. Gastroenterol. 2003, 9, 2490. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage Migration Inhibitory Factor Stimulates AMP-Activated Protein Kinase in the Ischaemic Heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Stosic-Grujicic, S.; Stojanovic, I.; Maksimovic-Ivanic, D.; Momcilovic, M.; Popadic, D.; Harhaji, L.; Miljkovic, D.; Metz, C.; Mangano, K.; Papaccio, G.; et al. Macrophage Migration Inhibitory Factor (MIF) Is Necessary for Progression of Autoimmune Diabetes Mellitus. J. Cell. Physiol. 2008, 215, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E.; Purucker, E.; Breuer, B.; Nguyen, H.N.; Heinrich, P.C.; Rose-John, S.; Matern, S. TIMP Expression in Toxic and Cholestatic Liver Injury in Rat. J. Hepatol. 1997, 27, 535–544. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, Z.; Li, H.; Lai, F.; Li, X.; Tang, Y.; Tian, M.; Wu, H. Antioxidant Mechanism of Betaine without Free Radical Scavenging Ability. J. Agric. Food Chem. 2016, 64, 7921–7930. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The Clinical Significance of Betaine, an Osmolyte with a Key Role in Methyl Group Metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Warskulat, U.; Wettstein, M.; Haussinger, D. Identification of Betaine as an Osmolyte in Rat Liver Macrophages (Kupffer Cells). Gastroenterology 1996, 110, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Chen, C.-Y.; Pan, Y.-H.; Wang, S.-H.; Mersmann, H.J.; Ding, S.-T. Alleviation of Carbon-Tetrachloride-Induced Liver Injury and Fibrosis by Betaine Supplementation in Chickens. Evid. Based Complement. Altern. Med. 2015, 2015, 725379. [Google Scholar] [CrossRef] [PubMed]
- Bingül, İ.; Başaran-Küçükgergin, C.; Aydın, A.F.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine Treatment Decreased Oxidative Stress, Inflammation, and Stellate Cell Activation in Rats with Alcoholic Liver Fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bingül, İ.; Aydın, A.F.; Başaran-Küçükgergin, C.; Doğan-Ekici, I.; Çoban, J.; Doğru-Abbasoğlu, S.; Uysal, M. High-Fat Diet plus Carbon Tetrachloride-Induced Liver Fibrosis Is Alleviated by Betaine Treatment in Rats. Int. Immunopharmacol. 2016, 39, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine Modulates Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Akt/MTOR Signaling in Methionine-Choline Deficiency-Induced Fatty Liver Disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fang, S.; Liu, Q.; Gao, J.; Wang, X.; Zhu, H.; Zhu, Z.; Ji, F.; Wu, J.; Ma, Y.; et al. TGF-β1/P65/MAT2A Pathway Regulates Liver Fibrogenesis via Intracellular SAM. EBioMedicine 2019, 42, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Seo, J.M.; Chae, Y.R.; Jung, Y.S.; Park, J.H.; Kim, Y.C. Alleviation of Dimethylnitrosamine-Induced Liver Injury and Fibrosis by Betaine Supplementation in Rats. Chem. Biol. Interact. 2009, 177, 204–211. [Google Scholar] [CrossRef] [PubMed]
- French, S. How to Prevent Alcoholic Liver Disase. Exp. Mol. Pathol. 2015, 98, 304–307. [Google Scholar] [CrossRef][Green Version]
- Gharbia, S.; Balta, C.; Herman, H.; Rosu, M.; Váradi, J.; Bácskay, I.; Vecsernyés, M.; Gyöngyösi, S.; Fenyvesi, F.; Voicu, S.N.; et al. Enhancement of Silymarin Anti-Fibrotic Effects by Complexation with Hydroxypropyl (HPBCD) and Randomly Methylated (RAMEB) β-Cyclodextrins in a Mouse Model of Liver Fibrosis. Front. Pharmacol. 2018, 9, 883. [Google Scholar] [CrossRef]
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Silymarin Inhibits Melanoma Cell Growth Both in Vitro and in Vivo by Targeting Cell Cycle Regulators, Angiogenic Biomarkers and Induction of Apoptosis. Mol. Carcinog. 2015, 54, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Niknahad, H.; Sadeghi, A.; Mohammadi, H.; Ghanbarinejad, V.; Ommati, M.M.; Hosseini, A.; Azarpira, N.; Khodaei, F.; Farshad, O.; et al. Betaine Treatment Protects Liver through Regulating Mitochondrial Function and Counteracting Oxidative Stress in Acute and Chronic Animal Models of Hepatic Injury. Biomed. Pharmacother. 2018, 103, 75–86. [Google Scholar] [CrossRef]
- Yang, W.; Huang, L.; Gao, J.; Wen, S.; Tai, Y.; Chen, M.; Huang, Z.; Liu, R.; Tang, C.; Li, J. Betaine Attenuates Chronic Alcohol-Induced Fatty Liver by Broadly Regulating Hepatic Lipid Metabolism. Mol. Med. Rep. 2017, 16, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, D.; Rovčanin, B.; Gopčević, K.; Stanković, S.; Vučević, D.; Jorgačević, B.; Mladenović, D.; Vesković, M.; Samardžić, J.; Ješić, R.; et al. The Role of MIF in Hepatic Function, Oxidative Stress, and Inflammation in Thioacetamide-Induced Liver Injury in Mice: Protective Effects of Betaine. Curr. Med. Chem. 2021, 28, 3249–3268. [Google Scholar] [CrossRef]
- Jorgačević, B.; Vučević, D.; Samardžić, J.; Mladenović, D.; Vesković, M.; Vukićević, D.; Ješić, R.; Radosavljević, T. The Effect of CB1 Antagonism on Hepatic Oxidative/Nitrosative Stress and Inflammation in Nonalcoholic Fatty Liver Disease. Curr. Med. Chem. 2021, 28, 169–180. [Google Scholar] [CrossRef]
- Jorgačević, B.; Mladenović, D.; Ninković, M.; Vesković, M.; Dragutinović, V.; Vatazević, A.; Vučević, D.; Ješić, R.; Radosavljević, T. Rimonabant Improves Oxidative/Nitrosative Stress in Mice with Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2015, 2015, 842108. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Guillot, A.; Tacke, F. Liver Macrophages: Old Dogmas and New Insights. Hepatol. Commun. 2019, 3, 730–743. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radosavljevic, T.; Vukicevic, D.; Djuretić, J.; Gopcevic, K.; Labudovic Borovic, M.; Stankovic, S.; Samardzic, J.; Radosavljevic, M.; Vucevic, D.; Jakovljevic, V. The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine. Biomedicines 2024, 12, 1337. https://doi.org/10.3390/biomedicines12061337
Radosavljevic T, Vukicevic D, Djuretić J, Gopcevic K, Labudovic Borovic M, Stankovic S, Samardzic J, Radosavljevic M, Vucevic D, Jakovljevic V. The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine. Biomedicines. 2024; 12(6):1337. https://doi.org/10.3390/biomedicines12061337
Chicago/Turabian StyleRadosavljevic, Tatjana, Dusan Vukicevic, Jasmina Djuretić, Kristina Gopcevic, Milica Labudovic Borovic, Sanja Stankovic, Janko Samardzic, Milica Radosavljevic, Danijela Vucevic, and Vladimir Jakovljevic. 2024. "The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine" Biomedicines 12, no. 6: 1337. https://doi.org/10.3390/biomedicines12061337
APA StyleRadosavljevic, T., Vukicevic, D., Djuretić, J., Gopcevic, K., Labudovic Borovic, M., Stankovic, S., Samardzic, J., Radosavljevic, M., Vucevic, D., & Jakovljevic, V. (2024). The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine. Biomedicines, 12(6), 1337. https://doi.org/10.3390/biomedicines12061337







