Abstract
Gastrointestinal cancers, which include a variety of esophageal and colorectal malignancies, present a global health challenge and require effective treatment strategies. In the evolving field of cancer immunotherapy, tissue-resident memory T cells (Trm cells) have emerged as important players in the immune response within nonlymphoid tissues. In this review, we summarize the characteristics and functions of Trm cells and discuss their profound implications for patient outcomes in gastrointestinal cancers. Positioned strategically in peripheral tissues, Trm cells have functions beyond immune surveillance, affecting tumor progression, prognosis, and response to immunotherapy. Studies indicate that Trm cells are prognostic markers and correlate positively with enhanced survival. Their presence in the tumor microenvironment has sparked interest in their therapeutic potential, particularly with respect to immune checkpoint inhibitors, which may improve cancer treatment. Understanding how Trm cells work will not only help to prevent cancer spread through effective treatment but will also contribute to disease prevention at early stages as well as vaccine development. The role of Trm cells goes beyond just cancer, and they have potential applications in infectious and autoimmune diseases. This review provides a thorough analysis of Trm cells in gastrointestinal cancers, which may lead to personalized and effective cancer therapies.
1. Introduction
Gastrointestinal cancers, such as pancreatic cancer [1] and colorectal cancer [2] (CRC), remain formidable medical challenges with a substantial effect on global public health. The rising incidence and often late-stage diagnosis of these malignancies underscore the urgency of developing effective treatment strategies. Recently, cancer immunotherapy has shown remarkable progress in the treatment of various cancers; however, the role of tissue-resident memory T cells (Trm cells) in gastrointestinal cancers has become a subject of increased interest [3,4,5,6].
Trm cells, initially described as a specialized subset of memory T cells, are uniquely located within nonlymphoid tissues, where they provide rapid and localized immune responses toward pathogens and malignancy [3,7,8,9]. They are primarily found in peripheral tissues, do not recirculate in the blood, and elicit a rapid immune response in the initial stages of pathogen invasion [4] (Figure 1). Trm cells are present in various solid tumors, correlate with an improved prognosis, and can defend against tumor attack in mice [10].
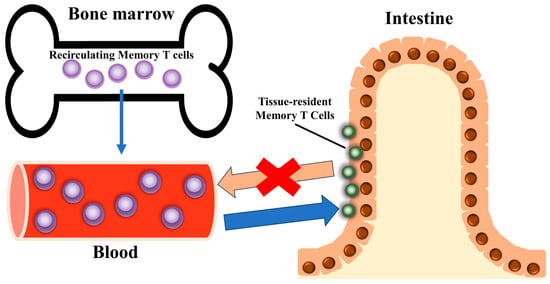
Figure 1.
Tissue-resident memory T cell (Trm cell) localization. Characteristically, they reside in specific tissues and organs, effectively modulate the immune response in those tissues, and do not recirculate into blood vessels.
The uptake and metabolism of exogenous lipids play an important role in the maintenance, longevity, and function of CD8+ Trm cells [11] and trigger the initial response to infections re-encountered on the body surface, where the clearance of pathogens occurs [7,12,13]. In the early stages of acute viral and bacterial pathogeneses, intestinal antigen-specific Trm cells were identified, including the most prominent Blimp1hiId3lo tissue-resident effector cell population. They exhibit various cytokine production abilities, secondary memory capacity, and transcriptional programs, including different roles for the transcriptional regulators, Blimp1, T-bet, Id2, and Id3, to support and maintain gut Trm cells [14]. These cells provide robust immune protection against a wide breadth of both viral and bacterial infections and/or reinfection because of their presence at sites of pathogen entry [15]. Active Trm cell responses also contribute to the development of various chronic respiratory diseases, including pulmonary sequelae following acute viral infection [16]. The phenotypic, transcriptional, and functional characteristics of Trm cells have been elucidated previously in mouse models of infection [17].
Trm cells also play an important role in the pathogenesis of host antifungal infections [18], antimicrobial infections, cancer immunotherapy, and various human autoimmune diseases, such as psoriasis [19,20], vitiligo [19,21], atopic dermatitis [22], lupus nephritis, antineutrophil cytoplasmic antibody-associated glomerulonephritis [23], rheumatoid arthritis [24,25], and inflammatory bowel disease [4]. In the perivascular lumen, cuffs containing CD8+ Trm cells have been observed in advanced multiple sclerosis, suggesting that they may be involved in local reactivation sites [26]. Trm cells may also contribute to autoimmune reactions during organ transplantation [27,28]. Combined single-cell RNA and T-cell receptor (TCR)-sequencing analyses of recipient-derived T cells from the bronchoalveolar lavage fluid of three recipients with lung transplant rejection revealed the accumulation of cytotoxic recipient-derived Trm cells within lung allografts, even after rejection treatment with high-dose systemic glucocorticoids [29].
Trm cell function extends beyond immune surveillance including CD8+CD69+CD103+ Trm cells [30], which renders them key players in the intricate immune landscape of gastrointestinal cancer. The abundance of skin Trm cells, which are CD69- or CD103-positive cells, was increased in skin lesions of cutaneous lupus erythematosus, whereas interferon (IFN)-α increased CD69 expression in T cells [31]. CD103 is not just a biomarker for Trm cells but provides substrate specificity for cell adhesion to e-cadherin, and CD49a is a collagen-binding integrin [32,33]. In contrast, Trm cells are characterized by downregulation of S1PR1 [34,35,36], S1PR5 [37], CD62L [34], CCR7 [34], KLF4 [35], and eomesodermin (EOMES) [38] (Figure 2). These new findings associated with CD8+ T-cell immunity will lead to the development of more effective preventive and therapeutic interventions against cancer and infectious diseases [39]. A recent study indicated that ICOS-PI3K signaling promotes the establishment of CD8+ Trm cells [40]. Dendritic cell-presented antigens and interleukin (IL)-15 play important roles in antigen persistence and the maintenance of CD8+ Trm cells during inflammation [41].
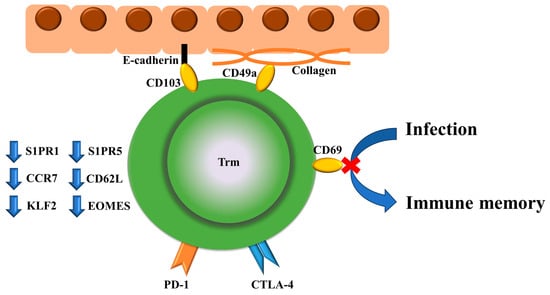
Figure 2.
Surface markers specific to Trm cells and their functions, and markers that are downregulated are shown. Trm cells play a role in PD-1/CTLA-4-mediated cancer immunity, infection defense, and thereby, immune memory. CD103 and CD49a are associated with E-cadherin and collagen, respectively. Trm cells are characterized by the downregulation of S1PR1, S1PR5, CD62L, CCR7, KLF4, and eomesodermin (EOMES).
Trm cells have been identified in the tumor microenvironment (TME) of pancreatic cancer [42] and CRC [4,43], which raises important questions regarding their contribution to antitumor immunity and potential for therapeutic manipulation. In this review, we discuss the emerging field of Trm cells in the context of gastrointestinal cancers. We aimed to comprehensively analyze the current knowledge on the role of Trm cells in these malignancies. We will examine their presence, functions, and potential effects on patient outcomes and discuss the prospects of exploiting Trm cells for novel treatment strategies. This analysis of the intersection of Trm cells and gastrointestinal cancers will contribute to a deeper understanding of the complex immunological dynamics that occur and stimulate further research in pursuit of more effective immunotherapies for cancer.
2. Trm Cells and Gastrointestinal Cancers
Trm cells have gained significant recognition as pivotal components of the immune response against various cancers, such as lung cancer [44,45]. In the intricate context of the gastrointestinal tract, particularly in pancreatic cancer [42] and CRC [43], the interactions between Trm cells and malignancies have taken center stage. In this section, we discuss the effect of Trm cells on gastrointestinal cancer and explore their role in tumor progression, patient prognosis, and as targets for therapeutic intervention.
2.1. Immune Surveillance by Trm Cells in the Gastrointestinal Tumor Microenvironment
The gastrointestinal TME provides a unique site for the interaction between Trm and cancer cells. The location of Trm cells in pancreatic cancer and CRC tissues has captured the attention of researchers and prompted studies regarding their spatial distribution and abundance. This important finding provides insights into the extent of Trm cell infiltration within these tumors.
Trm cells are known for their role in immune surveillance within tissues, and immune surveillance by cells with a Trm-like phenotype was enhanced in individuals with a history of smoking [46]. In gastrointestinal cancers, they actively survey the TME, equipped to detect malignant cells and initiate immune responses [30]. This proactive surveillance by Trm cells is integral to our understanding of their contribution to antitumor immunity.
2.2. Trm Cells as Prognostic Factors
Studies have indicated a significant influence of Trm cells on tumor progression and patient outcomes in pancreatic cancer and CRC. Trm cells in pancreatic ductal adenocarcinoma co-express anti-programmed cell death protein 1 (PD-1) and TIGIT (T Cell Immunoreceptor with Ig And ITIM Domains). Moreover, the combination of anti-PD-1 and TIGIT inhibition therapy enhances IFN-γ secretion and T-cell proliferation in the presence of PD-1 and TIGIT ligands [42]. With further study, it has become apparent that the abundance of Trm cells is a vital determinant of patient survival. In pancreatic cancer patients, those with a higher abundance of Trm cells tended to survive longer compared with those with lower numbers [47]. In left-sided CRC, the presence of activated Trm cells (not CD8 alone) was prognostically significant, and patients with low numbers of activated Trm cells exhibited a poor prognosis, even with high CD8 T-cell infiltration. Interestingly, patients with high CD8 T-cell infiltration and low numbers of activated Trm cells showed good prognosis in right-sided CRC [48].
Tumor invasion by Trm cells correlates with an increased response to current immunotherapies and is often associated with a favorable outcome [49]. Recent studies analyzing Trm cells from the intrinsic layer and epithelial compartment of the small intestine and colon have revealed molecular heterogeneity and a varying dependence on EOMES, which warrants further study [50]. Trm cell formation and maintenance are influenced by several factors, including inflammation, antigen induction, and tissue-specific cues, which suggests that these signals also contribute to heterogeneity within the Trm cell compartment [51].
Although not previously reported in gastrointestinal cancers, CD8+CD103+ tumor-infiltrating lymphocytes are memory T cells that reside in tumor-specific tissues and are considered a prognostic factor for survival in lung cancer patients [44]. The number of CD103+/CD8+ tumor-infiltrating lymphocytes (TILs) is a good prognostic and predictive factor for overall and relapse-free survival in patients with CRC [43]. Based on a combined analysis of single-cell and bulk RNA-sequencing data, the Trm-related gene risk score was closely correlated with the prognosis and treatment response in patients with CRC [52]. The percentage of resident memory CD103-expressing CD8+ and γδTCR+ intraepithelial lymphocytes was markedly reduced in the left and right colon of patients with familial adenomatous polyposis compared with healthy controls [53]. In esophageal cancer, patients with CD103high biopsy specimens showed a favorable prognosis, whereas chemotherapy increased the number of CD103+ cells [54]. The presence of CD103+CD8+ TILs, a Trm cell phenotype, and high expression of immune checkpoints PD-1 and TIM-3 in esophageal squamous cell carcinoma (ESCC) was positively correlated with the overall survival of ESCC patients.
This suggests that this cell population may induce strong proliferation, cytotoxic cytokine production, and antitumor immunity following anti-PD-1 inhibition [55]. Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, and the high frequency of CD103 in anti-PD-1+CD8+ T cells two weeks after nivolumab administration in patients with advanced gastric cancer (GC) may be a useful biomarker for predicting the efficacy of anti-PD-1 therapy [56]. In GC, low levels of tumor-infiltrating CD8+CD103+ Trm cells were positively correlated with GC progression and decreased patient survival [57]. CD103+ T cells are located around tertiary lymphoid structures (TLSs), and TLSs are more abundant in CD103high patients [58].
Furthermore, ZNF683 expression was identified as a candidate biomarker of cancer-specific Trm cells and a promising target for cancer immune regulation [43,59]. Some CD8+ T cells derived from colorectal liver metastases preferentially re-populate patient-derived autologous xenograft tumors as Trm cells [60]. In esophageal cancer, specimens rich in Trm cells showed reduced lymphatic invasion and lymph node metastases as well as prolonged survival compared with specimens with fewer Trm cells [61].
Changes in the gastric microbiome are linked to a decrease in CD8+ Trm cells in the TME of GC [62]. An analysis of the role of Trm cells in enhancing immunogenicity in CRC stratified by microsatellite instability (MSI) and BRAF status revealed that in both BRAF mutants and MSI-H BRAF wild-type MSI-H CRCs, CD8+ Trm cells were more abundant compared with the microsatellite stable group [63]. Ovarian cancer is an immunogenic disease that is dependent on approximately 13% of CD8+ tumor-infiltrating T cells that are initially stimulated against high-affinity antigens and maintain a wave of effector Trm-like cells [64], which was also found to be potentially applicable to other tumors.
Recent studies have revealed an intricate link between the abundance of Trm cells within the TME and favorable survival outcomes [65,66,67,68,69,70,71,72,73]. They have yielded valuable insight into the prognostic implications of Trm cell infiltration, which further underscores their significance in gastrointestinal malignancies.
2.3. Trm Cells in Hepatocellular Carcinoma and Other Liver Diseases
In hepatocellular carcinoma (HCC), patients with predominantly exhausted CD8+ T cells (TEX) showed lower survival rates compared with those with a predominance of Trm cells. These T-cell populations were considered important novel biomarkers [65,66]. The expansion of peripheral Vγ9Vδ2 T cells with aminobisphosphonate reproduces the Trm cell phenotype and increases their cytotoxic potential. When combined with intratumor delivery, they may achieve more effective HCC immunotherapy [74].
One study elucidated the mechanism through which Trm cells coordinate the enhancement of viral clearance by potentiating local lymphoid sites [75]. Because of the potent effector function of hepatic Trm cells, they are essential not only for HCC but also for chronic liver diseases, including viral and parasitic infections, autoimmune liver diseases, non-alcoholic fatty liver disease, and liver transplantation. Moreover, the manipulation of hepatic Trm cells offers a new strategy for precision immunotherapy of chronic liver diseases [76]. Single-cell transcriptome and fluorescence-activated cell sorting analyses revealed enriched CD69+CD103−CD8+ Trm cells in NASH resolution livers [77]. Further insight into intrahepatic Trm cells will lead to a better understanding of the pathophysiology of many liver diseases and the identification of potential drug targets for the development of new therapeutics [78].
3. Trm Cells and Therapeutic Implications
Trm cells have become an important player in the immune response against cancer, particularly within the complex milieu of gastrointestinal tumors, such as pancreatic cancer and CRC. The discovery of Trm cells within these malignancies has sparked considerable interest in their potential use in therapeutic applications. Intratumor Trm cells express checkpoint inhibitory receptors, such as PD-1 and LAG-3; however, their induction can cause dysfunction, often referred to as fatigue, which may limit the effectiveness of Trm cells in countering tumor growth [79].
3.1. Trm Cells in Gastrointestinal Tumors and Immunotherapy
Intestinal tissue is often a target for the local growth of pathogens and invasion prior to entering the systemic circulation. It is also a prominent site of tumorigenesis; thus, promoting Trm cell formation at this site represents an attractive therapeutic option [80]. In this section, we explore how harnessing Trm cells can transform the clinical management of gastrointestinal cancers and other multiple carcinomas, including their relationship with ICIs [81].
Immunotherapy has ushered in a new era of cancer treatment. The inclusion of Trm cells as a therapeutic target adds an intriguing dimension. PD-1 blockade promotes the proliferation of highly suppressive PD-1+ eTreg cells in hyperprogressive disease (HPD), which results in the inhibition of antitumor immunity and is a reliable marker of HPD. Moreover, the depletion of effector Treg (eTreg) cells in tumor tissues is effective for the treatment and prevention of HPD in PD-1 blockade cancer immunotherapy [81] (Figure 3).
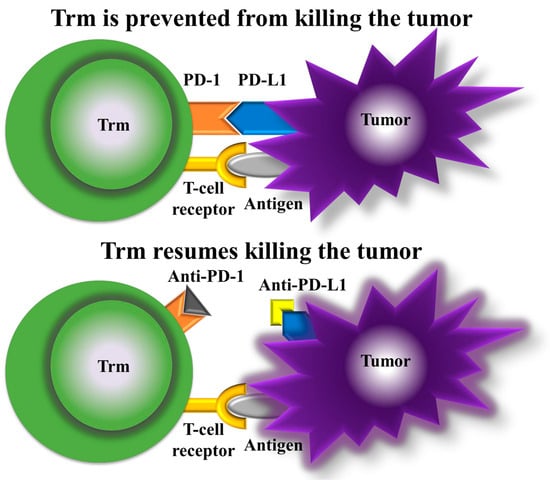
Figure 3.
Mechanism of action of anti-PD-1 and anti-PD-L1. With PD-1 and PD-L1 bound (top panel), Trm cells are prevented from killing the tumor, but with anti-PD-1 and anti-PD-L1 (bottom panel), Trm cells resume tumor killing.
3.2. Neoadjuvant Therapies and Clinical Outcomes of ICIs
Neoadjuvant anti-programmed death protein-1 (PD-1) or anti-PD-1/cytotoxic T-lymphocyte antigen-4 (CTLA-4) therapies in patients with oral cancer exhibit promising clinical activity [82]. The analysis of samples from a phase II clinical trial of head and neck oral squamous carcinoma patients who received neoadjuvant immune checkpoint blockade (ICB) therapy revealed an association of PD-1+/KLRG1- CD8+ T cells with pathologic response, which supports its use as a potential biomarker of ICB response [83].
ICIs targeting PD-1 and CTLA-4 significantly improve the outcomes of metastatic melanoma patients and reduce relapse in resected stage III disease [84]. In patients with metastatic vaginal melanoma, Trm cells are located in the tumor periphery. Trm cells exhibited an excellent functional response to autologous tumor cells and predicted neoantigens and melanoma differentiation antigens, with CD8+ Trm cells showing the highest tumor responsiveness. This suggests that Trm cells retain a strong antitumor T-cell functional response, which further increases following anti-PD-1 therapy [85].
3.3. Enhancing Trm Cell Function and Prognosis in Various Cancers
In mice with CRC liver metastases, the inhibition of the renin–angiotensin system reduced the abundance of immunosuppressive bone-marrow-derived suppressor cells and enhanced PD-1+ hepatic Trm cells, which suggests that it may be an effective adjuvant therapy for patients with CRC liver metastases [86]. ICIs, including nivolumab, have been approved for the treatment of esophageal cancer, and patients with an abundance of Trm cells have a better prognosis after starting nivolumab therapy. This suggests that Trm cells are important prognostic factors [87]. The prophylactic use of ICIs during the early stages of ESCC potentially provides long-term benefits to patients [88]. Thus, these CD103+CD8+ Trm cells may be regulated by retinoic acid metabolism [89]. These cells are pivotal in enhancing the effectiveness of ICIs, thereby expanding their utility in clinical practice. In addition, the development of Trm-cell-based therapies has the potential to provide novel treatments that enhance patient outcomes [90].
3.4. Trm Cells and Immune-Related Adverse Events
Moreover, our understanding of the etiology of ICI-induced colitis has improved. CD8+ Trm cell activation correlates with the severity of clinical and endoscopic ICI colitis, and activated CD8+ Trm cells express high levels of checkpoint inhibitors and IFN-γ transcripts in ICI colitis [90]. TCR-sequencing analysis identified cytokines, chemokines, and surface receptors that may serve as therapeutic targets for inflammatory side effects [91]. In addition, immune-related adverse events (irAEs), which are frequently caused by ICIs and can be life-threatening in some cases, as well as the expression of IFNγ, CXCL9, CXCL10, and TNFα in irAE dermatitis, have been confirmed, particularly in Trm cells [92].
3.5. The Impact of IL Pathways in Trm-Cell-Mediated Cancer Therapy and Psoriasis Treatment
The interaction between Trm cells and ICIs is a focal point in the evolving landscape of cancer therapy. Trm cells act as sentinel cells within the TME to promote an immune response against cancer cells when checkpoint inhibitors are administered. This interplay suggests the potential for synergy and improved therapeutic outcomes. Clonotype and trajectory analyses of the TME in GC revealed that Tc17 cells (IL-17 + CD8 + T cells) are derived from Trm cells and can subsequently differentiate into depleted T cells. This suggests the possibility of targeting IL17+ cells and associated signaling pathways as a therapeutic strategy for the treatment of GC [93]. In psoriasis, IL-17A-producing CD8+ Trm cells represent an attractive therapeutic target because they are considered one of the pathogenic populations in the skin [94]. Trm cells are classified as CD8+ Trm cells and are primarily distributed in the epidermis, whereas CD4+ Trm cells reside in the dermis. CD8+ Trm cells are derived from circulating memory T cells, and CD49a-CD8+ Trm cells have an important role in the recurrence of psoriasis because IL-23 can also activate Trm cells. Thus, neutralizing antibodies against IL-23 may be effective in the clinic [95].
3.6. Trm Cells in Infection, Immunity, and Prevention
In addition to therapy, a neoantigen peptide vaccine was developed for HCC against Trm cells. When combined with α-PD-1, it significantly increases the number of CD8+ Trm cells and exhibits potent tumor-killing ability [96]. In addition, the newly reported CD4+ Trm cells may be important for advancing the design of new vaccines and the development of new therapies for CD4+ T-cell-mediated autoimmune diseases [97].
Applying our knowledge of local Trm cell function not only enables tertiary prevention, such as the early treatment of tumors, but also plays a major role in secondary prevention, such as immune surveillance, particularly for early cancer detection [98]. Although Trm cells are key cell types involved in the early detection and restriction of mucosal pathogens, following tissue-specific infection or vaccination, they remain in the tissue and perform rapid sensing and alarm functions to control reinfection by various respiratory pathogens, such as influenza and respiratory syncytial agents [99]. Herpes simplex virus and cytomegalovirus infections in both mice and humans constitute particularly important viral sanctuaries and induce Trm cells in mucosal tissue, which is the site of entry associated with attack and overlapping infection [100]. Examination of CD4+ and CD8+ Trm cells in the lungs of BALB/c mice after acute respiratory syncytial virus (RSV) infection revealed enhanced viral clearance. This suggests that CD8+ Trm cells contribute to protection against secondary RSV infection, and given this protective capacity, they are considered a future RSV vaccine candidate [101]. Further understanding of the mechanisms of protective immune enhancement will improve rational vaccine development for primary prevention [96,102,103] (Figure 4).
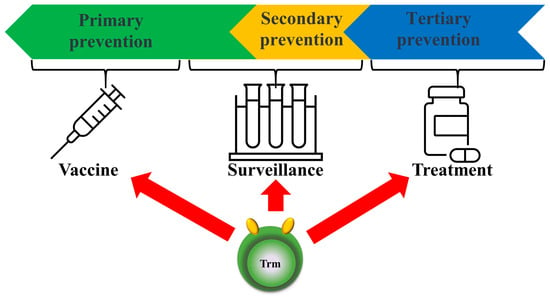
Figure 4.
Prospects for future clinical applications of Trm cells. The concept of Trm cells is not only used for treatment (tertiary prevention) but also surveillance for primary/secondary prevention and for vaccines (primary prevention).
3.7. Future Directions and Therapeutic Potential
The multifaceted understanding and application of Trm cells could be a major contributor to cancer therapy and the treatment of infectious and autoimmune diseases. A summary of the references that we have discussed regarding Trm cells in various cancers is listed in Table 1. As we explore the therapeutic implications of Trm cells in gastrointestinal cancers, the results indicate that these tissue-resident immune cells have the potential to revolutionize the treatment of patients with various types of cancers. Innovative treatment strategies that exploit Trm cells and their synergy with checkpoint inhibitors will lead to more effective and personalized cancer therapies.

Table 1.
Summary of references for Trm cells in various cancers.
4. Conclusions
In conclusion, the role of tissue-resident memory T (Trm) cells in gastrointestinal (GI) cancers, particularly pancreatic cancer and colorectal cancer (CRC), represents a promising frontier in cancer immunotherapy. The distinct ability of Trm cells to reside in nonlymphoid tissues and provide rapid, localized immune responses underscores their potential as pivotal players in the fight against GI malignancies. Recent studies have demonstrated that Trm cells in the tumor microenvironment (TME) of GI cancers can significantly influence tumor progression, patient outcomes, and responses to immunotherapy.
The presence of Trm cells within pancreatic cancer and CRC tissues has been correlated with improved prognosis and survival rates [42,47]. These cells exhibit unique phenotypic and functional characteristics that enable them to perform robust immune surveillance and mount effective antitumor responses [30]. For instance, the co-expression of inhibitory receptors such as PD-1 and TIGIT on Trm cells in pancreatic cancer highlights the potential of combined inhibition therapies to enhance antitumor immunity [42]. Moreover, the density and activation state of Trm cells in CRC have been linked to differential prognostic outcomes depending on the tumor location, further emphasizing their role as critical determinants of patient survival [48].
Trm cells also show promise as biomarkers for predicting the efficacy of immune checkpoint inhibitors (ICIs), such as PD-1 and CTLA-4 blockers. Patients with a higher abundance of Trm cells often exhibit better responses to ICIs and improved clinical outcomes [43,56]. This highlights the therapeutic potential of targeting Trm cells to enhance the effectiveness of current immunotherapy strategies. Additionally, the modulation of Trm cell activity through various adjuvant therapies, such as renin–angiotensin system inhibitors, has shown potential in augmenting the antitumor responses in CRC liver metastases [86].
As research advances, the therapeutic manipulation of Trm cells could revolutionize the treatment landscape for GI cancers. Developing strategies to increase the infiltration and activation of Trm cells within the TME, while mitigating the risk of immune-related adverse events, will be crucial. Furthermore, the integration of Trm-cell-targeted therapies with existing modalities, including ICIs and personalized vaccine approaches, offers a promising pathway toward more effective and durable cancer treatments.
In summary, the emerging insights into the role of Trm cells in GI cancers underscore their potential as powerful mediators of antitumor immunity. By leveraging their unique properties and understanding the mechanisms that regulate their function, we can pave the way for innovative and personalized therapeutic strategies that improve patient outcomes and advance the field of cancer immunotherapy.
Author Contributions
H.I. conceptualized the study objectives and obtained the funding. H.S., S.M., T.H. (Tomoaki Hara)., Y.T., Y.A. and H.I. contributed to drawing artwork, collecting information, and writing the manuscript. H.S., T.H. (Tomoaki Hara), K.S., S.K., Y.D., E.d.L., T.H. (Takaaki Hirotsu), T.S., H.E. and H.I. constructed the study design and outlined the content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (grant nos. 17cm0106414h0002, JP21lm0203007, 18KK0251, 19K22658, 20H00541, 21K19526, 22H03146, 22K19559, 23K19505, and 16H06279 (PAGS)). Partial support was offered by the Mitsubishi Foundation (2021-48), the Yokoyama Foundation for Clinical Pharmacology (2023), the Institute for Fermentation Osaka Research Fund (2023), and the Princess Takamatsu Cancer Research Fund (23-255001) to H.I.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are thankful to every lab member.
Conflicts of Interest
Partial institutional endowments were received from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan), Hirotsu Bio Science Inc. (Tokyo, Japan), Kinshu-kai Medical Corporation (Osaka, Japan), Kyowa-kai Medical Corporation (Osaka, Japan), IDEA Consultants Inc. (Tokyo, Japan), and Unitech Co. Ltd. (Chiba, Japan). E.d.L. is an employee and T.H. (Takaaki Hirotsu) is the CEO of Hirotsu Bio Science Inc. T.H. (Takaaki Hirotsu) and H.I. are guest editors of this journal. The remaining authors have no conflicts of interest for this article.
References
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Park, S.L.; Gebhardt, T.; Mackay, L.K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 2019, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Zhou, Y.; Shen, J. An Overview of Tissue-Resident Memory T Cells in the Intestine: From Physiological Functions to Pathological Mechanisms. Front. Immunol. 2022, 13, 912393. [Google Scholar] [CrossRef]
- Abdeljaoued, S.; Arfa, S.; Kroemer, M.; Ben Khelil, M.; Vienot, A.; Heyd, B.; Loyon, R.; Doussot, A.; Borg, C. Tissue-resident memory T cells in gastrointestinal cancer immunology and immunotherapy: Ready for prime time? J. Immunother. Cancer 2022, 10, e003472. [Google Scholar] [CrossRef]
- Liang, M.; Wang, X.; Cai, D.; Guan, W.; Shen, X. Tissue-resident memory T cells in gastrointestinal tumors: Turning immune desert into immune oasis. Front. Immunol. 2023, 14, 1119383. [Google Scholar] [CrossRef]
- Yenyuwadee, S.; Sanchez-Trincado Lopez, J.L.; Shah, R.; Rosato, P.C.; Boussiotis, V.A. The evolving role of tissue-resident memory T cells in infections and cancer. Sci. Adv. 2022, 8, eabo5871. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.; Masopust, D.; Schumacher, T.N. The precursors of CD8+ tissue resident memory T cells: From lymphoid organs to infected tissues. Nat. Rev. Immunol. 2022, 22, 283–293. [Google Scholar] [CrossRef]
- Asada, N.; Ginsberg, P.; Gagliani, N.; Mittrucker, H.W.; Panzer, U. Tissue-resident memory T cells in the kidney. Semin. Immunopathol. 2022, 44, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Mackay, L.K.; Waithman, J.; Gebhardt, T. Tissue-resident memory T cells orchestrate tumour-immune equilibrium. Cell Stress 2019, 3, 162–164. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Parga-Vidal, L.; van Aalderen, M.C.; Stark, R.; van Gisbergen, K. Tissue-resident memory T cells in the urogenital tract. Nat. Rev. Nephrol. 2022, 18, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Toma, C.; He, Z.; Kurd, N.S.; Nguyen, Q.P.; McDonald, B.; Quezada, L.; Widjaja, C.E.; Witherden, D.A.; Crowl, J.T.; et al. Heterogenous Populations of Tissue-Resident CD8+ T Cells Are Generated in Response to Infection and Malignancy. Immunity 2020, 52, 808–824.e7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Z.M.; Wakim, L.M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022, 15, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Cheon, I.S.; Son, Y.M.; Sun, J. Tissue-resident memory T cells and lung immunopathology. Immunol. Rev. 2023, 316, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S. Tissue-Resident Memory T Cells in Antifungal Immunity. Front. Immunol. 2021, 12, 693055. [Google Scholar] [CrossRef]
- Ryan, G.E.; Harris, J.E.; Richmond, J.M. Resident Memory T Cells in Autoimmune Skin Diseases. Front. Immunol. 2021, 12, 652191. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, P.; Chen, C.; Yan, B.; Chen, X.; Li, J.; Peng, C. Advancements in the characterization of tissue resident memory T cells in skin disease. Clin. Immunol. 2022, 245, 109183. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Z. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell. Mol. Immunol. 2020, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kupper, T.S. Metabolic Reprogramming and Longevity of Tissue-Resident Memory T Cells. Front. Immunol. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, P.; Panzer, U.; Asada, N. Tissue-resident memory T cells in renal autoimmune diseases. Front. Immunol. 2023, 14, 1111521. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Levescot, A.; Nelson-Maney, N.; Blaustein, R.B.; Winden, K.D.; Morris, A.; Wactor, A.; Balu, S.; Grieshaber-Bouyer, R.; Wei, K.; et al. Arthritis flares mediated by tissue-resident memory T cells in the joint. Cell Rep. 2021, 37, 109902. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zhao, W.; Wu, R.; Su, R.; Jin, R.; Luo, J.; Gao, C.; Li, X.; Wang, C. Tissue-resident memory T cells: The key frontier in local synovitis memory of rheumatoid arthritis. J. Autoimmun. 2022, 133, 102950. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Fransen, N.L.; Hsiao, C.C.; Hamann, J.; Huitinga, I. Perivascular tissue resident memory T cells as therapeutic target in multiple sclerosis. Expert Rev. Neurother. 2020, 20, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Abou-Daya, K.I.; Tieu, R.; Zhao, D.; Rammal, R.; Sacirbegovic, F.; Williams, A.L.; Shlomchik, W.D.; Oberbarnscheidt, M.H.; Lakkis, F.G. Resident memory T cells form during persistent antigen exposure leading to allograft rejection. Sci. Immunol. 2021, 6, eabc8122. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sykes, M. Emerging Concepts of Tissue-resident Memory T Cells in Transplantation. Transplantation 2022, 106, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.E.; Moghbeli, K.; Bondonese, A.; Craig, A.; Popescu, I.; Fan, L.; Tabib, T.; Lafyatis, R.; Chen, K.; Trejo Bittar, H.E.; et al. Modulation of tissue resident memory T cells by glucocorticoids after acute cellular rejection in lung transplantation. J. Exp. Med. 2022, 219, e20212059. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2020, 11, 618897. [Google Scholar] [CrossRef]
- Gu, H.J.; Song, S.; Roh, J.Y.; Jung, Y.; Kim, H.J. Expression pattern of tissue-resident memory T cells in cutaneous lupus erythematosus. Lupus 2021, 30, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Topham, D.J.; Reilly, E.C. Tissue-Resident Memory CD8+ T Cells: From Phenotype to Function. Front. Immunol. 2018, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.V.; Ruef, N.; Wissmann, S. Organ-Specific Surveillance and Long-Term Residency Strategies Adapted by Tissue-Resident Memory CD8+ T Cells. Front. Immunol. 2021, 12, 626019. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.A.K.; van der Geest, J.; Vastert, S.J.; van Loosdregt, J.; van Wijk, F. Tissue-Resident Memory T Cells in Chronic Inflammation-Local Cells with Systemic Effects? Cells 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, J.S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Shin, E.C.; Hong, S. Synovial fluid CD69+CD8+ T cells with tissue-resident phenotype mediate perforin-dependent citrullination in rheumatoid arthritis. Clin. Transl. Immunol. 2020, 9, e1140. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.G.; Pallett, L.J.; Li, X.; Davies, S.P.; Amin, O.E.; Gill, U.S.; Kucykowicz, S.; Patel, A.M.; Aliazis, K.; Liu, Y.S.; et al. The human liver microenvironment shapes the homing and function of CD4+ T-cell populations. Gut 2022, 71, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Hallisey, V.M.; Schwab, S.R. Blood-thirsty: S1PR5 and TRM. J. Exp. Med. 2022, 219, e20211971. [Google Scholar] [CrossRef]
- Mami-Chouaib, F.; Blanc, C.; Corgnac, S.; Hans, S.; Malenica, I.; Granier, C.; Tihy, I.; Tartour, E. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer 2018, 6, 87. [Google Scholar] [CrossRef]
- Buggert, M.; Price, D.A.; Mackay, L.K.; Betts, M.R. Human circulating and tissue-resident memory CD8+ T cells. Nat. Immunol. 2023, 24, 1076–1086. [Google Scholar] [CrossRef]
- Peng, C.; Huggins, M.A.; Wanhainen, K.M.; Knutson, T.P.; Lu, H.; Georgiev, H.; Mittelsteadt, K.L.; Jarjour, N.N.; Wang, H.; Hogquist, K.A.; et al. Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells. Immunity 2022, 55, 98–114.e5. [Google Scholar] [CrossRef]
- Tieu, R.; Zeng, Q.; Zhao, D.; Zhang, G.; Feizi, N.; Manandhar, P.; Williams, A.L.; Popp, B.; Wood-Trageser, M.A.; Demetris, A.J.; et al. Tissue-resident memory T cell maintenance during antigen persistence requires both cognate antigen and interleukin-15. Sci. Immunol. 2023, 8, eadd8454. [Google Scholar] [CrossRef] [PubMed]
- Pearce, H.; Croft, W.; Nicol, S.M.; Margielewska-Davies, S.; Powell, R.; Cornall, R.; Davis, S.J.; Marcon, F.; Pugh, M.R.; Fennell, E.; et al. Tissue-Resident Memory T Cells in Pancreatic Ductal Adenocarcinoma Coexpress PD-1 and TIGIT and Functional Inhibition Is Reversible by Dual Antibody Blockade. Cancer Immunol. Res. 2023, 11, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Uemura, M.; Hara, T.; Chijimatsu, R.; Motooka, D.; Hirai, T.; Konno, M.; Okuzaki, D.; Sekido, Y.; Hata, T.; et al. Cancer-specific tissue-resident memory T-cells express ZNF683 in colorectal cancer. Br. J. Cancer 2023, 128, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Djenidi, F.; Adam, J.; Goubar, A.; Durgeau, A.; Meurice, G.; de Montpreville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Yu, J.; Jiao, Z.; Li, J.; Wu, F.; Yan, R.; Huang, X.; Chen, C. The Roles of Tissue-Resident Memory T Cells in Lung Diseases. Front. Immunol. 2021, 12, 710375. [Google Scholar] [CrossRef] [PubMed]
- Weeden, C.E.; Gayevskiy, V.; Marceaux, C.; Batey, D.; Tan, T.; Yokote, K.; Ribera, N.T.; Clatch, A.; Christo, S.; Teh, C.E.; et al. Early immune pressure initiated by tissue-resident memory T cells sculpts tumor evolution in non-small cell lung cancer. Cancer Cell 2023, 41, 837–852.e6. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, T.; Akar, K.E.; Bagci, P.; Akgul Babacan, N.; Ozturk, M.A.; Ozturk, F.E.; Demircan, N.C.; Arikan, R.; Akin Telli, T.; Ercelep, O.; et al. Prognostic Value of Tissue-Resident Memory T Cells and Tumor Microenvironmental Features in Resected Pancreatic Adenocarcinoma. Balk. Med. J. 2022, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Talhouni, S.; Fadhil, W.; Mongan, N.P.; Field, L.; Hunter, K.; Makhsous, S.; Maciel-Guerra, A.; Kaur, N.; Nestarenkaite, A.; Laurinavicius, A.; et al. Activated tissue resident memory T-cells (CD8+CD103+CD39+) uniquely predict survival in left sided “immune-hot” colorectal cancers. Front. Immunol. 2023, 14, 1057292. [Google Scholar] [CrossRef] [PubMed]
- Okla, K.; Farber, D.L.; Zou, W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J. Exp. Med. 2021, 218, e20201605. [Google Scholar] [CrossRef]
- Lin, Y.H.; Duong, H.G.; Limary, A.E.; Kim, E.S.; Hsu, P.; Patel, S.A.; Wong, W.H.; Indralingam, C.S.; Liu, Y.C.; Yao, P.; et al. Small intestine and colon tissue-resident memory CD8+ T cells exhibit molecular heterogeneity and differential dependence on Eomes. Immunity 2023, 56, 207–223.e8. [Google Scholar] [CrossRef]
- van der Gracht, E.T.I.; Behr, F.M.; Arens, R. Functional Heterogeneity and Therapeutic Targeting of Tissue-Resident Memory T Cells. Cells 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Zheng, Y. Identification of a tissue resident memory CD8 T cell-related risk score signature for colorectal cancer, the association with TME landscapes and therapeutic responses. Front. Genet. 2022, 13, 1088230. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Durant, L.; Dilke, S.M.; Man, R.; Martin, I.; Patel, R.; Hoyles, L.; Pring, E.T.; Latchford, A.; Clark, S.K.; et al. Altered Mucosal Immune-Microbiota Interactions in Familial Adenomatous Polyposis. Clin. Transl. Gastroenterol. 2022, 13, e00428. [Google Scholar] [CrossRef]
- Natsuki, S.; Miki, Y.; Tanaka, H.; Nishiyama, M.; Kasashima, H.; Fukuoka, T.; Yoshii, M.; Tamura, T.; Shibutani, M.; Toyokawa, T.; et al. Usefulness of Biopsy Specimens for Evaluating CD103+ Tumor-resident Memory T Cells in Esophageal Cancer. Anticancer Res. 2023, 43, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, Q.L.; Zhou, X.M.; Shi, C.; Chen, G.Y.; Song, Y.P.; Yao, Y.J.; Zhao, Y.M.; Wen, X.Y.; Liu, S.L.; et al. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: May be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol. Immunother. 2020, 69, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Nose, Y.; Saito, T.; Yamamoto, K.; Yamashita, K.; Tanaka, K.; Yamamoto, K.; Makino, T.; Takahashi, T.; Kawashima, A.; Haruna, M.; et al. The tissue-resident marker CD103 on peripheral blood T cells predicts responses to anti-PD-1 therapy in gastric cancer. Cancer Immunol. Immunother. 2023, 72, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, X.L.; Li, Y.X.; Shan, Z.G.; Zhao, Y.L.; Cheng, P.; Zhao, Z.; Zhang, J.Y.; Chen, W.; Zhuang, Y.; et al. Distribution, phenotype, functional and clinical relevance of CD8+CD103+ tissue-resident memory T cells in human gastric cancer. Cancer Immunol. Immunother. 2022, 71, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tanaka, H.; Suzuki, S.; Deguchi, S.; Yamakoshi, Y.; Yoshii, M.; Miki, Y.; Tamura, T.; Toyokawa, T.; Lee, S.; et al. Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Sci. 2021, 112, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qin, S.; Si, W.; Wang, A.; Xing, B.; Gao, R.; Ren, X.; Wang, L.; Wu, X.; Zhang, J.; et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 2021, 374, abe6474. [Google Scholar] [CrossRef]
- Liang, F.; Nilsson, L.M.; Byvald, F.; Rezapour, A.; Taflin, H.; Nilsson, J.A.; Yrlid, U. A Fraction of CD8+ T Cells from Colorectal Liver Metastases Preferentially Repopulate Autologous Patient-Derived Xenograft Tumors as Tissue-Resident Memory T Cells. Cancers 2022, 14, 2882. [Google Scholar] [CrossRef]
- Natsuki, S.; Tanaka, H.; Nishiyama, M.; Mori, T.; Deguchi, S.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; et al. Prognostic relevance of tumor-resident memory T cells in metastatic lymph nodes of esophageal squamous cell carcinoma. Cancer Sci. 2023, 114, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Liu, S.; You, W.; Huang, Y.; Hu, C.; Gao, Y.; Jia, X.; Li, G.; Xu, Z.; Chen, Y. Gastric Microbiome Alterations Are Associated with Decreased CD8+ Tissue-Resident Memory T Cells in the Tumor Microenvironment of Gastric Cancer. Cancer Immunol. Res. 2022, 10, 1224–1240. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Ferguson, A.L.; Spring, K.J.; Mahajan, H.; Palendira, U. Cytotoxic CD8+ T cells and tissue resident memory cells in colorectal cancer based on microsatellite instability and BRAF status. World J. Clin. Oncol. 2021, 12, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Anadon, C.M.; Yu, X.; Hanggi, K.; Biswas, S.; Chaurio, R.A.; Martin, A.; Payne, K.K.; Mandal, G.; Innamarato, P.; Harro, C.M.; et al. Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells. Cancer Cell 2022, 40, 545–557.e13. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Barsch, M.; Salie, H.; Schlaak, A.E.; Zhang, Z.; Hess, M.; Mayer, L.S.; Tauber, C.; Otto-Mora, P.; Ohtani, T.; Nilsson, T.; et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022, 77, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Savas, P.; Sant, S.; Li, R.; Virassamy, B.; Luen, S.J.; Beavis, P.A.; Mackay, L.K.; Neeson, P.J.; Loi, S. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat. Rev. Clin. Oncol. 2020, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Martin, E.M.; Mellows, T.W.P.; Clarke, J.; Ganesan, A.P.; Wood, O.; Cazaly, A.; Seumois, G.; Chee, S.J.; Alzetani, A.; King, E.V.; et al. M1hot tumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. J. Immunother. Cancer 2020, 8, e000778. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Yu, Y.; Zeng, H.; Liu, Z.; You, R.; Zhang, H.; Liu, C.; Su, X.; Yan, S.; Chang, Y.; et al. CD103+CD8+ tissue-resident memory T cell infiltration predicts clinical outcome and adjuvant therapeutic benefit in muscle-invasive bladder cancer. Br. J. Cancer 2022, 126, 1581–1588. [Google Scholar] [CrossRef]
- La Manna, M.P.; Di Liberto, D.; Lo Pizzo, M.; Mohammadnezhad, L.; Shekarkar Azgomi, M.; Salamone, V.; Cancila, V.; Vacca, D.; Dieli, C.; Maugeri, R.; et al. The Abundance of Tumor-Infiltrating CD8+ Tissue Resident Memory T Lymphocytes Correlates with Patient Survival in Glioblastoma. Biomedicines 2022, 10, 2454. [Google Scholar] [CrossRef]
- Romagnoli, G.; D’Alessandris, Q.G.; Capone, I.; Tavilla, A.; Canini, I.; Lapenta, C.; Buccarelli, M.; Giordano, M.; Tirelli, V.; Sanchez, M.; et al. CD8+CD103+PD1+TIM3+ T cells in glioblastoma microenvironment correlate with prognosis. Immunology 2023, 171, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, C.; Goswami, S.; Huang, X.; Ma, L.; Guo, Y.; Yang, B.; Li, T.; Chi, Y.; Zhang, X.; et al. Tumoral PD-1hiCD8+ T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin. Sci. 2020, 134, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Young, R.J.; Bressel, M.; Cernelc, J.; Savas, P.; Liu, H.; Urban, D.; Thai, A.; Cooper, C.; Fua, T.; et al. Identification of an excellent prognosis subset of human papillomavirus-associated oropharyngeal cancer patients by quantification of intratumoral CD103+ immune cell abundance. Ann. Oncol. 2019, 30, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, N.; Hall, A.; Swadling, L.; Pallett, L.J.; Schmidt, N.M.; Diniz, M.O.; Kucykowicz, S.; Amin, O.E.; Gander, A.; Pinzani, M.; et al. Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat. Commun. 2022, 13, 1372. [Google Scholar] [CrossRef]
- Paik, D.H.; Farber, D.L. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 2021, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; You, Z.; Tang, R.; Ma, X. Tissue-resident memory T cells in chronic liver diseases: Phenotype, development and function. Front. Immunol. 2022, 13, 967055. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Teratani, T.; Chu, P.S.; Hagihara, Y.; Mikami, Y.; Harada, Y.; Tsujikawa, H.; Miyamoto, K.; Suzuki, T.; Taniki, N.; et al. CD8+ tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 2021, 12, 4474. [Google Scholar] [CrossRef]
- Bartsch, L.M.; Damasio, M.P.S.; Subudhi, S.; Drescher, H.K. Tissue-Resident Memory T Cells in the Liver-Unique Characteristics of Local Specialists. Cells 2020, 9, 2457. [Google Scholar] [CrossRef] [PubMed]
- Beumer-Chuwonpad, A.; Taggenbrock, R.; Ngo, T.A.; van Gisbergen, K. The Potential of Tissue-Resident Memory T Cells for Adoptive Immunotherapy against Cancer. Cells 2021, 10, 2234. [Google Scholar] [CrossRef]
- Cheng, L.; Becattini, S. Intestinal CD8+ tissue-resident memory T cells: From generation to function. Eur. J. Immunol. 2022, 52, 1547–1560. [Google Scholar] [CrossRef]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Wang, Y.; Gunasti, L.; Porter, C.B.M.; Nabilsi, N.; Tadros, J.; Ferretti, A.P.; Liao, S.; Gurer, C.; et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 2022, 185, 2918–2935.e29. [Google Scholar] [CrossRef]
- Tissue-Resident Memory T Cells Underlie Neoadjuvant Immunotherapy Response. Cancer Discov. 2022, 12, OF2. [CrossRef]
- Plunkett, K.R.; Armitage, J.D.; Inderjeeth, A.J.; McDonnell, A.M.; Waithman, J.; Lau, P.K.H. Tissue-resident memory T cells in the era of (Neo) adjuvant melanoma management. Front. Immunol. 2022, 13, 1048758. [Google Scholar] [CrossRef]
- Pizzolla, A.; Keam, S.P.; Vergara, I.A.; Caramia, F.; Thio, N.; Wang, M.; Kocovski, N.; Tantalo, D.; Jabbari, J.; Au-Yeung, G.; et al. Tissue-resident memory T cells from a metastatic vaginal melanoma patient are tumor-responsive T cells and increase after anti-PD-1 treatment. J. Immunother. Cancer 2022, 10, e004574. [Google Scholar] [CrossRef]
- Riddiough, G.E.; Walsh, K.A.; Fifis, T.; Kastrappis, G.; Tran, B.M.; Vincan, E.; Muralidharan, V.; Christophi, C.; Gordon, C.L.; Perini, M.V. Captopril, a Renin-Angiotensin System Inhibitor, Attenuates Tumour Progression in the Regenerating Liver Following Partial Hepatectomy. Int. J. Mol. Sci. 2022, 23, 5281. [Google Scholar] [CrossRef]
- Natsuki, S.; Tanaka, H.; Nishiyama, M.; Deguchi, S.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; Maeda, K. Significance of CD103+ tissue-resident memory T cells for predicting the effectiveness of immune checkpoint inhibitors in esophageal cancer. BMC Cancer 2023, 23, 1011. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, R.; Liu, H.; Huang, X.; Liang, Z.; An, G.; Ge, Y. Preventive Treatment with PD-1 Antibody Increases Tissue-resident Memory T Cells Infiltration and Delays Esophageal Carcinogenesis. Cancer Prev. Res. 2023, 16, 669–679. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Lu, X.F.; Chen, H.; Wang, X.Y.; Cheng, W.; Zhang, Q.W.; Chen, J.N.; Wang, X.Y.; Jin, J.Z.; Yan, F.R.; et al. Single-cell Transcriptomics Reveals Early Molecular and Immune Alterations Underlying the Serrated Neoplasia Pathway Toward Colorectal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 393–424. [Google Scholar] [CrossRef]
- Sasson, S.C.; Slevin, S.M.; Cheung, V.T.F.; Nassiri, I.; Olsson-Brown, A.; Fryer, E.; Ferreira, R.C.; Trzupek, D.; Gupta, T.; Al-Hillawi, L.; et al. Interferon-Gamma-Producing CD8+ Tissue Resident Memory T Cells Are a Targetable Hallmark of Immune Checkpoint Inhibitor-Colitis. Gastroenterology 2021, 161, 1229–1244.e9. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e22. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Shapiro, J.W.; Yu, J.; Rouhani, S.J.; Olson, D.J.; Zha, Y.; Gajewski, T.F. Checkpoint Blockade-Induced Dermatitis and Colitis Are Dominated by Tissue-Resident Memory T Cells and Th1/Tc1 Cytokines. Cancer Immunol. Res. 2022, 10, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xu, R.; Ma, F.; Yang, N.; Li, Y.; Sun, X.; Jin, P.; Kang, W.; Jia, L.; Xiong, J.; et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat. Commun. 2022, 13, 4943. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Koguchi-Yoshioka, H.; Watanabe, R. Skin-Resident Memory T Cells: Pathogenesis and Implication for the Treatment of Psoriasis. J. Clin. Med. 2021, 10, 3822. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lin, L.; Du, J. Characteristics and sources of tissue-resident memory T cells in psoriasis relapse. Curr. Res. Immunol. 2023, 4, 100067. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Qiu, L.; Dong, X.; Chen, G.; Shi, Y.; Cai, L.; Liu, W.; Ye, H.; Zhou, Y.; et al. Personalized neoantigen vaccine combined with PD-1 blockade increases CD8+ tissue-resident memory T-cell infiltration in preclinical hepatocellular carcinoma models. J. Immunother. Cancer 2022, 10, e004389. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Joern, R.R.; Forsthuber, T.G. Memory CD4+ T Cells in Immunity and Autoimmune Diseases. Cells 2020, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Palendira, U.; Tscharke, D.C.; Bedoui, S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 2018, 283, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Hassert, M.; Harty, J.T. Tissue resident memory T cells- A new benchmark for the induction of vaccine-induced mucosal immunity. Front. Immunol. 2022, 13, 1039194. [Google Scholar] [CrossRef]
- Thom, J.T.; Oxenius, A. Tissue-resident memory T cells in cytomegalovirus infection. Curr. Opin. Virol. 2016, 16, 63–69. [Google Scholar] [CrossRef]
- Luangrath, M.A.; Schmidt, M.E.; Hartwig, S.M.; Varga, S.M. Tissue-Resident Memory T Cells in the Lungs Protect against Acute Respiratory Syncytial Virus Infection. Immunohorizons 2021, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dumauthioz, N.; Labiano, S.; Romero, P. Tumor Resident Memory T Cells: New Players in Immune Surveillance and Therapy. Front. Immunol. 2018, 9, 2076. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).